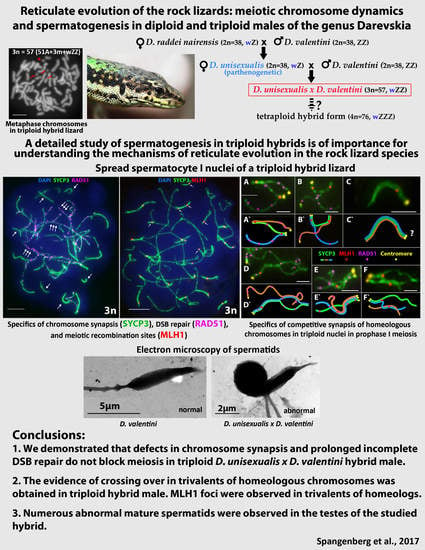
Reticulate Evolution of the Rock Lizards: Meiotic Chromosome Dynamics and Spermatogenesis in Diploid and Triploid Males of the Genus Darevskia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synaptonemal Complex Analysis
2.2. Electron Microscopy
3. Results
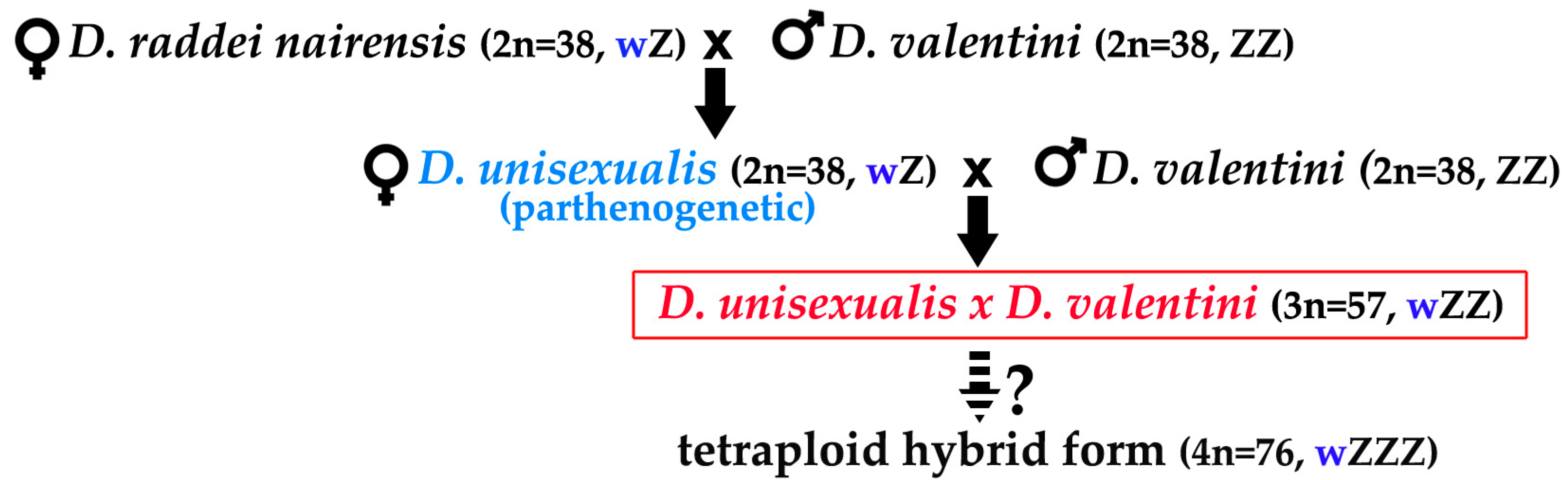
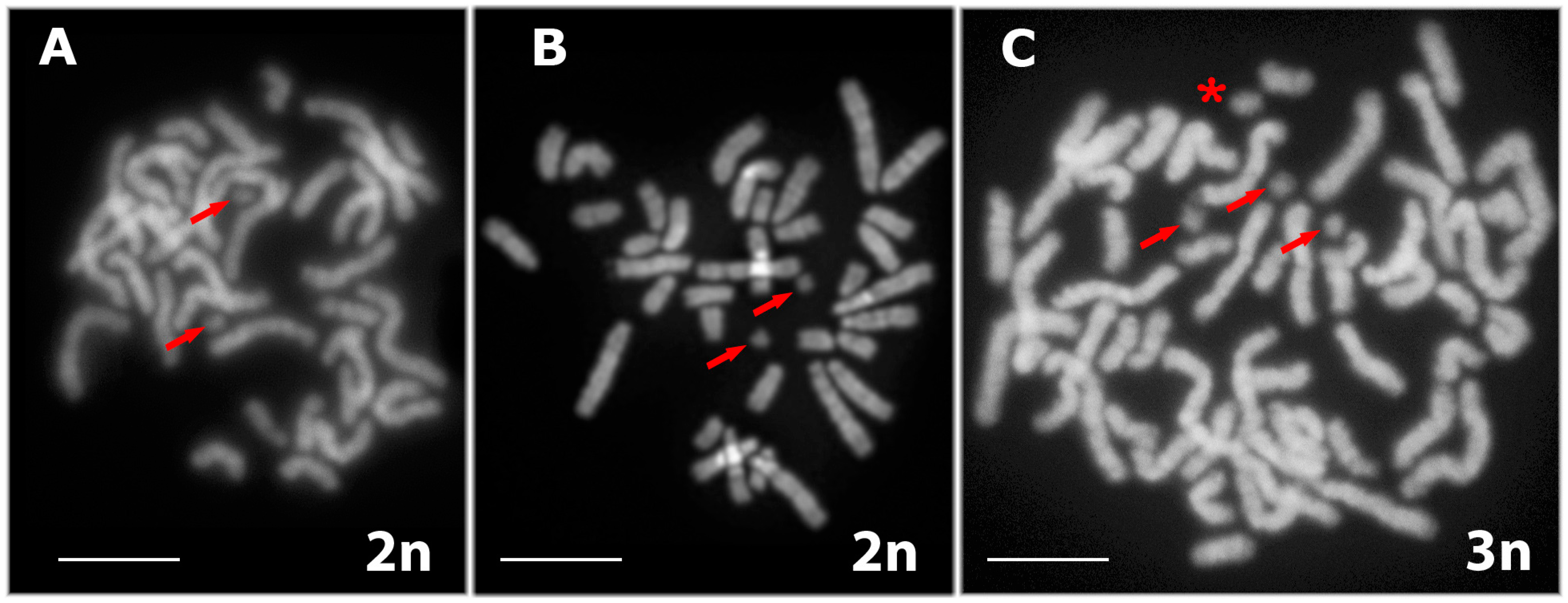
3.1. Karyotyping in Males of the Parental Species D. raddei nairensis and D. valentini and Triploid Hybrid D. unisexualis × D. valentini
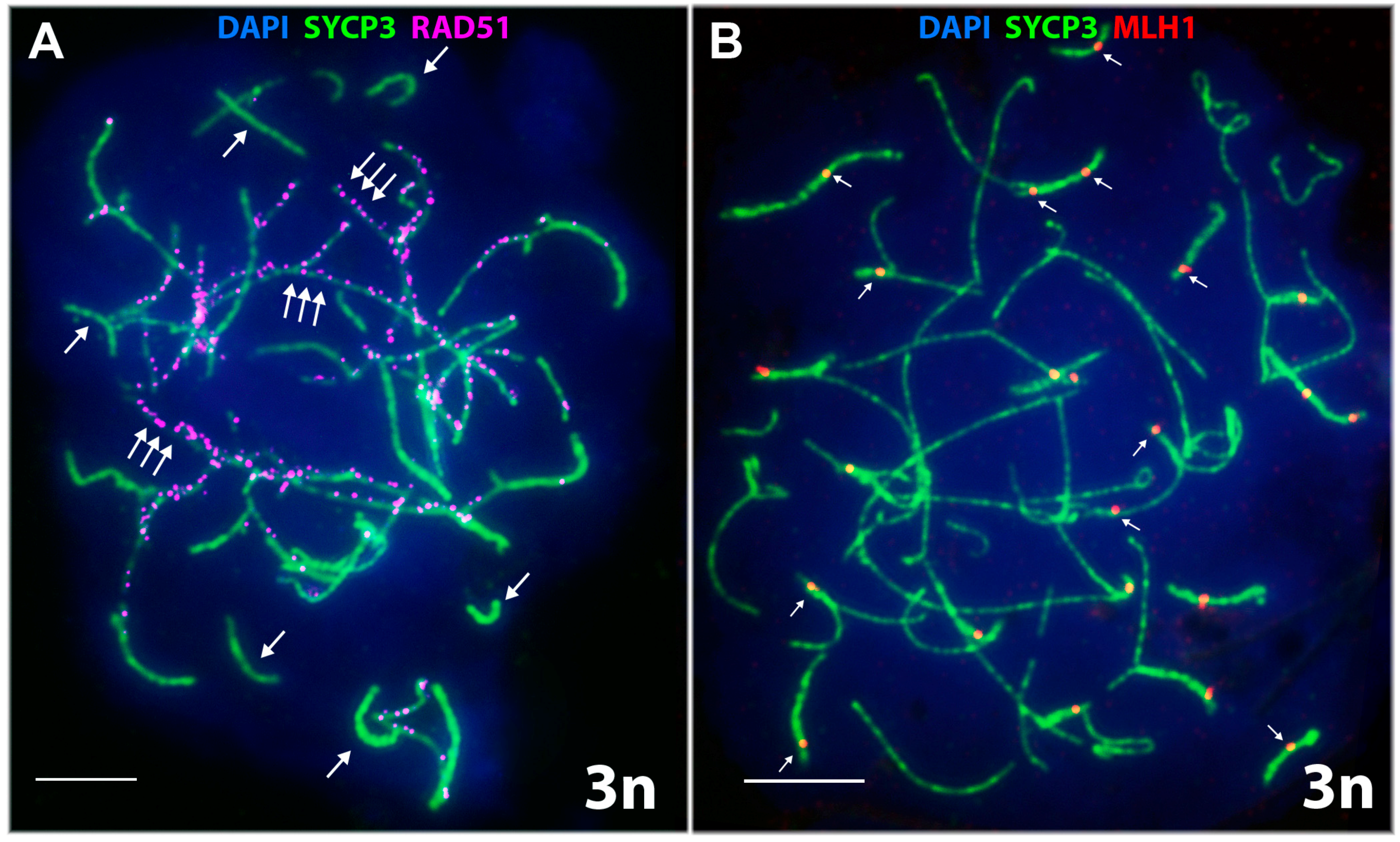
3.2. Specifics of Chromosome Synapsis, DSB Repair, and Recombination in Males of the Parental Species D. raddei nairensis and D. valentini and in Triploid D. unisexualis × D. valentini Hybrid Male
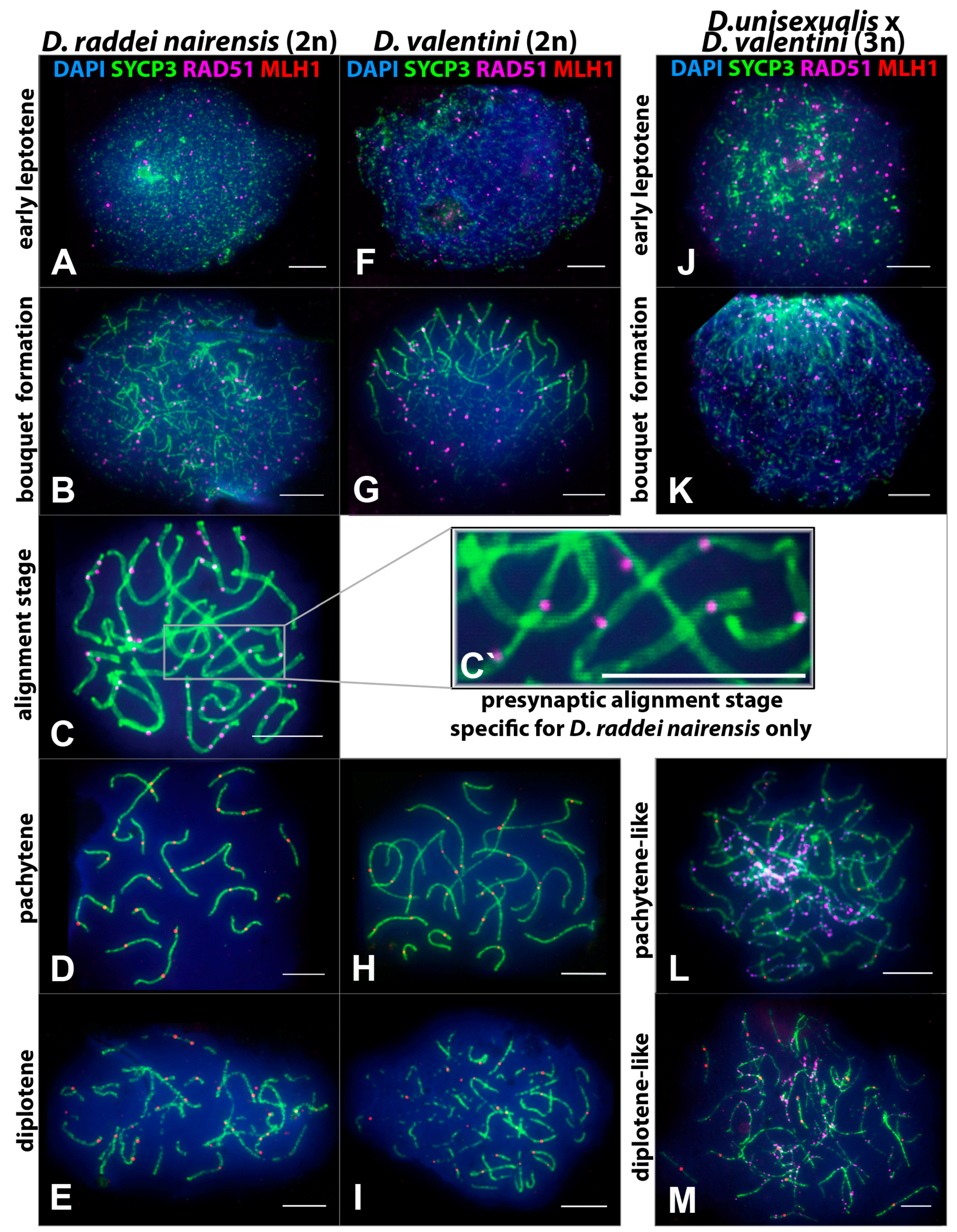
3.2.1. Specifics of Meiotic Prophase I in D. raddei nairensis Male
3.2.2. Specifics of Meiotic Prophase I in D. valentini Male
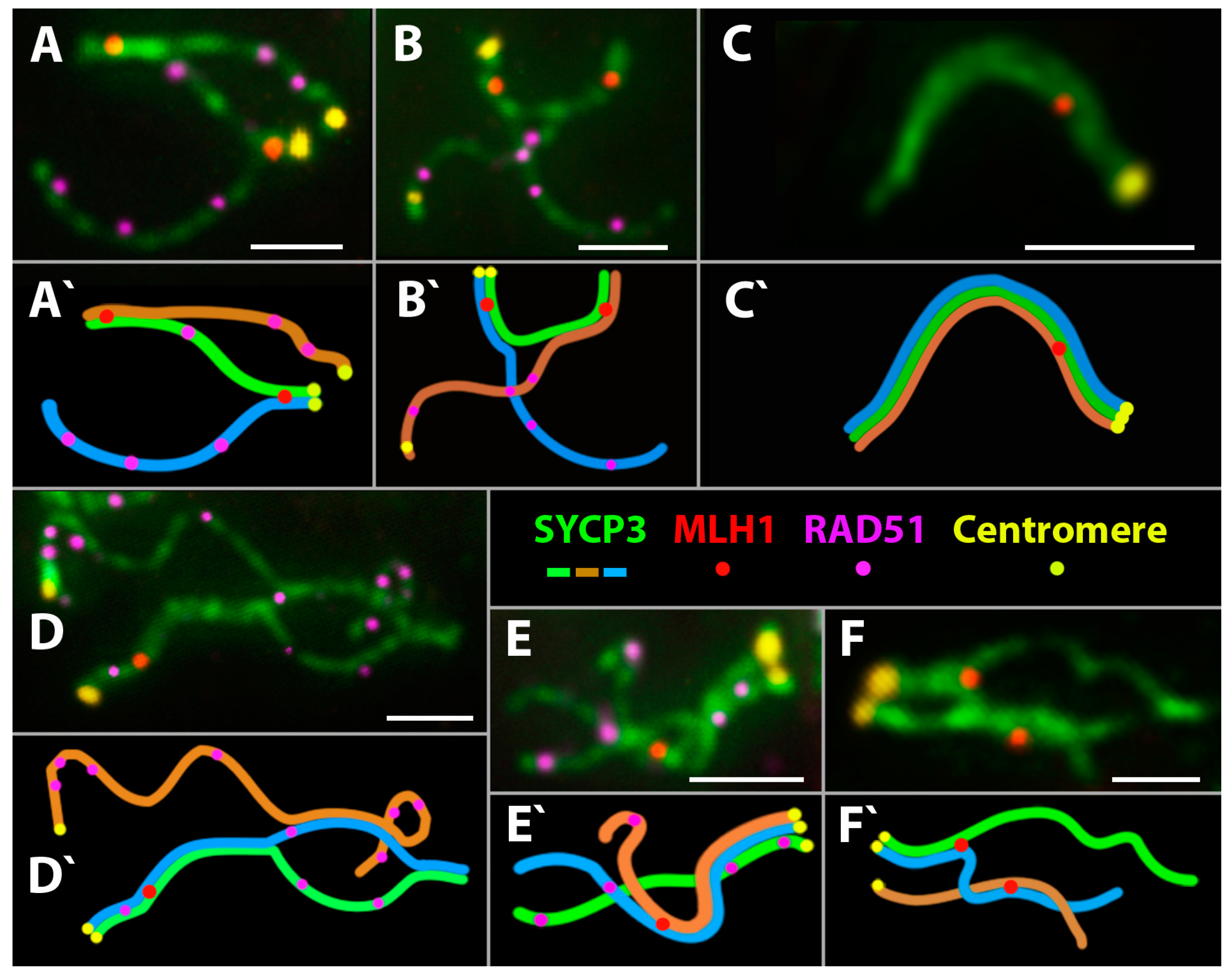
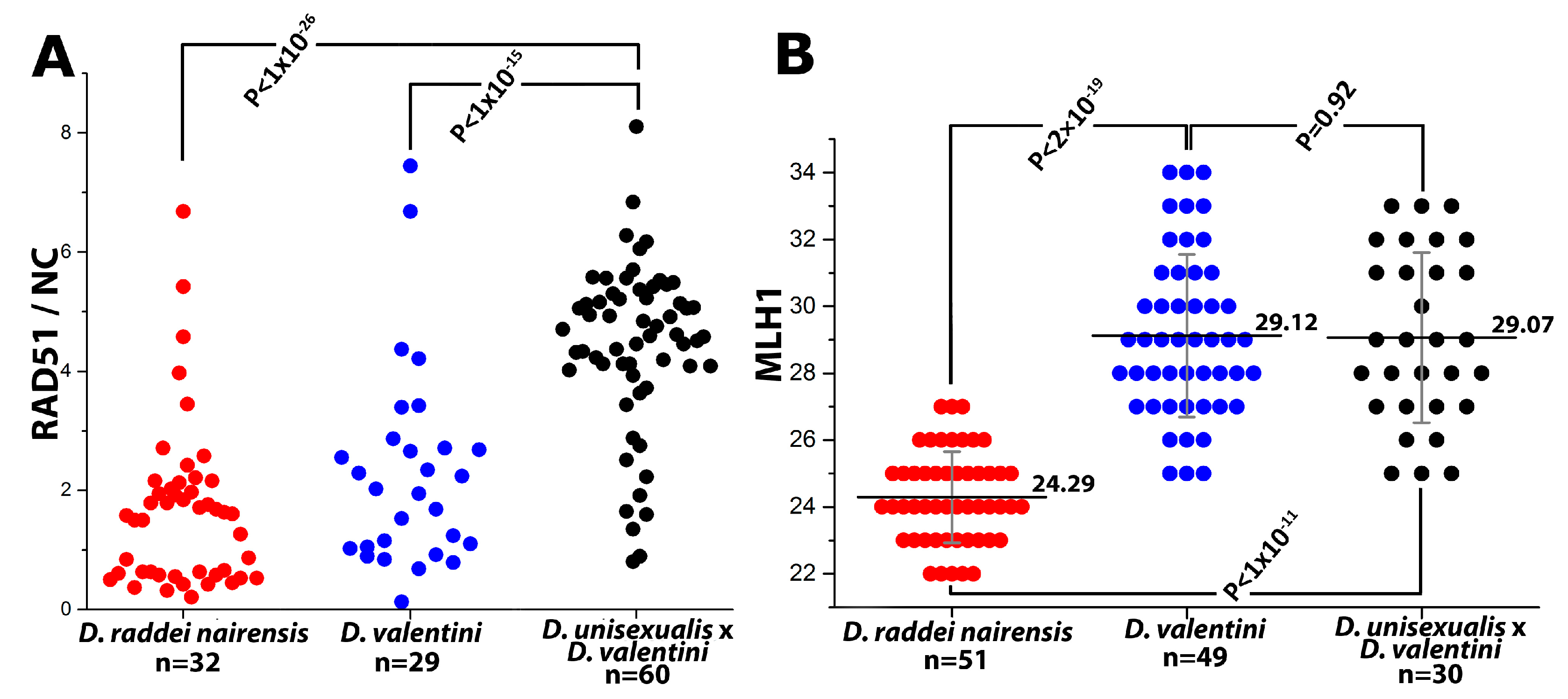
- Thus, main characteristics and specifics of meiotic prophase I in males were established for D. raddei nairensis and D. valentini. A significant difference (p < 0.01) was found between the levels of meiotic recombination (number of MLH1 foci) of the two parental species (Figure 6B). We also detected a specific stage of homologous chromosome alignment, which was characteristic of the D. raddei nairensis male only (Figure 3C,C`).
3.2.3. Specifics of Synapsis and DSB Repair in D. unisexualis × D. valentini Triploid Hybrid Male
- 1.
- Short chromosomes (from 0.5 to 1 μm) formed SC throughout their lengths in nuclei from the triploid male. These SC already lacked RAD51 foci, but had one or, rarely, two MLH1 foci (Figure 3L and Figure 4A,B). Staining with anti-SYCP3 antibodies demonstrated that the short SC differed in width from SC bivalents observed in the diploid lizards. Electron microscopy is necessary to confirm or reject a trivalent nature of the thickened SC (Figure 5C).
- 2.
- Medium-length chromosomes (from 1 to 3 μm) occurred more often at the periphery of a spread nucleus. These chromosomes showed side-by-side synapsis, or the SC formed only in the subtelomeric regions. However, synapsis of three medium-sized homeologs that was complete throughout the chromosome length was not observed. Likewise, RAD51 foci were not fully eliminated from the axial elements of chromosomes (Figure 3L, Figure 4А and Figure 5D,E).
- 3.
- Long chromosomes (from 3 to 5 μm) often grouped in the central region of a spread nucleus. Synapsis of these chromosomes was usually incomplete, the finding being supported by arrays of RAD51 foci observed in asynaptic axis regions (Figure 3L and Figure 4А). Synaptic configurations of the long chromosomes were difficult to analyze without electron microscopy.
3.2.4. Meiotic Recombination in Spermatocyte I Nuclei of the Triploid D. unisexualis × D. valentini Hybrid
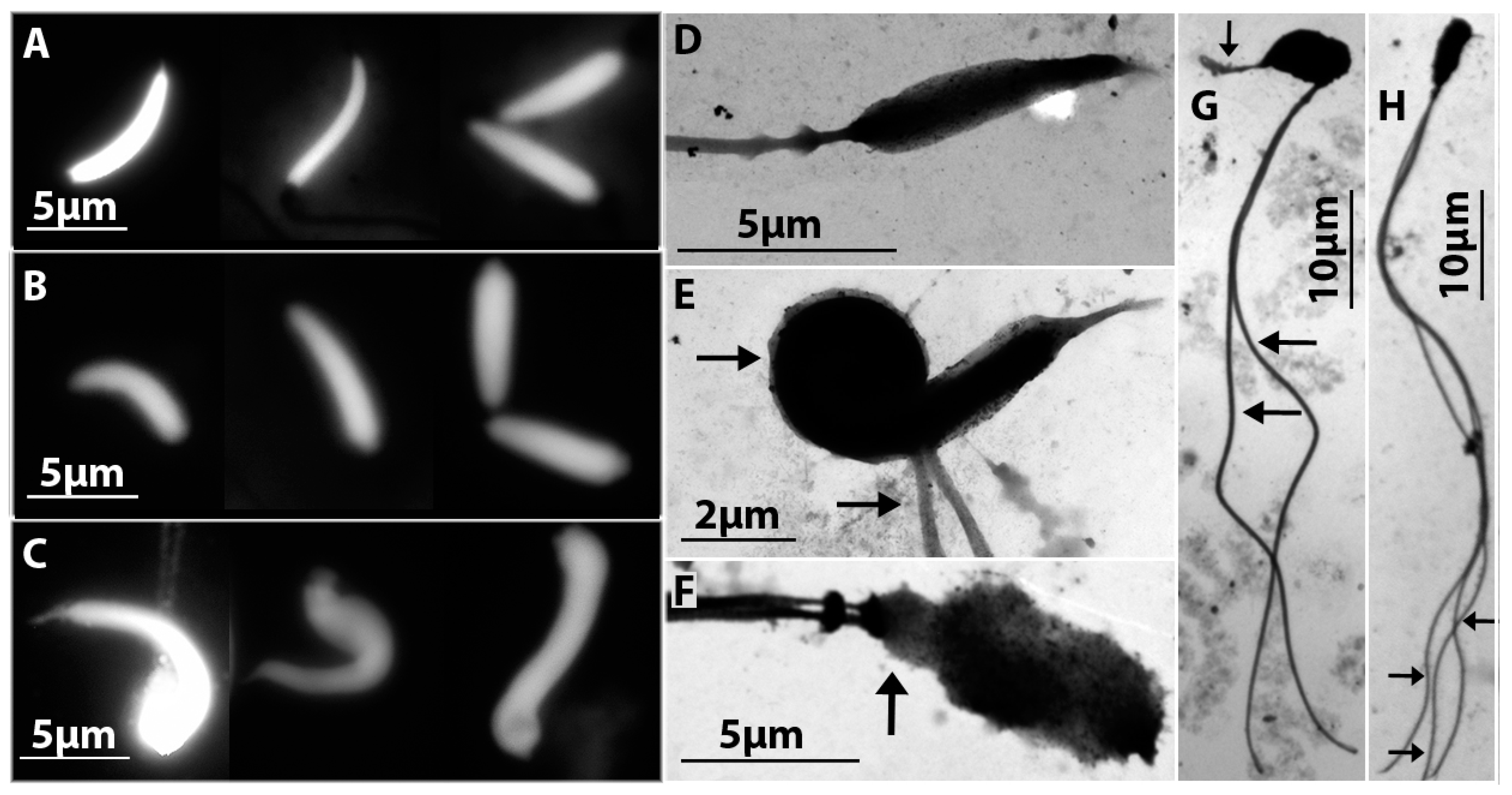
3.3. Comparative Study of Mature Spermatids of D. raddei nairensis and D. valentini and Hybrid D. unisexualis × D. valentini Male
4. Discussion
4.1. Meiosis in Bisexual Parental Species D. raddei nairensis and D. valentini Males
4.2. Meiotic Recombination in Spermatocytes of Triploid D. unisexualis × D. valentini Male
4.3. Comparison of Our Findings with Previous Observations
4.4. Is Morphology of Spermatids or Spermatozoa Indicative of Fertility or Infertility? What Is Necessary Bridging Cytogenetics of Spermatogenesis with Reticular Evolution?
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darevsky, I.S. Natural parthenogenesis in certain subspecies of rock lizards (Lacerta Saxicola Eversmann). Doklady Akademii Nauk SSSR 1958, 122, 730–732. (In Russian) [Google Scholar]
- Darevsky, I.S. Natural parthenogenesis in a polymorphic group of Caucasian rock lizard s related to Lacerta saxicola Eversmann. J. Ohio Herpetol. Soc. 1966, 5, 114–152. [Google Scholar] [CrossRef]
- Darevsky, I.S. Evolution and ecology of parthenogenesis in reptiles. Soc. Study Amphib. Reptiles Contr. Herpetol. 1992, 9, 21–39. [Google Scholar]
- Dobzhansky, T.G. Genetics and the Origin of Species; Columbia University Press: New York, NY, USA, 1937. [Google Scholar]
- Uzzell, T.; Darevsky, I.S. The evidence of the hybrid origin of parthenogenetic Caucasian rock lizards of the Lacerta genus. Zhurnal Obshchei Biol. 1974, 35, 553–561. (In Russian) [Google Scholar]
- Darevsky, I.S.; Kupriyanova, L.A.; Danielyan, F.D. New evidence of hybrid males of parthenogenetic species. In Studies in Herpetology; Rocek, Z., Ed.; Charles University: Prague, Czech Republic, 1986; pp. 207–212. [Google Scholar]
- Uzzell, T.; Darevsky, I.S. Biochemical evidence for the hybrid origin of the parthenogenetic species of the Lacerta saxicola complex (Sauria, Lacertidae), with a discussion of some ecological and evolutionary implications. Copeia 1975, 2, 204–222. [Google Scholar] [CrossRef]
- Borkin, L.J.; Darevsky, I.S. Reticular (hybridogeneous) speciation in vertebrate. Zhurnal Obshchei Biol. 1980, 41, 485–506. (In Russian) [Google Scholar]
- Danielyan, F.D. Theory of Hybrid Origin of Parthenogenesis in Group of Caucasian Rock Lizards. Ph.D. Thesis, Ukrainian Academy of Sciences, Kiev, Russia, 1989. (In Russian). [Google Scholar]
- Danielyan, F.D. Study of mixed populations of three parthenogenetic species of the Rock Lizards (Lacerta saxicola complex) in Armenia. Proc. Zool. Inst. 1987, 158, 77–83. (In Russian) [Google Scholar]
- Darevsky, I.S.; Danielyan, F.D. Evolution, Ecology and Biodiversity. In Proceedings of the Scientific Conference; Dedicated to Memory of Vorontsov; Krasilov, V.A., Ed.; Vladivostok, Russia; 2001; pp. 131–134. (In Russian) [Google Scholar]
- Arakelyan, M. Microevolutionary Processes in Sympatric Populations of Some Reptiles from Republic of Armenia and Adjacent Territories. D.Sc. Thesis, Yerevan State University, Yerevan, Armenia, 2012; p. 299. [Google Scholar]
- Kupriyanova, L.A. Concept of hybridogeneous speciation of vertebrate animals: Complex studies of unisexual species of reptilian. Proc. Zool. Inst. 2014, 318, 382–390. [Google Scholar]
- Hall, W.P. Three probable cases of parthenogenesis in lizards (Agamidae, Chamaeleontidae, Gekkonidae). Experientia 1970, 26, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Darevsky, I.S.; Kupriyanova, L.A. Two new all-female lizard species of the genus Leiolepis Cuvier, 1829 from Thailand and Vietnam (Squamata: Sauria: Uromastycinae). Herpetozoa 1993, 6, 3–20. [Google Scholar]
- Moritz, C.; Case, T.J.; Bolger, D.T.; Donnellan, S.C. Genetic diversity and the history of some Pacific island house geckos (Hemidactylus and Lepidodactylus). Biol. J. Linn. Soc. 1993, 48, 113–133. [Google Scholar] [CrossRef]
- Trifonov, V.A.; Paoletti, A.; Caputo Barucchi, V.; Kalinina, T.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; Giovannotti, M. Comparative Chromosome Painting and NOR Distribution Suggest a Complex Hybrid Origin of Triploid Lepidodactylus lugubris (Gekkonidae). PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Darevsky, I.S.; Kupriyanova, L.A.; Roshchin, V. A new all-female triploid species of gecko and karyological data on the bisexual Hemidactyltis frenatus from Vietnam. J. Herpetol. 1984, 18, 274–284. [Google Scholar] [CrossRef]
- Moritz, С. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae). Chromosoma 1984, 89, 151–162. [Google Scholar] [CrossRef]
- Cuellar, O. Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorus uniparens. J. Morphol. 1971, 133, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Serena, M. Distribution and habitats of parthenogenetic and sexual Cnemidophorus lemniscatus (Sauria: Teiidae) in Surinam. Copeia 1984, 1984, 713–719. [Google Scholar] [CrossRef]
- Lutes, A.A.; Baumann, D.P.; Neaves, W.B.; Baumann, P. Laboratory synthesis of an independently reproducing vertebrate species. Proc. Natl. Acad. Sci. USA 2011, 108, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, L. Some cytogenetic regular trends in reticular (hybridogeneous) speciation in unisexual lizards (Reptilia: Lacertidae) and other groups of vertebrates. Tsytologia 1997, 39, 1089–1108. (In Russian) [Google Scholar]
- Kupriyanova, L. Genetic variations in hybrid unisexual species and forms of the genus Lacerta (Lacertidae, Reptilia): Possible cytogenetic mechanisms, cytogenetics of meiosis in natural polyploidy forms. Tsytologia 1999, 41, 1038–1047. (In Russian) [Google Scholar]
- Tokarskaya, O.N.; Kan, N.G.; Petrosyan, V.G.; Martirosyan, I.A.; Grechko, V.V.; Danielyan, F.D.; Darevsky, I.S.; Ryskov, A.P. Genetic variation in parthenogenetic Caucasian rock lizards of the genus Lacerta (L. dahli, L. armeniaca, L. unisexualis) analyzed by DNA fingerprinting. Mol. Genet. Genom. 2001, 265, 1617–4615. [Google Scholar]
- Danielyan, F.; Arakelyan, M.; Stepanyan, I. Hybrids of Darevskia valentini, D. armeniaca and D. unisexualis from a sympatric population in Armenia. Amphibia-Reptilia 2008, 29, 487–504. [Google Scholar] [CrossRef]
- Moritz, C.; Uzzell, T.; Spolsky, C.; Hotz, H.; Darevsky, I.; Kupriyanova, L.; Danielyan, F. The material ancestry and approximate age of parthenogenetic species of Caucasian rock lizards (Lacerta: Lacertidae). Genetica 1992, 87, 53–62. [Google Scholar] [CrossRef]
- Tarkhnishvili, D.; Murtskhvaladze, M.; Anderson, C.L. Coincidence of genotypes at two loci in two parthenogenetic rock lizards: How backcrosses might trigger adaptive speciation. Biol. J. Linnean Soc. 2017, 20, 1–14. [Google Scholar] [CrossRef]
- Darevsky, I.S.; Danielyan, F.D. Diploid and triploid progeny arising from natural mating of parthenogenetic Lacerta armeniaca and L. unisexualis with bisexual L. saxicola valentini. J. Herp. 1968, 2, 65–69. [Google Scholar] [CrossRef]
- Darevsky, I.S.; Danielyan, F.D. Diploid and triploid specimens in progeny of parthenogenetic females of rocky lizards as a consequence of their natural crossing with males of closely related bisexual species. Doklady Akademii Nauk SSSR 1969, 184, 727–730. [Google Scholar]
- Cole, C.J.; Painter, C.W.; Dessauer, H.C.; Taylor, H.L. Hybridization between the Endangered Unisexual Gray-Checkered Whiptail Lizard (Aspidoscelis dixoni) and the Bisexual Western Whiptail Lizard (Aspidoscelis tigris) in Southwestern New Mexico; American Museum of Natural History: New York, NY, USA, 2007; No. 3555; pp. 1–31. [Google Scholar]
- Darevsky, I.S.; Uzzell, T.M.; Kupriyanova, L.A.; Danielyan, F.D. Triploid hybrid males in sympatric populations of some parthenogenetic and bisexual species of rock lizards of the genus Lacerta. Bull. Mosc. Soc. Nat. 1973, 78, 48–58. (In Russian) [Google Scholar]
- Darevsky, I.S.; Kupriyanova, L.A.; Uzzell, T. Parthenogenesis in reptiles. Biol. Reptil. 1985, 15, 411–526. [Google Scholar]
- Darevsky, I.S.; Kulikova, V.N. Taxonomic characters and certain peculiarities of the oogenesis of hybrids between bisexual and parthenogenetic forms of Lacerta saxicola Eversmann. Cytologia 1962, 5, 160–170. (In Russian) [Google Scholar]
- Darevsky, I.S.; Kulikova, V.N. Natural triploidy in polymorphic group of Caucasian rock lizards (Lacerta saxicola Eversmann) as result of hybridization of bisexual with parthenogenetic forms of these species. Doklady Akademii Nauk SSSR 1964, 158, 202–205. (In Russian) [Google Scholar]
- Grebelnyi, S.D. How many clonal species are there in the world. Invertebr. Zool. 2005, 2, 79–102. [Google Scholar]
- Kupriyanova, L. Cytogenetic evidence for genome interaction in hybrid lacertid lizards. Evol. Ecol. Unisexual Verteb. 1989, 466, 236–239. [Google Scholar]
- Darevsky, I.S.; Danielyan, F.D.; Sokolova, T.M.; Rozonov, Y.M. Intraclonal mating in the parthenogenetic lizard species Lacerta unisexualis. In Evolution and Ecology of Unisexual Vertebrates; Dawley, R.M., Bogart, J.P., Eds.; New York State Museum: New York, NY, USA, 1989; pp. 228–235. [Google Scholar]
- Lowe, C.H.; Wright, J.M. Evolution of parthenogenetic species of Cnemidophorus (whiptail lizards) in western North America. J. Ariz. Acad. Sci. 1966, 4, 81–87. [Google Scholar]
- Moritz, C.; Donnellan, S.; Adams, M.; Baverstock, P.R. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae): Extensive genotypic diversity among parthenogens. Evolution 1989, 43, 994–1003. [Google Scholar] [CrossRef]
- Ford, C.E.; Hamerton, J.L. A colchicine hypotonic citrate squash sequence for mammalian chromosomes. Stain Technol. 1956, 31, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Vidal, F.; Quitart, M.; Egozcue, J. A method for the sequential study of synaptonemal complexes by light and electron microscopy. Hum. Genet. 1981, 59, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Matveevsky, S.; Bakloushinskaya, I.; Kolomiets, O. Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation? Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gribbins, K.M. Reptilian spermatogenesis: A histological and ultrastructural perspective. Spermatogenesis 2011, 1, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Dolder, H. Ultrastructural analysis of spermiogenesis in Iguana iguana (Reptilia: Sauria: Iguanidae). Eur. J. Morphol. 2002, 40, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.H.; Colli, G.R.; Báo, S.N. The ultrastructure of the spermatozoon of the lizard Iguana iguana (Reptilia. Squamata, Iguanidae) and the variability of sperm morphology among iguanian lizards. J. Anat. 2004, 204, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Zickler, D.; Kleckner, N. Meiotic chromosomes: Integrating structure and function. Annu. Rev. Genet. 1999, 33, 603–754. [Google Scholar] [CrossRef] [PubMed]
- Moens, P.B. The fine structure of meiotic chromosomes: Polarization and pairing in Locusta migratoria spermatocytes. Chromosoma 1969, 28, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, O.L.; Fedotova, Yu. S.; Bogdanov, Yu. F. Natural degradation of synaptonemal complexes at diplotene stage of meiosis permits analyse of their ultrastructural components. Biolo. Membr. 2001, 18, 230–239. [Google Scholar]
- Cloutier, J.M.; Turner, J.M. Meiotic sex chromosome inactivation. Curr. Biol. 2010, 20, R962–R963. [Google Scholar] [CrossRef] [PubMed]
- Cuňado, N.; Terrones, J.; Sănchez, L.; Martinez, P.; Santos, J.L. Sex-dependent synaptic behaviour in triploid turbot, Scophthalmus maximus (Pisces, Scophthalmidae). Heredity 2002, 89, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Egozcue, S.; Blanco, J.; Vendrell, J.M.; Garcia, F.; Veiga, A.; Aran, B.; Barri, P.N.; Vidal, F.; Egozcue, J. Human male infertility: Chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum. Reprod. Update 2000, 6, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bocharova, E.N.; Kurilo, L.F.; Shileiko, L.V.; Bragina, E.E.; Yurov, Y.B.; Vorsanova, S.G.; Iourov, I.Y.; Klimova, R.R.; Kushch, A.A. Analysis of germ cell populations in ejaculate of men infected with herpes simplex virus. Russ. J. Dev. Biol. 2008, 39, 42–51. [Google Scholar] [CrossRef]
- Tassistro, V.; Ghalamoun-Slami, R.; Saias-Magnan, J.; Guichaoua, M.R. Chronology of meiosis and synaptonemal complex abnormalities in normal and abnormal spermatogenesis. Indian J. Med. Res. 2009, 129, 268–278. [Google Scholar] [PubMed]
- Röll, B.; von Düring, M.U. Sexual characteristics and spermatogenesis in males of the parthenogenetic gecko Lepidodactylus lugubris (Reptilia, Gekkonidae). Zoology 2008, 111, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Barringer, B.C.; Barbash, D.A. The pachytene checkpoint and its relationship to evolutionary patterns of polyploidization and hybrid sterility. Heredity 2009, 102, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Darevsky, I.S.; Kupriyanova, L.A.; Bakradze, M.A. Residual bisexuality in parthenogenetic species of lizards of the Lacerta genus. Zhurnal Obshchei Biol. 1977, 38, 772–780. (In Russian) [Google Scholar]
- Schultz, R.J. Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 1969, 103, 605–619. [Google Scholar] [CrossRef]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Jouannet, P.; Eustache, F. Another look at human sperm morphology. Hum. Reprod. 2016, 31, 10–23. [Google Scholar] [CrossRef] [PubMed]
| Species | Early Leptotene | Zygotene | Alignment | Pachytene | Diplotene | Total Nuclei |
|---|---|---|---|---|---|---|
| D. raddei nairensis | 5 (3%) | 26 (15.8%) | 32 (19.4%) | 61 (37%) | 41 (24.8%) | 165 |
| D. valentini | 5 (1.8%) | 63 (23.5%) | 0 | 139 (51.9%) | 61 (22.8%) | 268 |
| D. valentini × D. unisexualis | 20 (6.1%) | 88 (26.7%) | 0 | 150 (45.6%) 1 | 71 (21.6%) 2 | 329 |
| Species | Karyotype | N | MLH1 Focus Number Per Nucleus, Mean ± SD |
|---|---|---|---|
| D. raddei nairensis | 2n = 38 | 51 | 24.29 ± 1.36 |
| D. valentini | 2n = 38 | 49 | 29.12 ± 2.43 |
| D. valentini × D. unisexualis | 3n = 57 | 30 | 29.07 ± 2.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spangenberg, V.; Arakelyan, M.; Galoyan, E.; Matveevsky, S.; Petrosyan, R.; Bogdanov, Y.; Danielyan, F.; Kolomiets, O. Reticulate Evolution of the Rock Lizards: Meiotic Chromosome Dynamics and Spermatogenesis in Diploid and Triploid Males of the Genus Darevskia. Genes 2017, 8, 149. https://doi.org/10.3390/genes8060149
Spangenberg V, Arakelyan M, Galoyan E, Matveevsky S, Petrosyan R, Bogdanov Y, Danielyan F, Kolomiets O. Reticulate Evolution of the Rock Lizards: Meiotic Chromosome Dynamics and Spermatogenesis in Diploid and Triploid Males of the Genus Darevskia. Genes. 2017; 8(6):149. https://doi.org/10.3390/genes8060149
Chicago/Turabian StyleSpangenberg, Victor, Marine Arakelyan, Eduard Galoyan, Sergey Matveevsky, Ruzanna Petrosyan, Yuri Bogdanov, Felix Danielyan, and Oxana Kolomiets. 2017. "Reticulate Evolution of the Rock Lizards: Meiotic Chromosome Dynamics and Spermatogenesis in Diploid and Triploid Males of the Genus Darevskia" Genes 8, no. 6: 149. https://doi.org/10.3390/genes8060149