Identification and Analysis of RNA Editing Sites in the Chloroplast Transcripts of Aegilops tauschii L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prediction of RNA Editing Sites
2.2. Editing Site Detection by Read Mapping and SNP (Single Nucleotide Polymorphism) Calling
2.3. Protein Structure Analysis before and after Editing
2.4. Comparative Analysis of RNA Editing Sites in Cp Protein-Coding Genes among Poaceae Species
2.5. Validation of Partial RNA Editing Sites
3. Results and Discussion
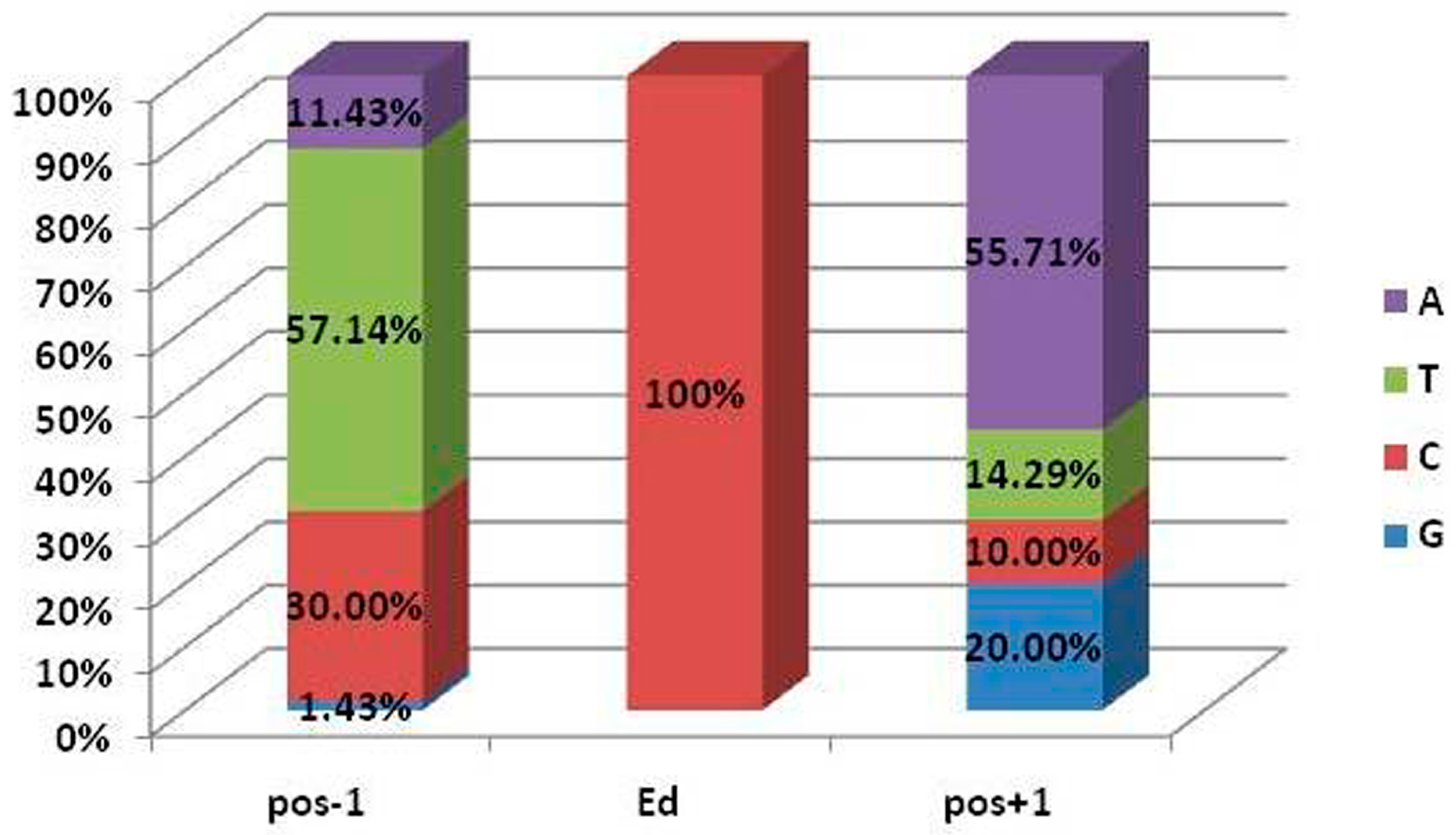
3.1. Prediction of RNA Editing Sites
3.2. Editing Site Detection Using Read Mapping and SNP Calling
3.3. Validation of the RNA Editing Sites by RT-PCR Analysis
3.4. Impact of RNA Editing on Protein Structure
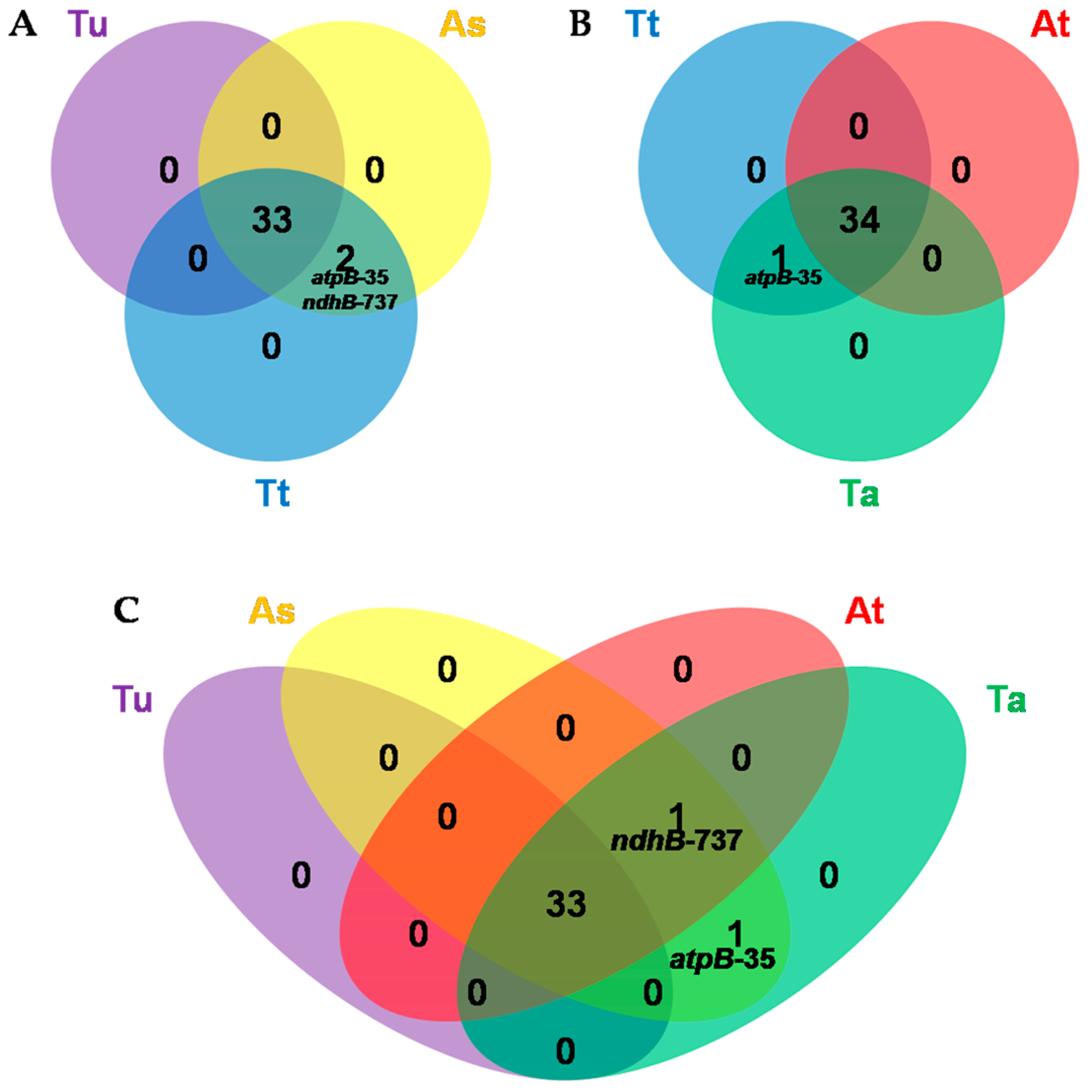
3.5. Comparative Analysis of Chloroplast RNA Editing Sites among Poaceae Species
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Xu, J.-R.; Liu, H. A-to-I RNA editing independent of adars in filamentous fungi. RNA Biol. 2016, 13, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, W.; Wu, Y.; Maliga, P.; Messing, J. RNA editing in chloroplasts of Spirodela polyrhiza, an aquatic monocotelydonous species. PLoS ONE 2015, 10, e0140285. [Google Scholar] [CrossRef] [PubMed]
- Brennicke, A.; Marchfelder, A.; Binder, S. RNA editing. FEMS Microbiol. Rev. 1999, 23, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Sutton, C.; Luis, B. Plant organelle gene expression: Altered by RNA editing. Trends Plant Sci. 1996, 1, 57–64. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; He, Y.; Chen, L.; Hao, C.; Jiang, C.; Li, Y.; Dai, Y.; Kang, Z.; Xu, J.-R. Genome-wide A-to-I RNA editing in fungi independent of ADAR enzymes. Genome Res. 2016, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Hoch, B.; Maier, R.M.; Appel, K.; Igloi, G.L.; Kossel, H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature 1991, 353, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Kusumegi, T.; Tsudzuki, T.; Sugiura, M. RNA editing sites in tobacco chloroplast transcripts: Editing as a possible regulator of chloroplast RNA polymerase activity. Mol. Gen. Genet. MGG 1999, 262, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yukawa, Y.; Miyamoto, T.; Obokata, J.; Sugiura, M. Identification of RNA editing sites in chloroplast transcripts from the maternal and paternal progenitors of tobacco (Nicotiana tabacum): Comparative analysis shows the involvement of distinct trans-factors for ndhB editing. Mol. Biol. Evol. 2003, 20, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Lutz, K.A.; Maliga, P. Lack of conservation of editing sites in mRNAs that encode subunits of the NAD(P)H dehydrogenase complex in plastids and mitochondria of Arabidopsis thaliana. Curr. Genet. 2001, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Sasaki, T.; Yukawa, M.; Tsudzuki, T.; Sugiura, M. A systematic search for RNA editing sites in pea chloroplasts: An editing event causes diversification from the evolutionarily conserved amino acid sequence. Plant Cell Physiol. 2004, 45, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Kahlau, S.; Aspinall, S.; Gray, J.C.; Bock, R. Sequence of the tomato chloroplast DNA and evolutionary comparison of solanaceous plastid genomes. J. Mol. Evol. 2006, 63, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yukawa, Y.; Wakasugi, T.; Yamada, K.; Sugiura, M. A simple in vitro RNA editing assay for chloroplast transcripts using fluorescent dideoxynucleotides: Distinct types of sequence elements required for editing of NDH transcripts. Plant J. 2006, 47, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Duff, R.J.; Moore, F.B.-G. Pervasive RNA editing among hornwort rbcL transcripts except leiosporoceros. J. Mol. Evol. 2005, 61, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Rowe, C.A.; Hasebe, M. High levels of RNA editing in a vascular plant chloroplast genome: Analysis of transcripts from the fern Adiantum capillus-veneris. Gene 2004, 339, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.B.; Goldschmidt-Clermont, M.; Hanson, M.R. Chloroplast RNA metabolism. Annu. Rev. Plant Biol. 2010, 61, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Freyer, R.; Kiefer-Meyer, M.-C.; Kössel, H. Occurrence of plastid RNA editing in all major lineages of land plants. Proc. Natl. Acad. Sci. USA 1997, 94, 6285–6290. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Neckermann, K.; Igloi, G.L.; Kössel, H. Complete sequence of the maize chloroplast genome: Gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J. Mol. Biol. 1995, 251, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Tsudzuki, T.; Wakasugi, T.; Sugiura, M. Comparative analysis of RNA editing sites in higher plant chloroplasts. J. Mol. Evol. 2001, 53, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. RNA editing in plant organelles: Machinery, physiological function and evolution. Cell. Mol. Life Sci. CMLS 2006, 63, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.C.; Gott, J.M.; Hanson, M.R. A guide to RNA editing. RNA 1997, 3, 1105–1123. [Google Scholar] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Kotera, E.; Tasaka, M.; Shikanai, T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 2005, 433, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A. RNA editing in plants and its evolution. Annu. Rev. Genet. 2013, 47, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A.; Takenaka, M. RNA editing competence of trans-factor MEF1 is modulated by ecotype-specific differences but requires the DYW domain. FEBS Lett. 2010, 584, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Salamini, F.; Ozkan, H.; Brandolini, A.; Schafer-Pregl, R.; Martin, W. Genetics and geography of wild cereal domestication in the near east. Nat. Rev. Genet. 2002, 3, 429–441. [Google Scholar] [PubMed]
- Jia, J.; Zhao, S.; Kong, X.; Li, Y.; Zhao, G.; He, W.; Appels, R.; Pfeifer, M.; Tao, Y.; Zhang, X.; et al. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 2013, 496, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.-C.; Gu, Y.Q.; You, F.M.; Deal, K.R.; Ma, Y.; Hu, Y.; Huo, N.; Wang, Y.; Wang, J.; Chen, S.; et al. A 4-gigabase physical map unlocks the structure and evolution of the complex genome of Aegilops tauschii, the wheat D-genome progenitor. Proc. Natl. Acad. Sci. USA 2013, 110, 7940–7945. [Google Scholar]
- Gornicki, P.; Zhu, H.; Wang, J.; Challa, G.S.; Zhang, Z.; Gill, B.S.; Li, W. The chloroplast view of the evolution of polyploid wheat. New Phytol. 2014, 204, 704–714. [Google Scholar]
- Wang, J.; Luo, M.-C.; Chen, Z.; You, F.M.; Wei, Y.; Zheng, Y.; Dvorak, J. Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat. New Phytol. 2013, 198, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P. The PREP suite: Predictive rna editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009, 37, W253–W259. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. Tophat: Discovering splice junctions with RNA-seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Conformational parameters for amino acids in helical, β-sheet, and random coil regions calculated from proteins. Biochemistry 1974, 13, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Prediction of protein conformation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Tsunewaki, K.; Matsuoka, Y.; Yamazaki, Y.; Ogihara, Y. Evolutionary dynamics of wheat mitochondrial gene structure with special remarks on the origin and effects of rna editing in cereals. Genes Genet. Syst. 2008, 83, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Middleton, C.P.; Senerchia, N.; Stein, N.; Akhunov, E.D.; Keller, B.; Wicker, T.; Kilian, B. Sequencing of chloroplast genomes from wheat, barley, rye and their relatives provides a detailed insight into the evolution of the Triticeae tribe. PLoS ONE 2014, 9, e85761. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, K.; Hodkinson, T.R.; Wolfe, K.H.; van den Bekerom, R.; Dix, P.J.; Barth, S. Complete chloroplast genome sequence of a major allogamous forage species, perennial ryegrass (Lolium perenne L.). DNA Res. 2009, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Corneille, S.; Lutz, K.; Maliga, P. Conservation of rna editing between rice and maize plastids: Are most editing events dispensable? Mol. Gen. Genet. MGG 2000, 264, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Calsa Júnior, T.; Carraro, D.M.; Benatti, M.R.; Barbosa, A.C.; Kitajima, J.P.; Carrer, H. Structural features and transcript-editing analysis of sugarcane (Saccharum officinarum L.) chloroplast genome. Curr. Genet. 2004, 46, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Henry, R.J. Molecular analysis of the DNA polymorphism of wild barley (Hordeum spontaneum) germplasm using the polymerase chain reaction. Genet. Resour. Crop Evol. 1995, 42, 273–280. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Ruwe, H.; Castandet, B.; Schmitz-Linneweber, C.; Stern, D.B. Arabidopsis chloroplast quantitative editotype. FEBS Lett. 2013, 587, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.B. The role of context-dependent mutations in generating compositional and codon usage bias in grass chloroplast DNA. J. Mol. Evol. 2003, 56, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.B.; Oberholzer, M.V.; Clegg, T.M. The influence of specific neighboring bases on substitution bias in noncoding regions of the plant chloroplast genome. J. Mol. Evol. 1997, 45, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Tillich, M.; Lehwark, P.; Morton, B.R.; Maier, U.G. The evolution of chloroplast RNA editing. Mol. Biol. Evol. 2006, 23, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Grosche, C.; Funk, H.T.; Maier, U.G.; Zauner, S. The chloroplast genome of Pellia endiviifolia: Gene content, RNA-editing pattern, and the origin of chloroplast editing. Genome Biol. Evol. 2012, 4, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Kugita, M.; Yamamoto, Y.; Fujikawa, T.; Matsumoto, T.; Yoshinaga, K. RNA editing in hornwort chloroplasts makes more than half the genes functional. Nucleic Acids Res. 2003, 31, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fan, S.L.; Song, M.Z.; Yu, J.N.; Yu, S.X. Identification of rna editing sites in cotton (Gossypium hirsutum) chloroplasts and editing events that affect secondary and three-dimensional protein structures. Genet. Mol. Res. GMR 2012, 11, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Li, Y. Prediction of C-to-U RNA editing sites in plant mitochondria using both biochemical and evolutionary information. J. Theor. Biol. 2008, 253, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, L.; Jiang, Y.; Lu, P.; Yu, J. RNA editing sites exist in protein-coding genes in the chloroplast genome of Cycas taitungensis. J. Integr. Plant Biol. 2011, 53, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Matzke, A.J.M.; Matzke, M. Complete sequence and comparative analysis of the chloroplast genome of coconut palm (Cocos nucifera). PLoS ONE 2013, 8, e74736. [Google Scholar] [CrossRef] [PubMed]
- Timme, R.E.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. A comparative analysis of the Lactuca and Helianthus (Asteraceae) plastid genomes: Identification of divergent regions and categorization of shared repeats. Am. J. Bot. 2007, 94, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zito, F.; Kuras, R.; Choquet, Y.; Kössel, H.; Wollman, F.-A. Mutations of cytochrome b6 in Chlamydomonas reinhardtii disclose the functional significance for a proline to leucine conversion by petB editing in maize and tobacco. Plant Mol. Biol. 1997, 33, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Hoch, B.; Zeltz, P.; Kössel, H. Internal editing of the maize chloroplast ndha transcript restores codons for conserved amino acids. Plant Cell 1992, 4, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Karcher, D.; Bock, R. The amino acid sequence of a plastid protein is developmentally regulated by RNA editing. J. Biol. Chem. 2002, 277, 5570–5574. [Google Scholar] [CrossRef] [PubMed]
- Maier, U.G.; Bozarth, A.; Funk, H.T.; Zauner, S.; Rensing, S.A.; Schmitz-Linneweber, C.; Börner, T.; Tillich, M. Complex chloroplast RNA metabolism: Just debugging the genetic programme? BMC Biol. 2008, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Material Name | GenBank Accession |
|---|---|
| Ae. tauschii (AL8/78) | KJ614412.1 |
| T. urartu (PI428335) | KJ614411.1 |
| Ae. speltoides ssp. ligustica (AE918) | KJ614404.1 |
| Ae. speltoides ssp. ligustica (TA1796) | KJ614405.1 |
| Ae. speltoides ssp. speltoides (PI487232) | KJ614406.1 |
| T. turgidum ssp. dicoccoides (TA0073) | KJ614400.1 |
| T. turgidum ssp. dicoccoides (TA0060) | KJ614401.1 |
| T. turgidum ssp. dicoccoides (TA1133) | KJ614402.1 |
| T. aestivum ssp. aestivum cv Chinese Spring (CS) (TA3008) | KJ614396.1 |
| Gene | Nucleotide Position | Amino Acid Position | Codon Conversion | Amino Acid Conversion |
|---|---|---|---|---|
| ndhA | 473 a | 158 | tCa>tTa | S>L |
| 563 a | 188 | tCa>tTa | S>L | |
| 1070 | 357 | tCt>tTt | S>F | |
| atpA | 1148 a | 383 | tCa>tTa | S>L |
| atpB | 1487 | 496 | tCg>tTg | S>L |
| matK | 1261 a | 421 | Cat>Tat | H>Y |
| ndhB | 149a | 50 | tCa>tTa | S>L |
| 467 a | 156 | cCa>cTa | P>L | |
| 586 a | 196 | Cat>Tat | H>Y | |
| 611 a | 204 | tCa>tTa | S>L | |
| 704 a | 235 | tCc>tTc | S>F | |
| 737 a | 246 | cCa>cTa | P>L | |
| 830 a | 277 | tCa>tTa | S>L | |
| 836 a | 279 | tCa>tTa | S>L | |
| 1481 a | 494 | cCa>cTa | P>L | |
| ndhD | 878 a | 293 | tCa>tTa | S>L |
| ndhF | 62 | 21 | tCa>tTa | S>L |
| 1487 | 496 | aCg>aTg | T>M | |
| petB | 662 | 221 | cCa>cTa | P>L |
| rpoC2 | 2009 | 670 | cCa>cTa | P>L |
| 2030 | 677 | cCa>cTa | P>L | |
| 2158 | 720 | Ccc>Tcc | P>S | |
| 3002 | 1001 | cCg>cTg | P>L | |
| 4031 | 1344 | tCg>tTg | S>L | |
| rpl2 | 62 | 21 | aCt>aTt | T>I |
| rpl20 | 308 | 103 | tCa>tTa | S>L |
| rpoA | 1009 | 337 | Ctc>Ttc | L>F |
| rpoB | 467 a | 156 | tCg>tTg | S>L |
| 545 a | 182 | tCa>tTa | S>L | |
| 560 a | 187 | tCg>tTg | S>L | |
| 617 b | 206 | cCg>cTg | P>L | |
| rps8 | 182 | 61 | tCa>tTa | S>L |
| ycf3 | 44 | 15 | tCc>tTc | S>F |
| 191 | 64 | aCg>aTg | T>M |
| Genome Position | Gene Position | Codon | Amino Acid | Leaf | Pistil | Root | Seed | Sheath | Spike | Stamen | Stem | ||||||||||||||||
| 1 b | 2 | 3 | 4 | 5 | 1 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 1 | 2 | 3 | ||||
| Sense-strand | |||||||||||||||||||||||||||
| 3090 | ~psbK | 22 | |||||||||||||||||||||||||
| 3551 | ~psbK | 7 | |||||||||||||||||||||||||
| 8259 | psbI~psbD | 35 c | 36 | 37 | 36 | 37 | 47 | 44 | 33 | 29 | 32 | 33 | 25 | 22 | |||||||||||||
| 8481 | psbI~psbD | 9 | |||||||||||||||||||||||||
| 11,311 | psbC~psbZ | 21 | 19 | 20 | 18 | 10 | 10 | 9 | |||||||||||||||||||
| 20,093 | rpoB-467 a | tCg | S>L | 38 | 28 | 66 | 75 | 78 | 90 | 72 | 32 | ||||||||||||||||
| 20,171 | rpoB-545 a | tCa | S>L | 48 | 70 | 86 | 88 | 78 | 71 | ||||||||||||||||||
| 20,186 | rpoB-560 a | tCg | S>L | 57 | 73 | 86 | 80 | 85 | 69 | ||||||||||||||||||
| 29,181 | rpoC2-4031 a | tCg | S>L | 94 | + | + | 91 | 98 | + | + | 82 | 71 | 60 | 73 | 97 | 99 | 93 | 97 | 92 | + | 92 | 99 | 98 | ||||
| 32,404 | atpH~atpF | 18 | 18 | 15 | |||||||||||||||||||||||
| 34,440 | atpA-234 | ggC | G>G | 11 | |||||||||||||||||||||||
| 35,354 | atpA-1148 a | tCa | S>L | 99 | 98 | 99 | 99 | 99 | 83 | 94 | 98 | 91 | 94 | 91 | 87 | 96 | 90 | 94 | 96 | 95 | 94 | 71 | 93 | 95 | 94 | ||
| 42,415 | atpA~trnS-GGA | 14 | |||||||||||||||||||||||||
| 57,960 | ycf4~cemA | 52 | 50 | 67 | 48 | 55 | 12 | 37 | 42 | 27 | 31 | 23 | 26 | ||||||||||||||
| 62,758 | petL-56 d | cCa | P>L | 83 | 75 | 62 | 77 | 60 | 58 | 36 | 30 | 83 | |||||||||||||||
| 65,352 | rps18-448 | Cga | R>Stop | 9 | |||||||||||||||||||||||
| 68,447 | psbB-414 | atC | L>L | 17 | |||||||||||||||||||||||
| 71,835 | petB-662 a | cCa | P>L | 96 | 98 | 97 | 98 | 90 | 43 | 27 | 34 | 66 | 58 | 26 | 13 | ||||||||||||
| 127,814 | ndhB-149 a | tCa | S>L | 94 | 96 | ||||||||||||||||||||||
| 128,251 | ndhB-586 a | Cat | H>Y | 95 | 88 | 91 | 93 | 93 | |||||||||||||||||||
| 128,276 | ndhB-611 a | tCa | S>L | 95 | 93 | 95 | 94 | ||||||||||||||||||||
| Genome Position | Gene Position | Codon | Amino Acid | Leaf | Pistil | Root | Seed | Sheath | Spike | Stamen | Stem | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 1 | 2 | 3 | ||||
| Sense-strand | |||||||||||||||||||||||||||
| 128,369 | ndhB-704 a | tCc | S>F | 92 | |||||||||||||||||||||||
| 129,858 | ndhB-1481 a | cCa | P>L | 96 | 99 | 99 | 97 | ||||||||||||||||||||
| Antisense-strand | |||||||||||||||||||||||||||
| 1956 | matK-1261 a | Cat | H>Y | 66 | 45 | 41 | 69 | 52 | 13 | 36 | 69 | 55 | 38 | 69 | 55 | 50 | 36 | 61 | 71 | ||||||||
| 5100 | rps16-intron | 19 | 17 | 22 | 23 | 22 | 89 | 54 | 51 | 50 | 65 | 36 | 33 | 32 | 37 | 83 | 83 | 60 | 66 | 70 | 60 | ||||||
| 5179 | rps16-intron | 15 | 15 | 23 | |||||||||||||||||||||||
| 36,673 | rps14~psaB | 45 | 45 | 45 | 44 | 45 | 20 | 20 | 14 | 19 | 23 | 8 | |||||||||||||||
| 42,982 | ycf3-191 a | aCg | T>M | 79 | 83 | 82 | 81 | 80 | 63 | 44 | 55 | 45 | 39 | 49 | 70 | 69 | 67 | 63 | 64 | 74 | 64 | 72 | 76 | ||||
| 43,880 | ycf3-44 a | tCc | S>F | 92 | 92 | 96 | 97 | 92 | 40 | 71 | 68 | 70 | 26 | 46 | 70 | 70 | 69 | 82 | 42 | 50 | 43 | 69 | 77 | 74 | |||
| 44,727 | ycf3~rps4 | 78 | 68 | 71 | 75 | 65 | 38 | 41 | 35 | 29 | 35 | 19 | 20 | 21 | |||||||||||||
| 44,868 | ycf3~rps4 | 11 | |||||||||||||||||||||||||
| 45,244 | rps4-305 | tCa | S>L | 20 | |||||||||||||||||||||||
| 49,393 | ndhK-125 d | cCa | P>L | 99 | 98 | 99 | 99 | 99 | + | 70 | + | 71 | 96 | 97 | 94 | 94 | + | 93 | 89 | 95 | 89 | ||||||
| 49,858 | ndhC-13 | Cac | H>Y | 98 | 98 | 98 | 99 | 99 | 75 | 86 | 83 | 63 | 63 | 86 | 86 | 92 | 85 | 91 | 56 | 80 | 83 | 63 | |||||
| 61,027 | psbL-111 | ttC | F>F | 77 | 83 | 83 | 81 | 49 | 76 | 47 | 45 | 33 | 29 | 56 | |||||||||||||
| 65,383 | trnP-GGG~rpl20 | 24 | |||||||||||||||||||||||||
| 65,632 | rpl20-308 a | tCa | S>L | 11 | 24 | 60 | 38 | 63 | 75 | 56 | |||||||||||||||||
| 67,968 | clpP~psbN | 31 | |||||||||||||||||||||||||
| 73,402 | psbN~rpoA | 45 | 50 | 51 | 51 | 45 | 35 | 19 | 12 | 13 | 21 | 20 | 15 | 14 | 22 | ||||||||||||
| 73,980 | rpoA-527 d | tCc | S>F | 30 | 34 | 30 | 28 | 28 | 23 | 18 | 31 | 35 | 79 | 64 | 72 | 81 | 84 | 81 | 90 | 84 | 75 | 85 | 41 | 87 | 82 | 86 | |
| 74,553 | rpoA~rps11 | 41 | 44 | 44 | 45 | 41 | 21 | 19 | 11 | 29 | 15 | 12 | 22 | 21 | 20 | 22 | 18 | 18 | 18 | 17 | 23 | 20 | 20 | ||||
| 76,074 | rps8-182 a | tCa | S>L | 89 | 90 | 89 | 90 | 90 | 96 | 83 | 86 | 83 | 84 | 96 | 96 | 95 | 97 | 97 | 97 | 97 | 93 | 95 | 93 | 96 | 95 | 95 | 95 |
| 77,285 | rpl16-36 | ccC | P>P | 18 | |||||||||||||||||||||||
| 78,313 | rpl16~rps3 | 92 | |||||||||||||||||||||||||
| Genome Position | Gene Position | Codon | Amino Acid | Leaf | Pistil | Root | Seed | Sheath | Spike | Stamen | Stem | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 1 | 2 | 3 | ||||
| Antisense-strand | |||||||||||||||||||||||||||
| 79,174 | rps3-30 d | ttC | F>F | 62 | 57 | 62 | 66 | 59 | 77 | 58 | 66 | 71 | 69 | 78 | 82 | 63 | 66 | 68 | 69 | 71 | 68 | 63 | 63 | 62 | 56 | 57 | 48 |
| 81,816 | rpl2~rpl23 | 82 | 87 | 88 | 88 | ||||||||||||||||||||||
| 86,095 | ndhB-836 a | tCa | S>L | 99 | |||||||||||||||||||||||
| 87,176 | ndhB-467 a | cCa | P>L | 99 | 95 | 96 | 97 | 98 | 96 | 89 | |||||||||||||||||
| 87,494 | ndhB-149 a | tCa | S>L | 95 | |||||||||||||||||||||||
| 96,705 | rps7~trnN-GUU | 6 | 12 | ||||||||||||||||||||||||
| 101,658 | ndhF-1891 | Cag | Q>Stop | 9 | |||||||||||||||||||||||
| 103,487 | ndhF-62 a | tCa | S>L | 45 | 43 | 44 | 49 | 51 | 28 | 39 | 39 | 43 | 18 | 21 | 17 | 44 | 37 | ||||||||||
| 106,742 | ndhD-1398 d | atC | L>L | 36 | 28 | 37 | 34 | 37 | 23 | ||||||||||||||||||
| 107,262 | ndhD-878 a,d | tCa | S>L | 99 | 98 | 99 | 99 | 98 | + | 89 | 95 | 89 | 81 | 89 | 77 | 84 | 77 | 94 | 89 | + | 83 | ||||||
| 109,393 | ndhE~ndhG | 32 | |||||||||||||||||||||||||
| 109,684 | ndhG-347 d | cCa | P>L | 98 | 97 | 97 | 98 | 96 | 65 | 59 | 64 | 72 | 40 | 78 | 65 | ||||||||||||
| 110,034 | ndhG~ndhI | 38 | 27 | 22 | 28 | 19 | |||||||||||||||||||||
| 110,946 | ndhA-1070 a | tCt | S>F | 75 | 64 | 92 | |||||||||||||||||||||
| 111,453 | ndhA-563 a | tCa | S>L | 13 | |||||||||||||||||||||||
| 112,572 | ndhA-473 a | tCa | S>L | + | 45 | 33 | |||||||||||||||||||||
| No. of site | 35 | 29 | 33 | 33 | 35 | 27 | 9 | 10 | 16 | 11 | 16 | 7 | 8 | 28 | 28 | 28 | 29 | 13 | 3 | 19 | 25 | 20 | 9 | 21 | |||
| Gene | Site | Codon Position | Edited Codon | Amino Acid Conversion | Ae. tauschii | H. vulgare | L. perenne | O. sativa | S. officinarum | Z. mays |
|---|---|---|---|---|---|---|---|---|---|---|
| matK | 1 | 420 (421) | Cat | H→Y | + | + | + a | + | ||
| rpoB | 1 | 156 | tCa | S→L | + | + | + a | + a | + | + |
| 2 | 182 | tCa | S→L | + | + | + a | + a | + | + | |
| 3 | 187 | tCg | S→L | + | + | + a | + a uCa | + | + | |
| 4 | 206 | cCg | P→L | + | − | − | − | − | + | |
| rps14 | 1 | 27 | tCa | S→L | (−) | (−) | + | + a | + | |
| atpA | 1 | 383 | tCa | S→L | + | + | + | + | + | |
| ycf3 | 1 | 15 | tCc | S→F | + | + | (−) | − | + | |
| 2 | 62 (64) | aCg | T→M | + | + | + a | + | + a | ||
| ndhG | 1 | 116 | cCa | P→L | + a | + | + | (−) | ||
| rpl20 | 1 | 103 | tCa | S→L | + | + a | (−) | +a | + | |
| psbL | 1 | 37 | ttC | F→F | + a | + a | (−) | |||
| rps8 | 1 | 61 | tCa | S→L | + | + | + | + | + | |
| rpl2 | 1 | 1 | aCg | T→M | (−) | + | + a | + a | +a | + |
| ndhB | 1 | 50 | tCa | S→L | + | + | + | (−) | (−) | (−) |
| 2 | 156 | cCa | P→L | + | + | + | + | + | + | |
| 3 | 196 | Cat | H→Y | + | + | + | + | + | + | |
| 4 | 204 | tCa | S→L | + | + | + | + a | + | + | |
| 5 | 235 | tCc | S→F | + | + | + | + | (−) | (−) | |
| 6 | 246 | cCa | P→L | + | + | + | + | + | + | |
| 7 | 277 | tCa | S→L | + | + | + | + | + | + | |
| 8 | 279 | tCa | S→L | + | + | + | + | (−) | (−) | |
| 9 | 494 | cCa | P→L | + | + | + | + a | + | + | |
| ndhF | 1 | 21 | tCa | S→L | + | + | + | + a | + | |
| ndhD | 1 | 295 (293) | tCa | S→L | + | + | + a | + | + | + |
| ndhA | 1 | 17 | tCa | S→L | (−) | + | + | (−) | + | + |
| 2 | 158 | tCa | S→L | + | + | + | + | + | + | |
| 3 | 188 | tCa | S→L | + | + | + | + | + | + | |
| 4 | 357 | tCc | S→F | + | + | (−) | + | + | + | |
| ndhK | 1 | 2 | gtC | V→V | − | + | ||||
| 2 | 43(42) | cCa | P→L | + | + |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Liu, H.; Ge, L.; Xing, G.; Wang, M.; Weining, S.; Nie, X. Identification and Analysis of RNA Editing Sites in the Chloroplast Transcripts of Aegilops tauschii L. Genes 2017, 8, 13. https://doi.org/10.3390/genes8010013
Wang M, Liu H, Ge L, Xing G, Wang M, Weining S, Nie X. Identification and Analysis of RNA Editing Sites in the Chloroplast Transcripts of Aegilops tauschii L. Genes. 2017; 8(1):13. https://doi.org/10.3390/genes8010013
Chicago/Turabian StyleWang, Mengxing, Hui Liu, Lingqiao Ge, Guangwei Xing, Meng Wang, Song Weining, and Xiaojun Nie. 2017. "Identification and Analysis of RNA Editing Sites in the Chloroplast Transcripts of Aegilops tauschii L." Genes 8, no. 1: 13. https://doi.org/10.3390/genes8010013
APA StyleWang, M., Liu, H., Ge, L., Xing, G., Wang, M., Weining, S., & Nie, X. (2017). Identification and Analysis of RNA Editing Sites in the Chloroplast Transcripts of Aegilops tauschii L. Genes, 8(1), 13. https://doi.org/10.3390/genes8010013







