Characterization and Functional Analysis of Fads Reveals Δ5 Desaturation Activity during Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Dwarf Surf Clam Mulinia lateralis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Identification and Sequence Analysis of Fads in M. lateralis
2.2. Phylogenetic Analysis of Fads
2.3. Spatiotemporal Expression of Fads in M. lateralis
2.4. Expression Analysis of Fads in M. lateralis under Two Unialgal Diets
2.5. Functional Characterization via Heterologous Expressions of M. lateralis Fads Open Reading Frame in Yeasts
2.6. Fatty Acid Analysis Using GC-MS
2.7. Statistical Analyses
3. Results
3.1. Gene Identification and Sequence Analysis of Fads in M. lateralis
3.2. Phylogenetic Analysis
3.3. Spatiotemporal Expression of MulFads
3.4. Effects of Different Unialgal Diets on Fads Gene Expression in M. lateralis
3.5. Functional Characterization of M. lateralis Fads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Burdge, G. Long-chain n-3 PUFA: Plant v. marine sources. Proc. Nutr. Soc. 2006, 65, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ugoala, C.; Ndukwe, G.; Audu, T. Comparison of fatty acids profile of some freshwater and marine fishes. Internet J. Food Saf. 2008, 10, 9–17. [Google Scholar]
- Pirestani, S.; Sahari, M.; Barzegar, M.; Nikoopour, H. Lipid, cholesterol and fatty acid profile of some commercially important fish species from south Caspian Sea. J. Food Biochem. 2010, 34, 886–895. [Google Scholar] [CrossRef]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates. Part II: Mollusca. Prog. Lipid Res. 1982, 21, 109–153. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Cook, H.W.; McMaster, C.R. Fatty acid desaturation and chain elongation in eukaryotes. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 181–204. [Google Scholar]
- Sprecher, H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta 2000, 1486, 219–231. [Google Scholar] [CrossRef]
- Monroig, O.; Navarro, J.C.; Dick, J.R.; Alemany, F.; Tocher, D.R. Identification of a Δ5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar. Biotechnol. 2012, 14, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mai, K.; He, G.; Ai, Q.; Zhang, W.; Xu, W.; Wang, J.; Liufu, Z.; Zhang, Y.; Zhou, H. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannai Ino). Aquaculture 2013, 416, 48–56. [Google Scholar] [CrossRef]
- Ran, Z.; Xu, J.; Liao, K.; Li, S.; Chen, S.; Yan, X. Biosynthesis of polyunsaturated fatty acids in the razor clam Sinonovacula constricta: Characterization of Δ5 and Δ6 fatty acid desaturases. J. Agric. Food Chem. 2018, 66, 4592–4601. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Zheng, H.; Wang, S.; Guo, Z.; Zhang, G. PUFA biosynthesis pathway in marine scallop Chlamys nobilis Reeve. J. Agric. Food Chem. 2014, 62, 12384–12391. [Google Scholar] [CrossRef]
- Caers, M.; Coutteau, P.; Sorgeloos, P. Dietary impact of algal and artificial diets, fed at different feeding rations, on the growth and fatty acid composition of Tapes philippinarum (L.) spat. Aquaculture 1999, 170, 307–322. [Google Scholar] [CrossRef]
- Lee, M.C.; Park, J.C.; Lee, J.S. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 2018, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V. Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ran, Z.; Wu, S.; Xie, H.; Li, Y.; Liao, K.; Xu, J.; Yan, X. Biosynthesis of LC-PUFA in Ruditapes philippinarum: Cloning and tissue distribution of Fad and Elovl, and effects of microalgae diets varied in LC-PUFA composition on their expressions and fatty acids profile of this bivalve. Front. Mar. Sci. 2023, 10, 1141231. [Google Scholar] [CrossRef]
- Walker, R.L.; Tenore, K.R. Growth and production of the dwarf surf clam Mulinia lateralis (Say 1822) in a Georgia estuary. Gulf Caribb. Res. 1984, 7, 357–363. [Google Scholar] [CrossRef]
- Calabrese, A. Reproductive cycle of the coot clam, Mulinia lateralis (Say), in Long Island Sound. Veliger 1970, 12, 265–269. [Google Scholar]
- Santos, S.; Simon, J. Response of soft-bottom benthos to annual catastrophic disturbance in a south Florida estuary. Mar. Ecol. Prog. Ser. 1980, 3, 347–355. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Wang, H.; Pan, H.; Wang, X.; Teng, M.; Ren, Q.; Bao, Z. Effects of microalgae diets and stocking density on larval growth, survival and metamorphosis of dwarf surfclam, Mulinia lateralis. Aquaculture 2021, 536, 736440. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Li, M.; Teng, M.; Wang, X.; Zhao, A.; Huang, X.; Hu, J.; Bao, Z. Optimizing Microalgae Diet, Temperature, and Salinity for Dwarf Surf Clam, Mulinia lateralis, Spat Culture. Front. Mar. Sci. 2022, 8, 823112. [Google Scholar] [CrossRef]
- Woffelman, C. DNAMAN7.0 for Windows, Lynon Biosoft; Institute of Molecular Plant Sciences, Leiden University: Leiden, The Netherlands, 2004. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Surm, J.M.; Prentis, P.J.; Pavasovic, A. Comparative analysis and distribution of omega-3 lcPUFA biosynthesis genes in marine molluscs. PLoS ONE 2015, 10, e0136301. [Google Scholar] [CrossRef] [PubMed]
- Surm, J.M.; Toledo, T.M.; Prentis, P.J.; Ana, P. Insights into the phylogenetic and molecular evolutionary histories of Fad and Elovl gene families in Actiniaria. Ecol. Evol. 2018, 8, 5323. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Llorens, M.; Hontoria, F.; Navarro, J.; Ferrier, D.; Monroig, Ó. Functionally diverse front-end desaturases are widespread in the phylum Annelida. BBA—Mol. Cell Biol. Lipids 2023, 10, 159377. [Google Scholar] [CrossRef]
- Hastings, N.; Agaba, M.; Tocher, D.R.; Leaver, M.J.; Dick, J.R.; Sargent, J.R.; Teale, A.J. A vertebrate fatty acid desaturase with Δ5 and Δ6 activities. Proc. Natl. Acad. Sci. USA 2001, 98, 14304–14309. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Monroig, Ó.; Li, Y.; Tocher, D.R. Delta-8 desaturation activity varies among fatty acyl desaturases of teleost fish: High activity in delta-6 desaturases of marine species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 206–213. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Δ8-desaturates 20: 2n-6 and 20: 3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.P.; Nakamura, M.T.; Clarke, S.D. Cloning, expression, and nutritional regulation of the mammalian Δ-6 desaturase. J. Biol. Chem. 1999, 274, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, A.; Stöhr, H.; White, K.; Weber, B.H. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics 2000, 66, 175–183. [Google Scholar] [CrossRef]
- Li, Y.; Monroig, O.; Zhang, L.; Wang, S.; Zheng, X.; Dick, J.R.; You, C.; Tocher, D.R. Vertebrate fatty acyl desaturase with Δ4 activity. Proc. Natl. Acad. Sci. USA 2010, 107, 16840–16845. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Tocher, D.R.; Hontoria, F.; Navarro, J.C. Functional characterisation of a Fads2 fatty acyl desaturase with Δ6/Δ8 activity and an Elovl5 with C16, C18 and C20 elongase activity in the anadromous teleost meagre (Argyrosomus regius). Aquaculture 2013, 412, 14–22. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, C.-R.; Wang, Y.-X.; Xu, Z.-M.; Li, S.-Q.; Li, J.-C.; Sun, X.-Q.; Wang, H.-W.; Wang, Q.; Zhang, Y. Fads2b Plays a Dominant Role in ∆6/∆5 Desaturation Activities Compared with Fads2a in Common Carp (Cyprinus carpio). Int. J. Mol. Sci. 2023, 24, 10638. [Google Scholar] [CrossRef]
- Monroig, Ó.; Hontoria, F.; Varó, I.; Tocher, D.R.; Navarro, J.C. Investigating the essential fatty acids in the common cuttlefish Sepia officinalis (Mollusca, Cephalopoda): Molecular cloning and functional characterisation of fatty acyl desaturase and elongase. Aquaculture 2016, 450, 38–47. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Zheng, H.; Wang, S.; Wang, Y.; Liu, W.; Zhang, G. Functional characterization of a Δ5-like fatty acyl desaturase and its expression during early embryogenesis in the noble scallop Chlamys nobilis Reeve. Mol. Biol. Rep. 2014, 41, 7437–7445. [Google Scholar] [CrossRef]
- Monroig, Ó.; Shu-Chien, A.; Kabeya, N.; Tocher, D.R.; Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog. Lipid Res. 2022, 86, 101157. [Google Scholar] [CrossRef]
- Monroig, Ó.; Rotllant, J.; Sánchez, E.; Cerdá-Reverter, J.M.; Tocher, D.R. Expression of long-chain polyunsaturated fatty acid (LC-PUFA) biosynthesis genes during zebrafish Danio rerio early embryogenesis. Biochim. Biophys. Acta 2009, 1791, 1093–1101. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, H.; Wang, S.; Wang, Y.; Li, S.; Liu, W.; Zhang, G. Cloning and functional characterization of a polyunsaturated fatty acid elongase in a marine bivalve noble scallop Chlamys nobilis Reeve. Aquaculture 2013, 416, 146–151. [Google Scholar] [CrossRef]
- Sarker, M.A.-A.; Yamamoto, Y.; Haga, Y.; Sarker, M.S.A.; Miwa, M.; Yoshizaki, G.; Satoh, S. Influences of low salinity and dietary fatty acids on fatty acid composition and fatty acid desaturase and elongase expression in red sea bream Pagrus major. Fish. Sci. 2011, 77, 385–396. [Google Scholar] [CrossRef]
- Wu, D.L.; Huang, Y.H.; Liu, Z.Q.; Yu, P.; Gu, P.H.; Fan, B.; Zhao, Y.L. Molecular cloning, tissue expression and regulation of nutrition and temperature on Delta 6 fatty acyl desaturase-like gene in the red claw crayfish (Cherax quadricarinatus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 225, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hao, M.; Zhu, D.; Li, S.; Wen, X. Molecular cloning, mRNA expression and nutritional regulation of a Δ6 fatty acyl desaturase-like gene of mud crab, Scylla paramamosain. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 208, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Hu, C.B.; Zheng, Y.J.; Xia, X.A.; Xu, W.J.; Wang, S.Q.; Chen, W.Z.; Sun, Z.W.; Huang, J.H. The effects of dietary fatty acids on liver fatty acid composition and Delta(6)-desaturase expression differ with ambient salinities in Siganus canaliculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Monroig, S.; Zheng, X.; Morais, S.; Leaver, M.J.; Taggart, J.B.; Tocher, D.R. Multiple genes for functional 6 fatty acyl desaturases (Fad) in Atlantic salmon (Salmo salar L.): Gene and cDNA characterization, functional expression, tissue distribution and nutritional regulation. BBA—Mol. Cell Biol. Lipids 2010, 1801, 1072–1081. [Google Scholar] [CrossRef]
- Gregory, M.K.; Collins, R.O.; Tocher, D.R.; James, M.J.; Turchini, G.M. Nutritional regulation of long-chain PUFA biosynthetic genes in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2016, 115, 1721–1729. [Google Scholar] [CrossRef]
- Yang, C.; Hao, R.; He, C.; Deng, Y.; Wang, Q. Cloning and functional characterization of PmΔ5FAD in pearl oyster Pinctada fucata martensii. Aquac. Rep. 2022, 23, 101036. [Google Scholar] [CrossRef]
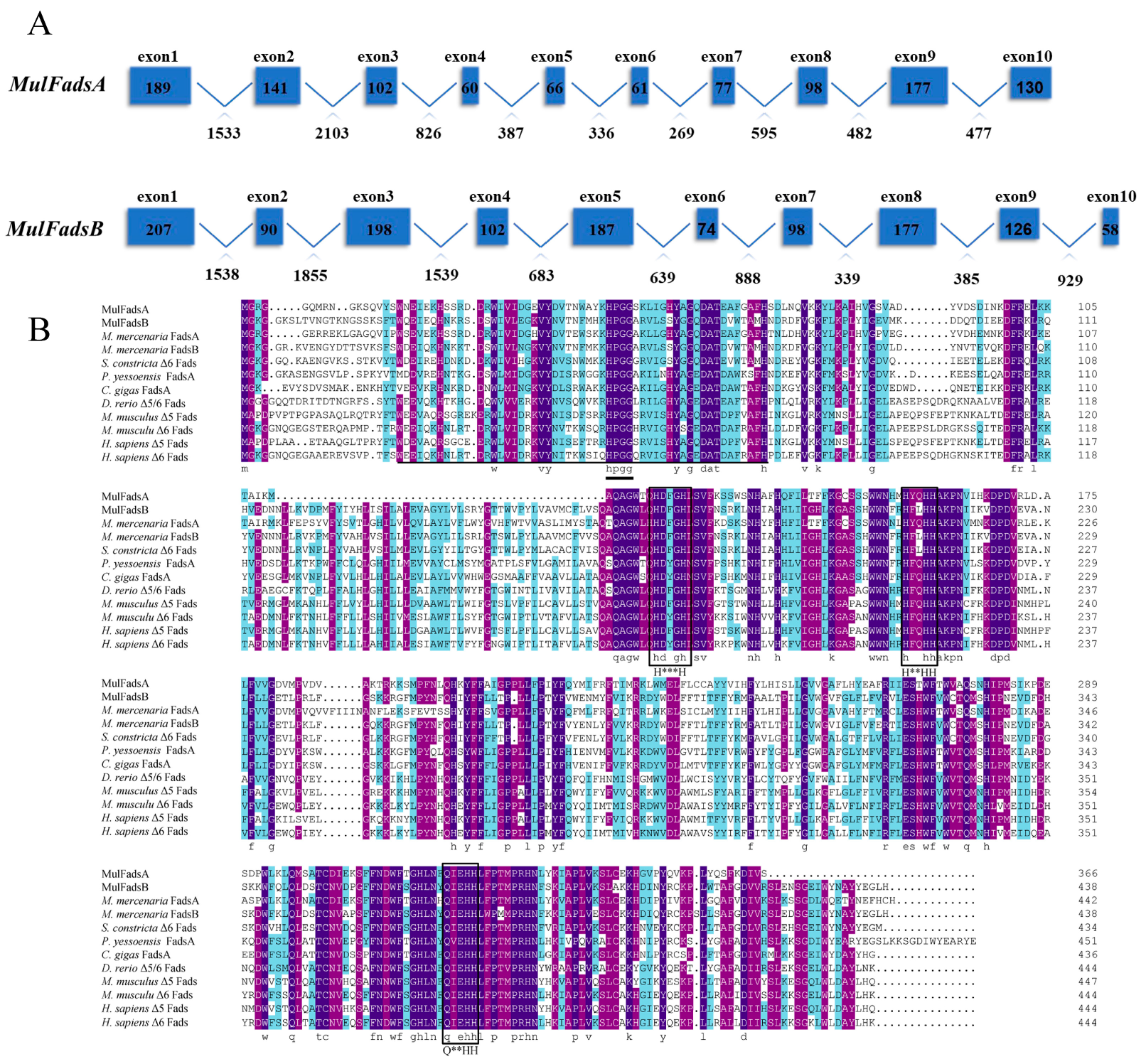
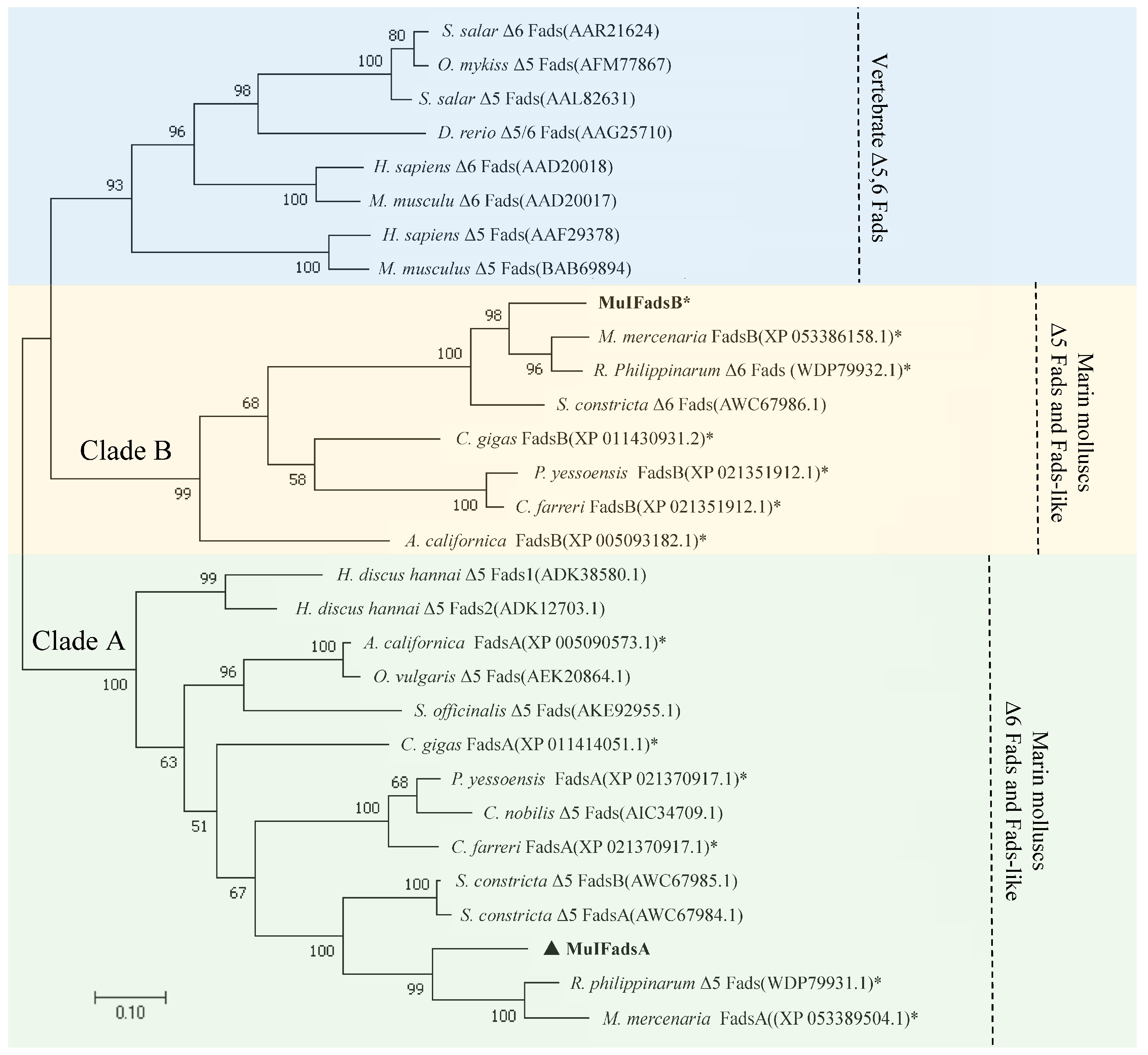
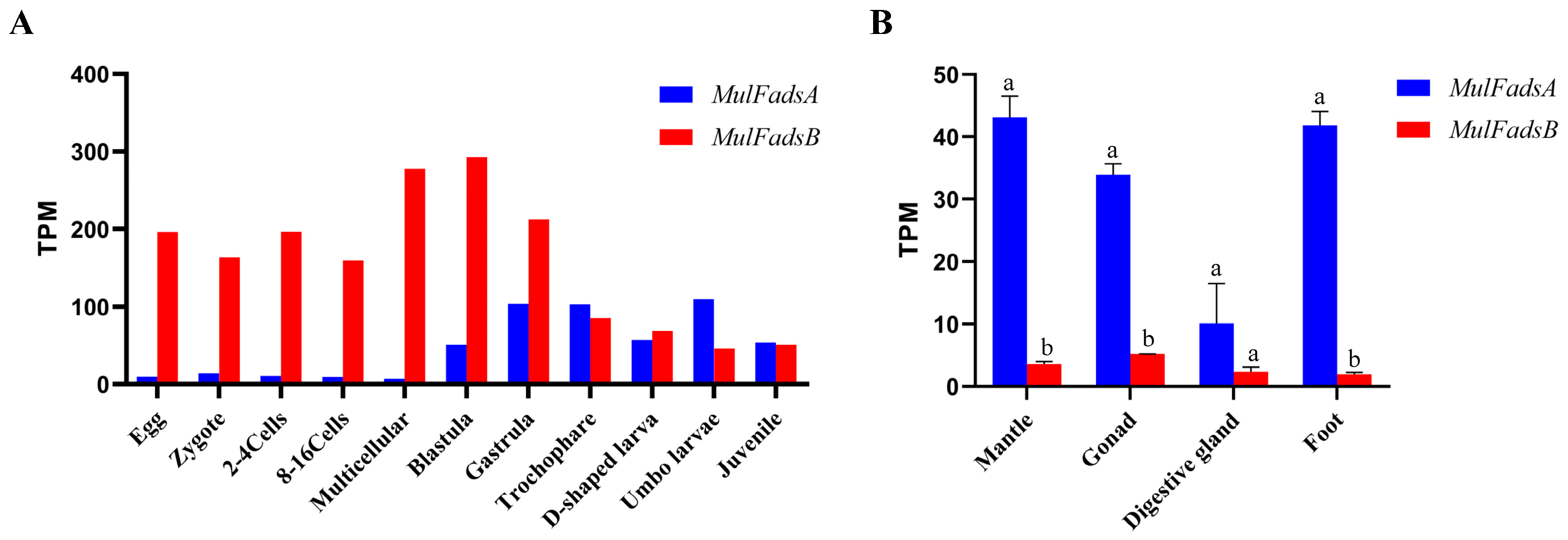
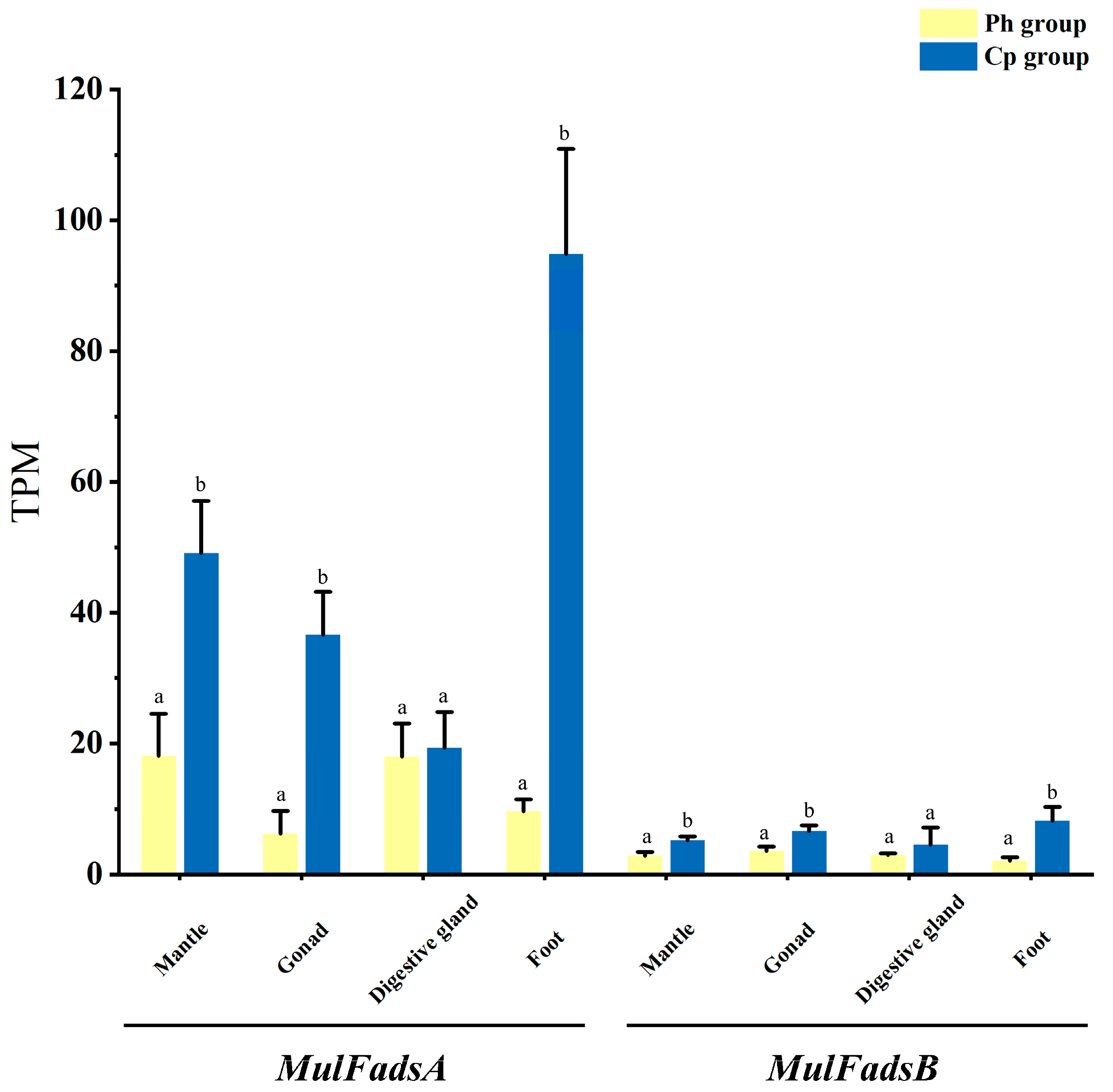

| Gene Name | cDNA Length (bp) | ORF Length (bp) | Exon No. | Intron No. | Amino Acid No. | Molecular Weight (kDa) | Theoretical pI | α No. | Extended No. | Colis No. | Turn No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MulFadsA | 1175 | 1101 | 11 | 10 | 366 | 42.8 | 8.85 | 166 | 41 | 147 | 12 |
| MulFadsB | 4510 | 1317 | 11 | 10 | 438 | 51.38 | 8.45 | 207 | 51 | 163 | 17 |
| Substrate | Product | Conversion Rate | Activity |
|---|---|---|---|
| C18:2n-6 | C18:3n-6 | 0% | Δ6 |
| C18:2n-6 | C18:3n-6 | 0% | Δ6 |
| C18:3n-3 | C18:4n-3 | 0% | Δ6 |
| C20:3n-3 | C20:4n-3 | 0% | Δ8 |
| C20:3n-6 | C20:4n-6 | 26% | Δ5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, T.; Zheng, Z.; Jiao, W.; Liu, N.; Wang, A.; Liu, M.; Xie, L.; Yang, Z.; Hu, J.; Bao, Z. Characterization and Functional Analysis of Fads Reveals Δ5 Desaturation Activity during Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Dwarf Surf Clam Mulinia lateralis. Genes 2024, 15, 365. https://doi.org/10.3390/genes15030365
Teng T, Zheng Z, Jiao W, Liu N, Wang A, Liu M, Xie L, Yang Z, Hu J, Bao Z. Characterization and Functional Analysis of Fads Reveals Δ5 Desaturation Activity during Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Dwarf Surf Clam Mulinia lateralis. Genes. 2024; 15(3):365. https://doi.org/10.3390/genes15030365
Chicago/Turabian StyleTeng, Tianhao, Zhenghua Zheng, Wenqian Jiao, Na Liu, Ao Wang, Mengjiao Liu, Le Xie, Zujing Yang, Jingjie Hu, and Zhenmin Bao. 2024. "Characterization and Functional Analysis of Fads Reveals Δ5 Desaturation Activity during Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Dwarf Surf Clam Mulinia lateralis" Genes 15, no. 3: 365. https://doi.org/10.3390/genes15030365




