The Risk Genes for Neuropsychiatric Disorders negr1 and opcml Are Expressed throughout Zebrafish Brain Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Whole-Mount In Situ Hybridisation
2.3. PCR
3. Results
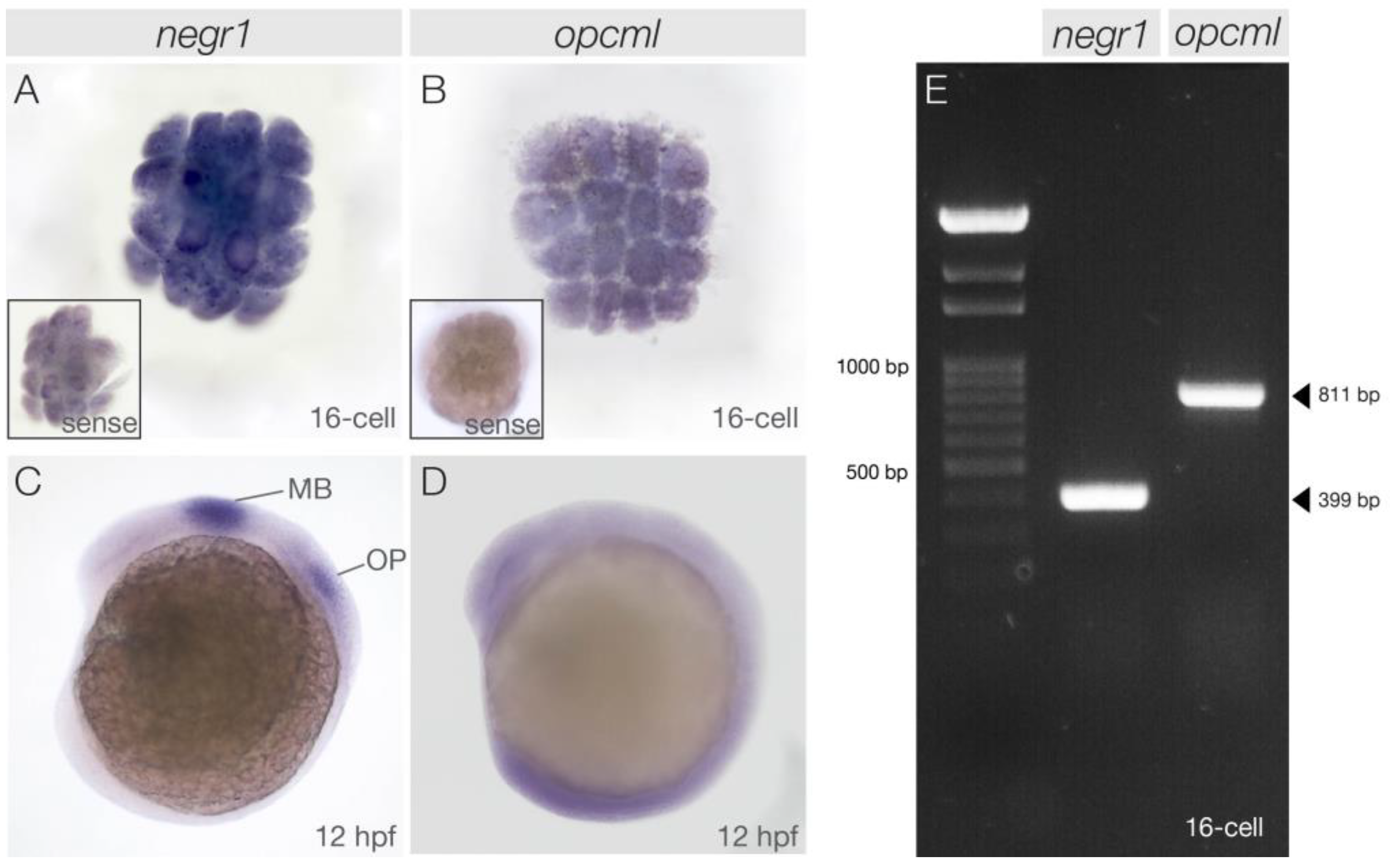
3.1. negr1 and opcml Transcripts Are Maternally Deposited in the Early Embryo
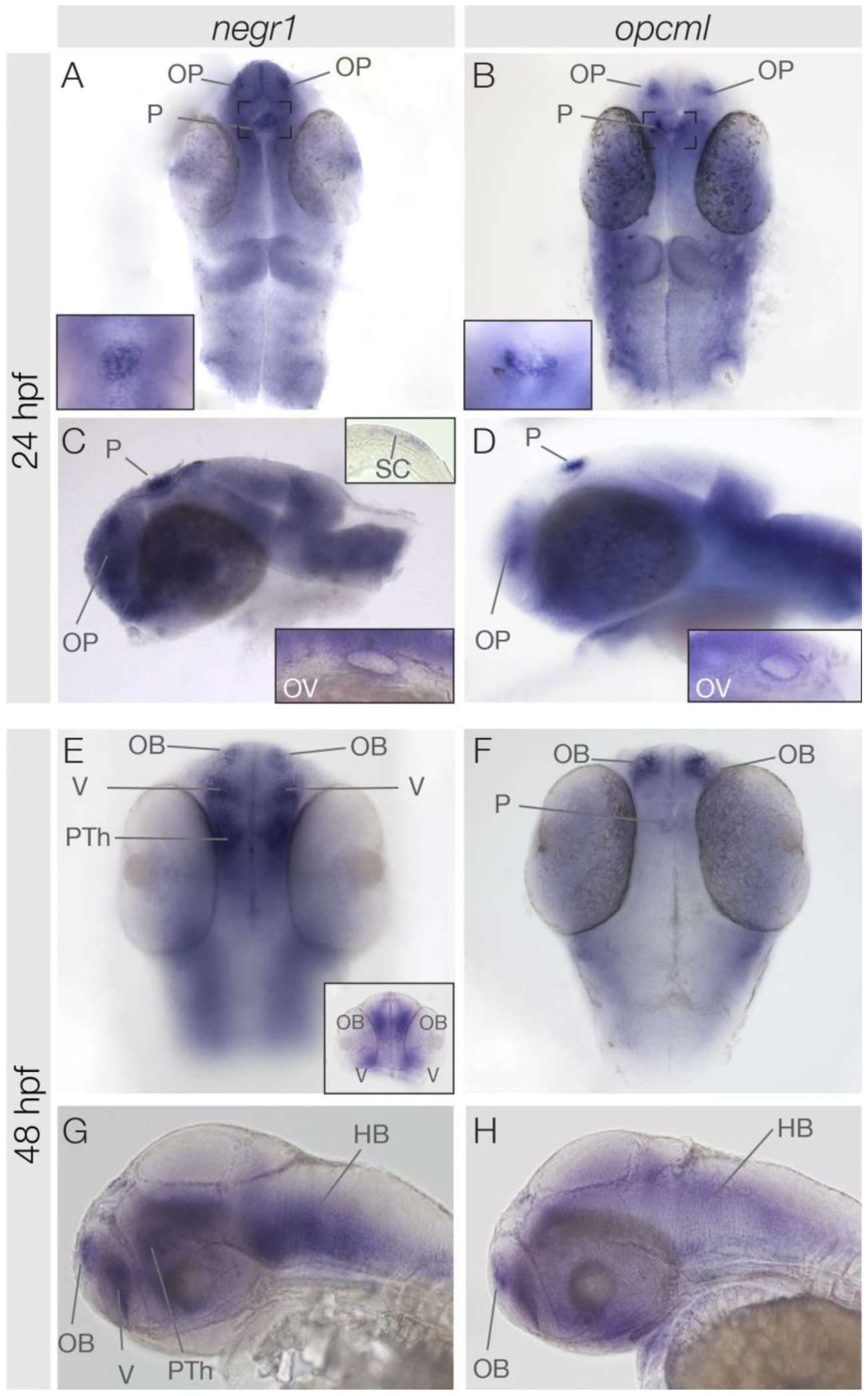
3.2. Similarities and Differences in negr1 and opcml Expression at 24 hpf and 48 hpf
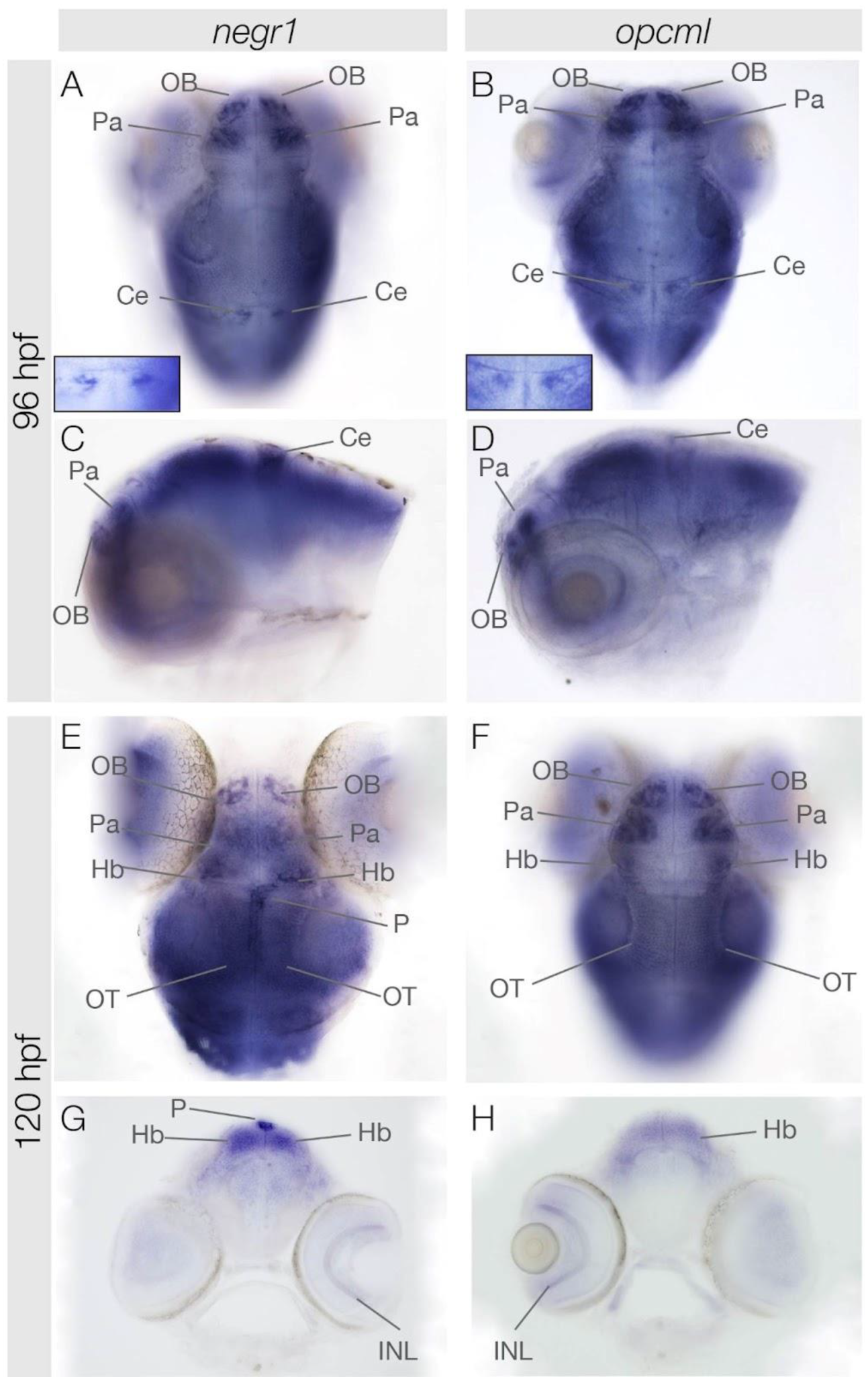
3.3. negr1 and opcml Expression in the Brain at 96 hpf and 120 hpf
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schofield, P.R.; McFarland, K.C.; Hayflick, J.S.; Wilcox, J.N.; Cho, T.M.; Roy, S.; Lee, N.M.; Loh, H.H.; Seeburg, P.H. Molecular Characterization of a New Immunoglobulin Superfamily Protein with Potential Roles in Opioid Binding and Cell Contact. EMBO J. 1989, 8, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Struyk, A.; Canoll, P.; Wolfgang, M.; Rosen, C.; D’Eustachio, P.; Salzer, J. Cloning of Neurotrimin Defines a New Subfamily of Differentially Expressed Neural Cell Adhesion Molecules. J. Neurosci. 1995, 15, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Horton, H.; Levitt, P. A Unique Membrane Protein Is Expressed on Early Developing Limbic System Axons and Cortical Targets. J. Neurosci. 1988, 8, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, N.; Miyata, S.; Kumanogoh, H.; Shigeta, M.; Hamada, K.; Endo, Y.; Sokawa, Y.; Maekawa, S. Characterization of a Novel Rat Brain Glycosylphosphatidylinositol-Anchored Protein (Kilon), a Member of the IgLON Cell Adhesion Molecule Family. J. Biol. Chem. 1999, 274, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, J.; Gordon, L.A.; Olsen, A.; Terry, A.; Schmutz, J.; Lamerdin, J.; Hellsten, U.; Goodstein, D.; Couronne, O.; Tran-Gyamfi, M.; et al. The DNA Sequence and Biology of Human Chromosome 19. Nature 2004, 428, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sellar, G.C.; Watt, K.P.; Rabiasz, G.J.; Stronach, E.A.; Li, L.; Miller, E.P.; Massie, C.E.; Miller, J.; Contreras-Moreira, B.; Scott, D.; et al. OPCML at 11q25 Is Epigenetically Inactivated and Has Tumor-Suppressor Function in Epithelial Ovarian Cancer. Nat. Genet. 2003, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Gil, O.D.; Zanazzi, G.; Struyk, A.F.; Salzer, J.L. Neurotrimin Mediates Bifunctional Effects on Neurite Outgrowth via Homophilic and Heterophilic Interactions. J. Neurosci. 1998, 18, 9312–9325. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamada, M.; Maekawa, S.; Nakashima, T.; Miyata, S. IgLON Cell Adhesion Molecule Kilon Is a Crucial Modulator for Synapse Number in Hippocampal Neurons. Brain Res. 2008, 1224, 1–11. [Google Scholar] [CrossRef]
- Sanz, R.; Ferraro, G.B.; Fournier, A.E. IgLON Cell Adhesion Molecules Are Shed from the Cell Surface of Cortical Neurons to Promote Neuronal Growth. J. Biol. Chem. 2015, 290, 4330–4342. [Google Scholar] [CrossRef]
- Schäfer, M.; Bräuer, A.U.; Savaskan, N.E.; Rathjen, F.G.; Brümmendorf, T. Neurotractin/Kilon Promotes Neurite Outgrowth and Is Expressed on Reactive Astrocytes after Entorhinal Cortex Lesion. Mol. Cell. Neurosci. 2005, 29, 580–590. [Google Scholar] [CrossRef]
- Cui, Y.; Ying, Y.; van Hasselt, A.; Ng, K.M.; Yu, J.; Zhang, Q.; Jin, J.; Liu, D.; Rhim, J.S.; Rha, S.Y.; et al. OPCML Is a Broad Tumor Suppressor for Multiple Carcinomas and Lymphomas with Frequently Epigenetic Inactivation. PLoS ONE 2008, 3, e2990. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.-S.; Lee, B.; Hong, J.; Lee, S. Newly Identified Cancer-Associated Role of Human Neuronal Growth Regulator 1 (NEGR1). J. Cancer 2014, 5, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Ntougkos, E.; Rush, R.; Scott, D.; Frankenberg, T.; Gabra, H.; Smyth, J.F.; Sellar, G.C. The IgLON Family in Epithelial Ovarian Cancer: Expression Profiles and Clinicopathologic Correlates. Clin. Cancer Res. 2005, 11, 5764–5768. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.E.; Dunn, J.R.; du Plessis, D.G.; Shaw, E.J.; Reeves, P.; Gee, A.L.; Warnke, P.C.; Sellar, G.C.; Moss, D.J.; Walker, C. Expression of Cellular Adhesion Molecule “OPCML” Is down-Regulated in Gliomas and Other Brain Tumours. Neuropathol. Appl. Neurobiol. 2007, 33, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Ghirelli, A.; Tripathi, V.; Piccoli, G. Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics 2023, 15, 2307. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.L.; Nagle, M.W.; Tian, C.; Chen, X.; Paciga, S.A.; Wendland, J.R.; Tung, J.Y.; Hinds, D.A.; Perlis, R.H.; Winslow, A.R. Identification of 15 Genetic Loci Associated with Risk of Major Depression in Individuals of European Descent. Nat. Genet. 2016, 48, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-Wide Association Analyses Identify 44 Risk Variants and Refine the Genetic Architecture of Major Depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C.; et al. Bi-Ancestral Depression GWAS in the Million Veteran Program and Meta-Analysis in >1.2 Million Individuals Highlight New Therapeutic Directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Schol-Gelok, S.; Janssens, A.C.J.W.; Tiemeier, H.; Liu, F.; Lopez-Leon, S.; Zorkoltseva, I.V.; Axenovich, T.I.; van Swieten, J.C.; Uitterlinden, A.G.; Hofman, A.; et al. A Genome-Wide Screen for Depression in Two Independent Dutch Populations. Biol. Psychiatry 2010, 68, 187–196. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, M.; Wang, J.; Xie, M.; Huang, Y.; Yuan, F.; Jia, Y.; Zhang, X.; Liu, N.; Zhang, N. Shared Genetics between Classes of Obesity and Psychiatric Disorders: A Large-Scale Genome-Wide Cross-Trait Analysis. J. Psychosom. Res. 2022, 162, 111032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Li, Q.; Li, J.; Wu, Y.; Liu, J. MicroRNA-25–5p Counteracts Oxidized LDL-Induced Pathological Changes by Targeting Neuronal Growth Regulator 1 (NEGR1) in Human Brain Micro-Vessel Endothelial Cells. Biochimie 2019, 165, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Athanasiu, L.; Mattingsdal, M.; Kähler, A.K.; Brown, A.; Gustafsson, O.; Agartz, I.; Giegling, I.; Muglia, P.; Cichon, S.; Rietschel, M.; et al. Gene Variants Associated with Schizophrenia in a Norwegian Genome-Wide Study Are Replicated in a Large European Cohort. J. Psychiatr. Res. 2010, 44, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Panichareon, B.; Nakayama, K.; Thurakitwannakarn, W.; Iwamoto, S.; Sukhumsirichart, W. OPCML Gene as a Schizophrenia Susceptibility Locus in Thai Population. J. Mol. Neurosci. 2012, 46, 373–377. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, M.C.; Craddock, N.; Norton, N.; Williams, H.; Peirce, T.; Moskvina, V.; Nikolov, I.; Hamshere, M.; Carroll, L.; Georgieva, L.; et al. Identification of Loci Associated with Schizophrenia by Genome-Wide Association and Follow-Up. Nat. Genet. 2008, 40, 1053–1055. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Holmans, P.; Pocklington, A.J.; Escott-Price, V.; Ripke, S.; Carrera, N.; Legge, S.E.; Bishop, S.; Cameron, D.; Hamshere, M.L.; et al. Common Schizophrenia Alleles Are Enriched in Mutation-Intolerant Genes and in Regions under Strong Background Selection. Nat. Genet. 2018, 50, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Minhas, H.M.; Pescosolido, M.F.; Schwede, M.; Piasecka, J.; Gaitanis, J.; Tantravahi, U.; Morrow, E.M. An Unbalanced Translocation Involving Loss of 10q26.2 and Gain of 11q25 in a Pedigree with Autism Spectrum Disorder and Cerebellar Juvenile Pilocytic Astrocytoma. Am. J. Med. Genet. A 2013, 161, 787–791. [Google Scholar] [CrossRef]
- Szczurkowska, J.; Pischedda, F.; Pinto, B.; Managò, F.; Haas, C.A.; Summa, M.; Bertorelli, R.; Papaleo, F.; Schäfer, M.K.; Piccoli, G.; et al. NEGR1 and FGFR2 Cooperatively Regulate Cortical Development and Core Behaviours Related to Autism Disorders in Mice. Brain 2018, 141, 2772–2794. [Google Scholar] [CrossRef]
- Steiger, H.; Booij, L.; Thaler, L.; St-Hilaire, A.; Israël, M.; Casey, K.F.; Oliverio, S.; Crescenzi, O.; Lee, V.; Turecki, G.; et al. DNA Methylation in People with Anorexia Nervosa: Epigenome-Wide Patterns in Actively Ill, Long-Term Remitted, and Healthy-Eater Women. World J. Biol. Psychiatry 2023, 24, 254–259. [Google Scholar] [CrossRef]
- Huckins, L.M.; Hatzikotoulas, K.; Southam, L.; Thornton, L.M.; Steinberg, J.; Aguilera-McKay, F.; Treasure, J.; Schmidt, U.; Gunasinghe, C.; Romero, A.; et al. Investigation of Common, Low-Frequency and Rare Genome-Wide Variation in Anorexia Nervosa. Mol. Psychiatry 2018, 23, 1169–1180. [Google Scholar] [CrossRef]
- Raghavan, N.S.; Brickman, A.M.; Andrews, H.; Manly, J.J.; Schupf, N.; Lantigua, R.; Wolock, C.J.; Kamalakaran, S.; Petrovski, S.; Tosto, G.; et al. Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer’s Disease. Ann. Clin. Transl. Neurol. 2018, 5, 832–842. [Google Scholar] [CrossRef]
- Liu, F.; Arias-Vásquez, A.; Sleegers, K.; Aulchenko, Y.S.; Kayser, M.; Sanchez-Juan, P.; Feng, B.-J.; Bertoli-Avella, A.M.; van Swieten, J.; Axenovich, T.I.; et al. A Genomewide Screen for Late-Onset Alzheimer Disease in a Genetically Isolated Dutch Population. Am. J. Hum. Genet. 2007, 81, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Salluzzo, M.; Vianello, C.; Abdullatef, S.; Rimondini, R.; Piccoli, G.; Carboni, L. The Role of IgLON Cell Adhesion Molecules in Neurodegenerative Diseases. Genes 2023, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Park, J.-C.; Han, J.-S.; Lee, S.J. From Bound Cells Comes a Sound Mind: The Role of Neuronal Growth Regulator 1 in Psychiatric Disorders. Exp. Neurobiol. 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Piccoli, G. The IgLON Family Member Negr1 Promotes Neuronal Arborization Acting as Soluble Factor via FGFR2. Front. Mol. Neurosci. 2016, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Vanaveski, T.; Singh, K.; Narvik, J.; Eskla, K.-L.; Visnapuu, T.; Heinla, I.; Jayaram, M.; Innos, J.; Lilleväli, K.; Philips, M.-A.; et al. Promoter-Specific Expression and Genomic Structure of IgLON Family Genes in Mouse. Front. Neurosci. 2017, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Hachisuka, A.; Nakajima, O.; Yamazaki, T.; Sawada, J. Developmental Expression of Opioid-Binding Cell Adhesion Molecule (OBCAM) in Rat Brain. Dev. Brain Res. 2000, 122, 183–191. [Google Scholar] [CrossRef]
- Singh, K.; Jayaram, M.; Kaare, M.; Leidmaa, E.; Jagomäe, T.; Heinla, I.; Hickey, M.A.; Kaasik, A.; Schäfer, M.K.; Innos, J.; et al. Neural Cell Adhesion Molecule Negr1 Deficiency in Mouse Results in Structural Brain Endophenotypes and Behavioral Deviations Related to Psychiatric Disorders. Sci. Rep. 2019, 9, 5457. [Google Scholar] [CrossRef]
- Kubick, N.; Brösamle, D.; Mickael, M.-E. Molecular Evolution and Functional Divergence of the IgLON Family. Evol. Bioinform. 2018, 14, 117693431877508. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Bühler, A.; Carl, M. Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders. Biomolecules 2021, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- McNabb, A.; Scott, K.; von Ochsenstein, E.; Seufert, K.; Carl, M. Don’t Be Afraid to Set Up Your Fish Facility. Zebrafish 2012, 9, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Thisse, C.; Thisse, B. High-Resolution in Situ Hybridization to Whole-Mount Zebrafish Embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Kane, D.A.; Kimmel, C.B. The Zebrafish Midblastula Transition. Development 1993, 119, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, S.; Rampon, C.; Salvert, D.; Fort, P.; Sarda, N. Impaired Hippocampal Plasticity and Altered Neurogenesis in Adult Ube3a Maternal Deficient Mouse Model for Angelman Syndrome. Exp. Neurol. 2009, 220, 341–348. [Google Scholar] [CrossRef]
- Campinho, M.A.; Saraiva, J.; Florindo, C.; Power, D.M. Maternal Thyroid Hormones Are Essential for Neural Development in Zebrafish. Mol. Endocrinol. 2014, 28, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, F.; Campos-Ortega, J.A. Maternal Effects of Zygotic Mutants Affecting Early Neurogenesis in Drosophila. Wilehm Roux Arch. Dev. Biol. 1982, 191, 191–201. [Google Scholar] [CrossRef]
- Lee, A.W.S.; Hengstler, H.; Schwald, K.; Berriel-Diaz, M.; Loreth, D.; Kirsch, M.; Kretz, O.; Haas, C.A.; de Angelis, M.H.; Herzig, S.; et al. Functional Inactivation of the Genome-Wide Association Study Obesity Gene Neuronal Growth Regulator 1 in Mice Causes a Body Mass Phenotype. PLoS ONE 2012, 7, e41537. [Google Scholar] [CrossRef]
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11, 703. [Google Scholar] [CrossRef]
- Tayanloo-Beik, A.; Hamidpour, S.K.; Abedi, M.; Shojaei, H.; Tavirani, M.R.; Namazi, N.; Larijani, B.; Arjmand, B. Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective. Front. Psychiatry 2022, 13, 911770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ye, M.; Li, Q.; You, Y.; Yu, H.; Ma, Y.; Mei, L.; Sun, X.; Wang, L.; Yue, W.; et al. The Schizophrenia Susceptibility Gene OPCML Regulates Spine Maturation and Cognitive Behaviors through Eph-Cofilin Signaling. Cell Rep. 2019, 29, 49–61.e7. [Google Scholar] [CrossRef] [PubMed]
- Jagomäe, T.; Singh, K.; Philips, M.-A.; Jayaram, M.; Seppa, K.; Tekko, T.; Gilbert, S.F.; Vasar, E.; Lilleväli, K. Alternative Promoter Use Governs the Expression of IgLON Cell Adhesion Molecules in Histogenetic Fields of the Embryonic Mouse Brain. Int. J. Mol. Sci. 2021, 22, 6955. [Google Scholar] [CrossRef] [PubMed]
- Sudarov, A. Defining the Role of Cerebellar Purkinje Cells in Autism Spectrum Disorders. Cerebellum 2013, 12, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like Behaviour and Cerebellar Dysfunction in Purkinje Cell Tsc1 Mutant Mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Sapède, D.; Chaigne, C.; Blader, P.; Cau, E. Functional Heterogeneity in the Pineal Projection Neurons of Zebrafish. Mol. Cell. Neurosci. 2020, 103, 103468. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.L.; Russell, C.; Regan, J.C.; Tawk, M.; Sidi, S.; Gilmour, D.T.; Kapsimali, M.; Sumoy, L.; Goldstone, K.; Amaya, E.; et al. Local Tissue Interactions across the Dorsal Midline of the Forebrain Establish CNS Laterality. Neuron 2003, 39, 423–438. [Google Scholar] [CrossRef]
- Gamse, J.T.; Thisse, C.; Thisse, B.; Halpern, M.E. The Parapineal Mediates Left-Right Asymmetry in the Zebrafish Diencephalon. Development 2003, 130, 1059–1068. [Google Scholar] [CrossRef]
- Beretta, C.A.; Dross, N.; Guglielmi, L.; Bankhead, P.; Soulika, M.; Gutierrez-Triana, J.A.; Paolini, A.; Poggi, L.; Falk, J.; Ryu, S.; et al. Early Commissural Diencephalic Neurons Control Habenular Axon Extension and Targeting. Curr. Biol. 2017, 27, 270–278. [Google Scholar] [CrossRef]
- Hüsken, U.; Stickney, H.L.; Gestri, G.; Bianco, I.H.; Faro, A.; Young, R.M.; Roussigne, M.; Hawkins, T.A.; Beretta, C.A.; Brinkmann, I.; et al. Tcf7l2 Is Required for Left-Right Asymmetric Differentiation of Habenular Neurons. Curr. Biol. 2014, 24, 2217–2227. [Google Scholar] [CrossRef]
- Aizawa, H.; Goto, M.; Sato, T.; Okamoto, H. Temporally Regulated Asymmetric Neurogenesis Causes Left-Right Difference in the Zebrafish Habenular Structures. Dev. Cell 2007, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cui, Y.; Yang, Y. Circuits and Functions of the Lateral Habenula in Health and in Disease. Nat. Rev. Neurosci. 2020, 21, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, A.; Henn, F.A. Deep Brain Stimulation of the Lateral Habenula in Treatment Resistant Major Depression. Med. Hypotheses 2007, 69, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Kiening, K.; Sartorius, A. A New Translational Target for Deep Brain Stimulation to Treat Depression. EMBO Mol. Med. 2013, 5, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Vendrell Llopis, N.; Carl, M.; Yaksi, E.; Wilson, S.W. Left-Right Asymmetry Is Required for the Habenulae to Respond to Both Visual and Olfactory Stimuli. Curr. Biol. 2014, 24, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Amo, R.; Okamoto, H. Phylogeny and Ontogeny of the Habenular Structure. Front. Neurosci. 2011, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Beretta, C.A.; Dross, N.; Guiterrez-Triana, J.A.; Ryu, S.; Carl, M. Habenula Circuit Development: Past, Present, and Future. Front. Neurosci. 2012, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Wilson, S.W. The Habenular Nuclei: A Conserved Asymmetric Relay Station in the Vertebrate Brain. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1005–1020. [Google Scholar] [CrossRef]
- Barros, F.; Figueiredo, C.; Costa, A.; Soares, S.C. Sensory Processing in the Autism Spectrum: The Role of Attention to Detail and Somatic Trait Anxiety in the Olfactory Perception of the General Population. J. Autism Dev. Disord. 2021, 51, 2338–2353. [Google Scholar] [CrossRef]
- Thyme, S.B.; Pieper, L.M.; Li, E.H.; Pandey, S.; Wang, Y.; Morris, N.S.; Sha, C.; Choi, J.W.; Herrera, K.J.; Soucy, E.R.; et al. Phenotypic Landscape of Schizophrenia-Associated Genes Defines Candidates and Their Shared Functions. Cell 2019, 177, 478–491.e20. [Google Scholar] [CrossRef]
| Human (Homo sapiens) NEGR1 (IgLON4, KILON, NTRA) | Mice (Mus musculus) Negr1 (Ntra, neurotractin) | Rat (Rattus norvegicus) Negr1 | |
| Zebrafish (Danio rerio) negr1 | 64.1% similarity (bp sequence) 78.1% similarity (aa sequence) | 61.7% similarity (bp sequence) 75.8% similarity (aa sequence) | 63% similarity (bp sequence) 75.8% similarity (aa sequence) |
| Human (Homo sapiens) OPCML (IGLON1, OBCAM, OPCM) | Mice (Mus musculus) Opcml (Obcam) | Rat (Rattus norvegicus) Opcml | |
| Zebrafish (Danio rerio) opcml | 58.7% similarity (bp sequence) 78.3% similarity (aa sequence) | 61.4% similarity (bp sequence) 77.8% similarity (aa sequence) | 62.2% similarity (bp sequence) 77.8% similarity (aa sequence) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habicher, J.; Sanvido, I.; Bühler, A.; Sartori, S.; Piccoli, G.; Carl, M. The Risk Genes for Neuropsychiatric Disorders negr1 and opcml Are Expressed throughout Zebrafish Brain Development. Genes 2024, 15, 363. https://doi.org/10.3390/genes15030363
Habicher J, Sanvido I, Bühler A, Sartori S, Piccoli G, Carl M. The Risk Genes for Neuropsychiatric Disorders negr1 and opcml Are Expressed throughout Zebrafish Brain Development. Genes. 2024; 15(3):363. https://doi.org/10.3390/genes15030363
Chicago/Turabian StyleHabicher, Judith, Ilaria Sanvido, Anja Bühler, Samuele Sartori, Giovanni Piccoli, and Matthias Carl. 2024. "The Risk Genes for Neuropsychiatric Disorders negr1 and opcml Are Expressed throughout Zebrafish Brain Development" Genes 15, no. 3: 363. https://doi.org/10.3390/genes15030363







