The Impact of Blood Sample Processing on Ribonucleic Acid (RNA) Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Ribonucleic Acid (RNA) Isolation and Complementary Deoxyribonucleic Acid (cDNA) Library Construction
2.3. Bioinformatics Analysis
2.4. Analysis of the Similarities and Differences
2.4.1. Correlation Analysis
2.4.2. Gene Clustering
2.4.3. Differential Gene Screening
2.4.4. Time-Sensitive Genes
2.4.5. Gene Enrichment Analysis
3. Results
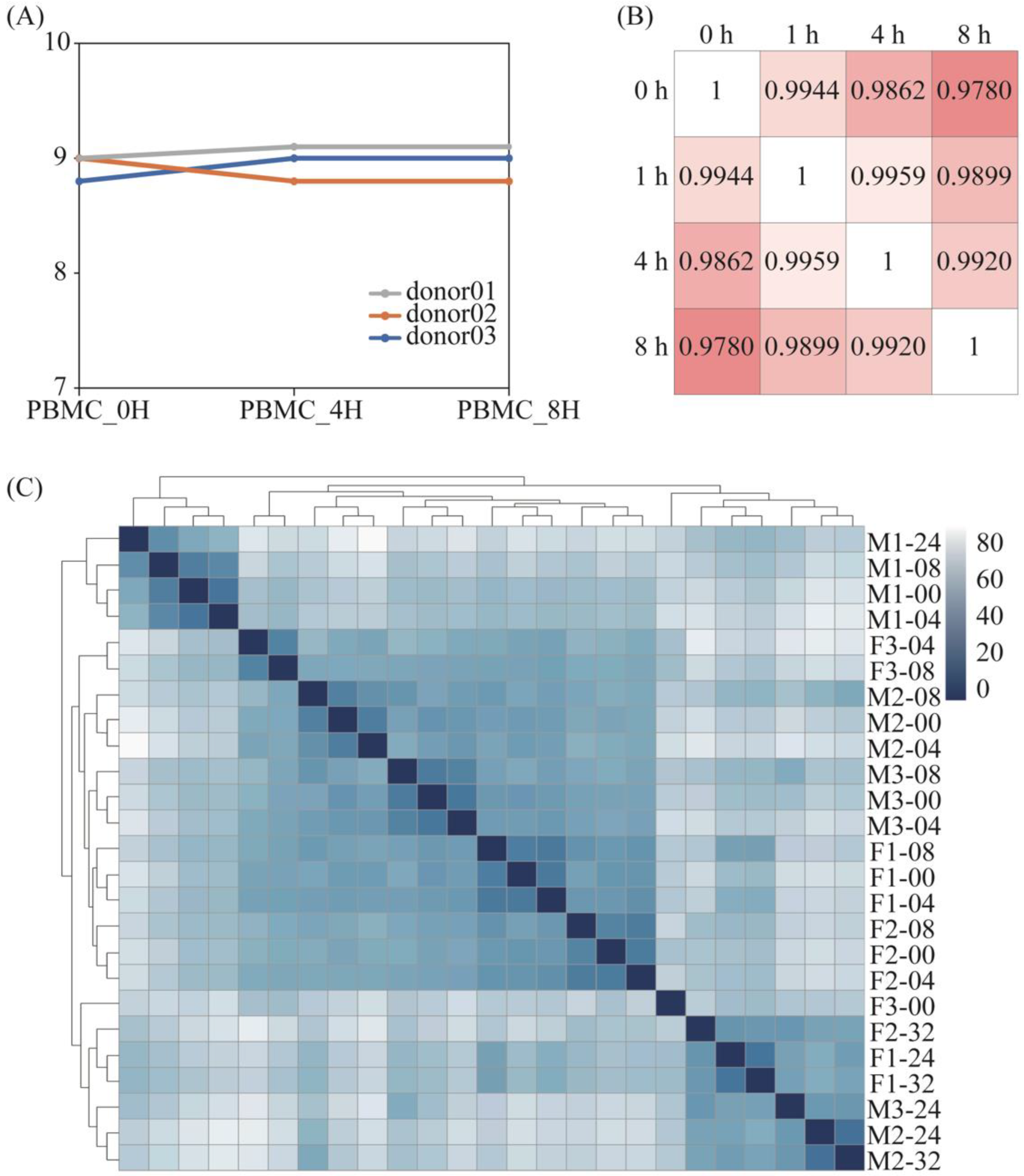
3.1. Gene Expression Changes along with Storage Times
3.2. Five Genes Are Significantly Different within Eight Hours in Peripheral Blood Mononuclear Cells (PBMCs)
3.3. Sequencing Differences between Whole Blood and Peripheral Blood Mononuclear Cells (PBMCs)
4. Discussion
- Try to select individuals with similar age ranges, genders and health statuses as blood sequencing samples. It is necessary to classify and discuss them when conducting research;
- After the blood sample is collected, try to extract RNA as soon as possible. If the experiment cannot be carried out immediately, it can be stored in an ice box at about 4 °C and added later with stabilizer RNA and anticoagulant;
- When studying genes or diseases related to leukemia, immune neutropenia and other blood and immune diseases and inflammatory reactions, the consistency of storage time should be strictly controlled;
- When extracting RNA from whole blood, try to select a reagent kit that can remove hemoglobin as much as possible.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, L.; Kehl, T.; Thedinga, K.; Grammes, N.L.; Backes, C.; Mohr, C.; Schubert, B.; Lenhof, K.; Gerstner, N.; Hartkopf, A.D.; et al. ClinOmicsTrailbc: A visual analytics tool for breast cancer treatment stratification. Bioinformatics 2019, 35, 5171–5181. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Feng, W.X.; Hu, R.; Ge, Q.Y.; Ma, W.F.; Zhang, W.; Xu, S.M.; Zhan, B.L.; Zhang, L.; Sun, X.F.; et al. RNA-seq profiling reveals PBMC RNA as a potential biomarker for hepatocellular carcinoma. Sci. Rep. 2021, 11, 17797. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhang, B.; Li, P.; Zhao, Y. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed. Pharmacother. 2023, 163, 114786. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, S.; Yin, H.; Zhuo, Z.; Meng, G. A comprehensive benchmarking of differential splicing tools for RNA-seq analysis at the event level. Brief. Bioinform. 2023, 24, bbad121. [Google Scholar] [CrossRef] [PubMed]
- Pepke, S.; Wold, B.; Mortazavi, A. Computation for ChIP-seq and RNA-seq studies. Nat. Methods 2009, 6, S22–S32. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Bolger, A.M.; Nagel, A.; Fernie, A.R.; Lunn, J.E.; Stitt, M.; Usadel, B. RobiNA: A user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012, 40, W622–W627. [Google Scholar] [CrossRef] [PubMed]
- Frazee, A.C.; Jaffe, A.E.; Langmead, B.; Leek, J.T. Polyester: Simulating RNA-seq datasets with differential transcript expression. Bioinformatics 2015, 31, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhou, Q.; Chen, Y. Impact of RNA degradation on next-generation sequencing transcriptome data. Genomics 2022, 114, 110429. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, M.; Ma, S.; Yang, E.W.; Jiang, T.; Li, W. Accurate inference of isoforms from multiple sample RNA-Seq data. BMC Genom. 2015, 16 (Suppl. S2), S15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Felts, S.J.; Van Keulen, V.P.; Pease, L.R.; Zhang, Y. Exploring the effect of library preparation on RNA sequencing experiments. Genomics 2019, 111, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.; Jia, E.; Pan, M.; Bai, Y.; Ge, Q. Bias in RNA-seq Library Preparation: Current Challenges and Solutions. Biomed. Res. Int. 2021, 2021, 6647597. [Google Scholar] [CrossRef] [PubMed]
- Dvinge, H.; Ries, R.E.; Ilagan, J.O.; Stirewalt, D.L.; Meshinchi, S.; Bradley, R.K. Sample processing obscures cancer-specific alterations in leukemic transcriptomes. Proc. Natl. Acad. Sci. USA 2014, 111, 16802–16807. [Google Scholar] [CrossRef] [PubMed]
- Pogosova-Agadjanyan, E.L.; Moseley, A.; Othus, M.; Appelbaum, F.R.; Chauncey, T.R.; Chen, I.L.; Erba, H.P.; Godwin, J.E.; Fang, M.; Kopecky, K.J.; et al. Impact of Specimen Heterogeneity on Biomarkers in Repository Samples from Patients with Acute Myeloid Leukemia: A SWOG Report. Biopreserv Biobank 2018, 16, 42–52. [Google Scholar] [CrossRef]
- Fowles, J.S.; How, J.; Allen, M.J.; Oh, S.T. Young versus old age at diagnosis confers distinct genomic profiles in patients with polycythemia vera. Leukemia 2019, 33, 1522–1526. [Google Scholar] [CrossRef]
- Imbeaud, S.; Graudens, E.; Boulanger, V.; Barlet, X.; Zaborski, P.; Eveno, E.; Mueller, O.; Schroeder, A.; Auffray, C. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res. 2005, 33, e56. [Google Scholar] [CrossRef]
- Gallego Romero, I.; Pai, A.A.; Tung, J.; Gilad, Y. RNA-seq: Impact of RNA degradation on transcript quantification. BMC Biol. 2014, 12, 42. [Google Scholar] [CrossRef]
- Jia, E.; Zhou, Y.; Liu, Z.; Wang, L.; Ouyang, T.; Pan, M.; Bai, Y.; Ge, Q. Transcriptomic Profiling of Circular RNA in Different Brain Regions of Parkinson’s Disease in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 3006. [Google Scholar] [CrossRef] [PubMed]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef] [PubMed]



| Donor | Sex | Sample Type | Treatment | Hours | Million Reads | RIN |
|---|---|---|---|---|---|---|
| 01 | XY | PBMCs | room temperature | 0 | 23.9 | 8.8 |
| 1 | 24.0 | 9.1 | ||||
| 4 | 24.0 | 9 | ||||
| 8 | 23.9 | 9 | ||||
| 02 1 | XX | PBMCs | room temperature | 0 | 35.4 | 9.0 |
| 4 | 33.0 | 8.8 | ||||
| 8 | 36.9 | 8.8 | ||||
| 03 1 | XY | PBMCs | room temperature | 0 | 48.2 | 9.0 |
| 4 | 45.1 | 9.1 | ||||
| 8 | 46.3 | 9.1 | ||||
| 04 | XX | blood | 4 °C | 0 | 45.4 | 8.9 |
| 4 | 48.0 | 9.0 | ||||
| 8 | 49.0 | 8.7 | ||||
| 24 | 48.2 | 8.0 | ||||
| 05 | XX | blood | 4 °C | 0 | 48.7 | 8.8 |
| 4 | 47.0 | 8.9 | ||||
| 8 | 48.7 | 8.6 | ||||
| 24 | 47.0 | 8.3 | ||||
| 06 | XX | blood | 4 °C | 0 | 48.1 | 9.0 |
| 4 | 46.1 | 8.7 | ||||
| 8 | 47.4 | 8.7 | ||||
| 24 | 47.1 | 8.5 | ||||
| 32 | 45.9 | 7.9 | ||||
| 07 | XY | blood | 4 °C | 0 | 45.5 | 8.9 |
| 4 | 47.2 | 8.7 | ||||
| 8 | 47.0 | 8.5 | ||||
| 24 | 47.3 | 8.5 | ||||
| 32 | 46.0 | 8.0 | ||||
| 08 | XY | blood | 4 °C | 0 | 46.1 | 9.2 |
| 4 | 48.7 | 9.1 | ||||
| 8 | 48.6 | 8.7 | ||||
| 32 | 46.8 | 7.8 | ||||
| 09 | XY | blood | 4 °C | 0 | 45.7 | 9.1 |
| 4 | 47.5 | 9.0 | ||||
| 8 | 45.9 | 8.6 |
| Name | Description | Diseases | Pathways | GO Annotations |
|---|---|---|---|---|
| PI3 | Peptidase Inhibitor 3 | Adult Respiratory Distress Syndrome; Bacterial Infectious Disease; Cystic Fibrosis; Inflammatory Bowel Disease; Joubert Syndrome 7; Lung Disease; Pasteurellosis; Preterm Premature Rupture of The Membranes; Skin Disease | Keratinization; Innate Immune System; Defensins; Nervous system development | endopeptidase inhibitor activity; serine-type endopeptidase inhibitor activity; structural constituent of skin epidermis; peptidase inhibitor activity |
| ALPL | Alkaline Phosphatase, Biomineralization Associated | Hypophosphatasia, Childhood; Hypophosphatasia, Infantile; Hypophosphatasia, Adult; Prenatal Benign Hypophosphatasia; Primary Bone Dysplasia | Endochondral ossification with skeletal dysplasias; Metabolism of proteins; Phenytoin Pathway, Pharmacokinetics; NAD metabolism; Post-translational modification: synthesis of GPI-anchored proteins; FGF23 signaling in hypophosphatemic rickets and related disorders; Netrin-UNC5B signaling pathway; NOTCH1 regulation of endothelial cell calcification; OSX and miRNAs in tooth development | alkaline phosphatase activity; inorganic diphosphate phosphatase activity; calcium ion binding; protein binding; pyrophosphatase activity |
| FCGR3B | Fc Fragment of IgG Receptor IIIb | Paroxysmal Nocturnal Hemoglobinuria; Neutropenia; Cryptococcosis; Poliomyelitis; Hemoglobinuria; Hypersensitivity Vasculitis; Peritonitis | Innate Immune System; Metabolism of proteins; GPCR Pathway; Fc-GammaR Pathway; RhoGDI Pathway; Integrin family cell surface interactions; Post-translational modification: synthesis of GPI-anchored proteins | transmembrane signaling receptor activity; IgG receptor activity; IgG binding; GPI anchor binding |
| TNFRSF10C | TNF Receptor Superfamily Member 10c | 46, Xy Sex Reversal 8; Ovarian Cancer; Myelodysplastic Syndrome; Prostate Cancer; Myeloma, Multiple | Gene expression (Transcription); Akt Signaling; TGF-β Pathway; MIF Mediated Glucocorticoid Regulation; TNFR1 Pathway; TP53 Regulates Transcription of Cell Death Genes; PAK Pathway; ERK Signaling; Death Receptor Signaling Pathway; Regulation by c-FLIP | transmembrane signaling receptor activity; protein binding; TRAIL binding |
| MME | Membrane Metalloendopeptidase | Spinocerebellar Ataxia 43; Charcot-Marie-Tooth Disease, Axonal, Type 2t; Charcot-Marie-Tooth Disease, Axonal, Type 2e; Membranous Nephropathy; Peripheral Nervous System Disease | Innate Immune System; Cardiac conduction; Peptide hormone metabolism; Alzheimer’s disease and miRNA effects; ACE Inhibitor Pathway, Pharmacodynamics; Metabolism of proteins; A-β Plaque Formation and APP Metabolism; | peptidase activity metallopeptidase activity; protein binding; cardiolipin binding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ouyang, T.; Yang, Y.; Sheng, Y.; Shi, H.; Liu, Q.; Bai, Y.; Ge, Q. The Impact of Blood Sample Processing on Ribonucleic Acid (RNA) Sequencing. Genes 2024, 15, 502. https://doi.org/10.3390/genes15040502
Liu Z, Ouyang T, Yang Y, Sheng Y, Shi H, Liu Q, Bai Y, Ge Q. The Impact of Blood Sample Processing on Ribonucleic Acid (RNA) Sequencing. Genes. 2024; 15(4):502. https://doi.org/10.3390/genes15040502
Chicago/Turabian StyleLiu, Zhiyu, Tinglan Ouyang, Yuwei Yang, Yuqi Sheng, Huajuan Shi, Quanjun Liu, Yunfei Bai, and Qinyu Ge. 2024. "The Impact of Blood Sample Processing on Ribonucleic Acid (RNA) Sequencing" Genes 15, no. 4: 502. https://doi.org/10.3390/genes15040502





