R Loops in the Regulation of Antibody Gene Diversification
Abstract
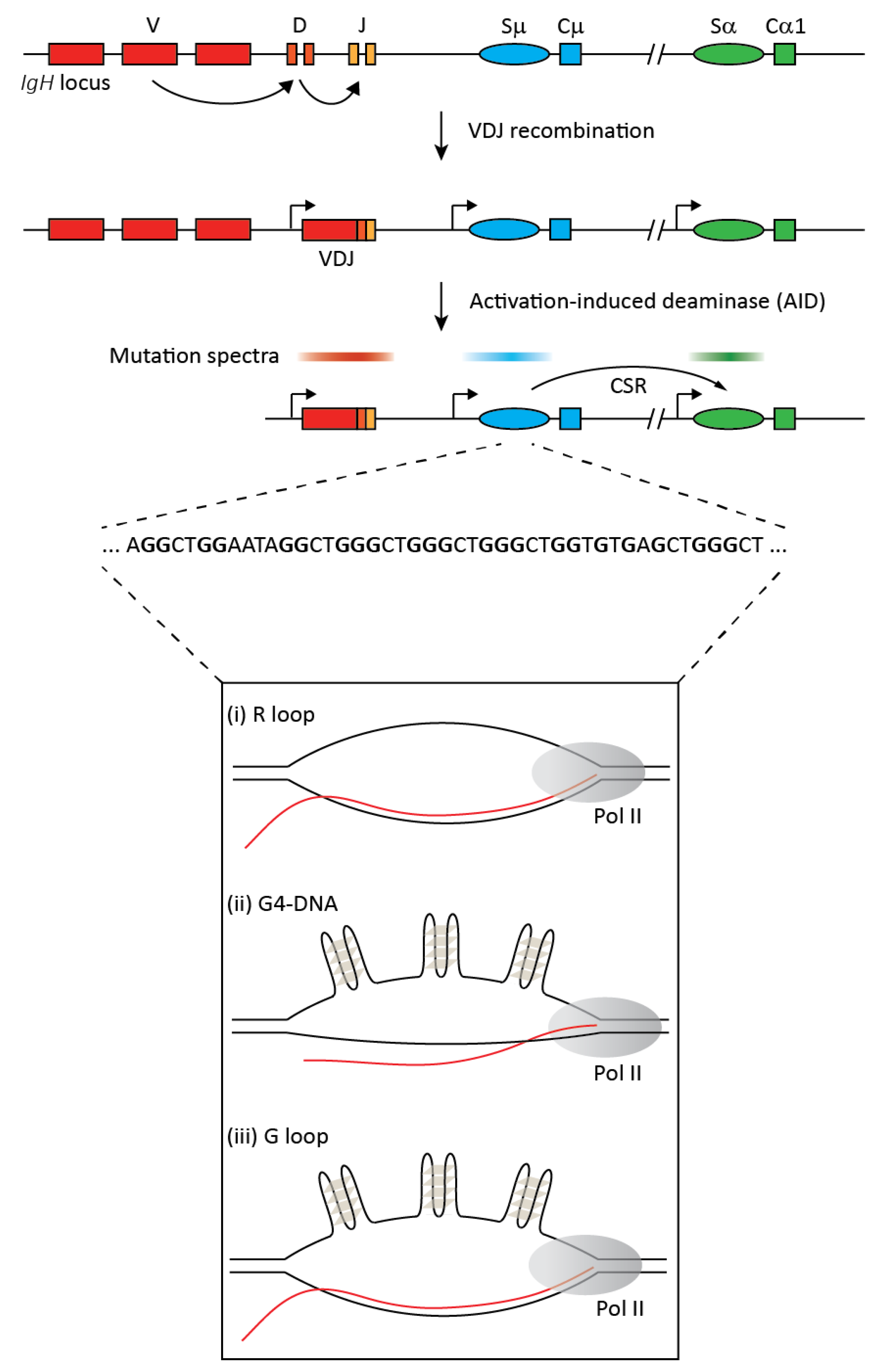
:1. Introduction
2. Transcription in CSR and SHM
3. Discovery of R Loops in IgH Switch Regions
4. R Loop Frequency Correlates with CSR Efficiency
5. Are R Loops Required for IgH Switch Region Mutagenesis? Probably Not
6. R Loops Influence CSR by Regulating the DNA Replication Landscape at the IgH Locus
7. G-Quadruplexes in IgH Switch DNA and Their Functional Implications vis-à-vis R Loops
8. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Chedin, F. Nascent Connections: R-Loops and Chromatin Patterning. Trends Genet. 2016, 32, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.B.; Chen, H.V.; Acharya, D.; Rando, O.J.; Fazzio, T.G. R loops regulate promoter-proximal chromatin architecture and cellular differentiation. Nat. Struct. Mol. Biol. 2015, 22, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.; Lufino, M.M.; Wade-Martins, R.; Gromak, N. R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 2014, 10, e1004318. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Kamieniarz-Gdula, K.; Proudfoot, N.J. R-loops induce repressive chromatin marks over mammalian gene terminators. Nature 2014, 516, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Proudfoot, N.J.; Gromak, N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell 2011, 42, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, J.M.; Aguilera, A. R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Proudfoot, N.J. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014, 28, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Becherel, O.J.; Yeo, A.J.; Stellati, A.; Heng, E.Y.; Luff, J.; Suraweera, A.M.; Woods, R.; Fleming, J.; Carrie, D.; McKinney, K.; et al. Senataxin plays an essential role with DNA damage response proteins in meiotic recombination and gene silencing. PLoS Genet. 2013, 9, e1003435. [Google Scholar] [CrossRef]
- Morales, J.C; Richard, P.; Patidar, P.L.; Motea, E.A.; Dang, T.T.; Manley, J.L.; Boothman, D.A. XRN2 Links Transcription Termination to DNA Damage and Replication Stress. PLoS Genet. 2016, 12, e1006107. [Google Scholar] [CrossRef]
- Yang, Y.; McBride, K.M.; Hensley, S.; Lu, Y.; Chedin, F.; Bedford, M. Arginine methylation facilitates the recruitment of TOP3B to chromatin to prevent R loop accumulation. Mol. Cell 2014, 53, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Barroso, S.I.; Garcia-Rubio, M.L.; Tumini, E.; Herrera-Moyano, E.; Aguilera, A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature 2014, 511, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Chao, J.; Rabadan, R.; Economides, A.N.; Basu, U. Noncoding RNA transcription targets AID to divergently transcribed loci in B cells. Nature 2014, 514, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Giri, P.K.; Kazadi, D.; Laffleur, B.; Zhang, W.; Grinstein, V.; Pefanis, E.; Brown, L.M.; Ladewig, E.; Martin, O.; et al. Nuclear Proximity of Mtr4 to RNA Exosome Restricts DNA Mutational Asymmetry. Cell 2017, 169, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Manley, J.L. R Loops and Links to Human Disease. J. Mol. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stavnezer, J.; Schrader, C.E. IgH chain class switch recombination: Mechanism and regulation. J. Immunol. 2014, 193, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zan, H.; Pone, E.J.; Mai, T.; Casali, P. Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat. Rev. Immunol. 2012, 12, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Muramatsu, M.; Sankaranand, V.S.; Anant, S.; Sugai, M.; Kinoshita, K.; Davidson, N.O.; Honjo, T. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 1999, 274, 18470–18476. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Tian, M.; Khuong, C.; Chua, K.; Pinaud, E.; Alt, F.W. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature 2003, 422, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.K.; Market, E.; Besmer, E.; Papavasiliou, F.N. AID mediates hypermutation by deaminating single stranded DNA. J. Exp. Med. 2003, 197, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Petersen-Mahrt, S.K.; Harris, R.S.; Neuberger, M.S. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 2002, 418, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Bransteitter, R.; Petruska, J.; Goodman, M.F. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 2003, 424, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, A.R.; Stavropoulos, P.; Jankovic, M.; Nussenzweig, M.C. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat. Immunol. 2003, 4, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Klapacz, J.; Samaranayake, M.; Ullah, A.; Bhagwat, A.S. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Res. 2003, 31, 2990–2994. [Google Scholar] [CrossRef] [PubMed]
- Stavnezer-Nordgren, J.; Sirlin, S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986, 5, 95–102. [Google Scholar] [PubMed]
- Yancopoulos, G.D.; DePinho, R.A.; Zimmerman, K.A.; Lutzker, S.G.; Rosenberg, N.; Alt, F.W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986, 5, 3259–3266. [Google Scholar] [PubMed]
- Pavri, R.; Nussenzweig, M.C. AID targeting in antibody diversity. Adv. Immunol. 2011, 110, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Stavnezer, J. Antibody class switching. Adv. Immunol. 1996, 61, 79–146. [Google Scholar] [PubMed]
- Storb, U. The molecular basis of somatic hypermutation of immunoglobulin genes. Curr. Opin. Immunol. 1996, 8, 206–214. [Google Scholar] [CrossRef]
- Odegard, V.H.; Schatz, D.G. Targeting of somatic hypermutation. Nat. Rev. Immunol. 2006, 6, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Reaban, M.E.; Griffin, J.A. Induction of RNA-stabilized DNA conformers by transcription of an immunoglobulin switch region. Nature 1990, 348, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.A.; Lieber, M.R. RNA/DNA complex formation upon transcription of immunoglobulin switch regions: Implications for the mechanism and regulation of class switch recombination. Nucleic Acids Res. 1995, 23, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Chedin, F.; Hsieh, C.L.; Wilson, T.E.; Lieber, M.R. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat. Immunol. 2003, 4, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Maul, R.W.; Chon, H.; Sakhuja, K.; Cerritelli, S.M.; Gugliotti, L.A.; Gearhart, P.J.; Crouch, R.J. R-Loop Depletion by Over-expressed RNase H1 in Mouse B Cells Increases Activation-Induced Deaminase Access to the Transcribed Strand without Altering Frequency of Isotype Switching. J. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Pannunzio, N.R.; Lu, Z.; Hsu, E.; Yu, K.; Lieber, M.R. The repetitive portion of the Xenopus IgH Mu switch region mediates orientation-dependent class switch recombination. Mol. Immunol. 2015, 67, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Yu, K.; Lieber, M.R. Mechanism of R-loop formation at immunoglobulin class switch sequences. Mol. Cell. Biol. 2008, 28, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Pannunzio, N.R.; Hsieh, C.L.; Yu, K.; Lieber, M.R. The role of G-density in switch region repeats for immunoglobulin class switch recombination. Nucleic Acids Res. 2014, 42, 13186–13193. [Google Scholar] [CrossRef] [PubMed]
- Shinkura, R.; Tian, M.; Smith, M.; Chua, K.; Fujiwara, Y.; Alt, F.W. The influence of transcriptional orientation on endogenous switch region function. Nat. Immunol. 2003, 4, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Meng, F.L.; Keim, C.; Grinstein, V.; Pefanis, E.; Eccleston, J.; Zhang, T.; Myers, D.; Wasserman, C.R.; Wesemann, D.R.; et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell 2011, 144, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Pannunzio, N.R.; Han, L.; Hsieh, C.L.; Yu, K.; Lieber, M.R. The strength of an Ig switch region is determined by its ability to drive R loop formation and its number of WGCW sites. Cell Rep. 2014, 8, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Ginno, P.A.; Lott, P.L.; Christensen, H.C.; Korf, I.; Chedin, F. R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol. Cell 2012, 45, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Parsa, J.Y.; Ramachandran, S.; Zaheen, A.; Nepal, R.M.; Kapelnikov, A.; Belcheva, A.; Berru, M.; Ronai, D.; Martin, A. Negative supercoiling creates single-stranded patches of DNA that are substrates for AID-mediated mutagenesis. PLoS Genet. 2017, 8, e1002518. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, E.M.; Peycheva, M.; Pavri, R. DNA Replication Origins in Immunoglobulin Switch Regions Regulate Class Switch Recombination in an R-Loop-Dependent Manner. Cell Rep. 2016, 17, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.D.; Garboczi, D.N.; Singh, K.; Hu, Z.; Leppla, S.H.; Leysath, C.E. The sub-nanomolar binding of DNA-RNA hybrids by the single-chain Fv fragment of antibody S9.6. J. Mol. Recognit. 2013, 26, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Methot, S.P.; Di Noia, J.M. Molecular Mechanisms of Somatic Hypermutation and Class Switch Recombination. Adv. Immunol. 2017, 133, 37–87. [Google Scholar] [CrossRef] [PubMed]
- Ronai, D.; Iglesias-Ussel, M.D.; Fan, M.; Li, Z.; Martin, A.; Scharff, M.D. Detection of chromatin-associated single-stranded DNA in regions targeted for somatic hypermutation. J. Exp. Med. 2007, 204, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; Schiavone, D.; Frey, A.; Sale, J.E. Histone H3.3 promotes IgV gene diversification by enhancing formation of AID-accessible single-stranded DNA. EMBO J. 2016, 35, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Yeap, L.S.; Hwang, J.K.; Du, Z.; Meyers, R.M.; Meng, F.L.; Jakubauskaite, A.; Liu, M.; Mani, V.; Neuberg, D.; Kepler, T.B.; et al. Sequence-Intrinsic Mechanisms that Target AID Mutational Outcomes on Antibody Genes. Cell 2015, 163, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Duquette, M.L.; Pham, P.; Goodman, M.F.; Maizels, N. AID binds to transcription-induced structures in c-MYC that map to regions associated with translocation and hypermutation. Oncogene 2005, 24, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Uong, B.Q.; Vaidyanathan, B.; Lin, J.Y.; Huang, F.T.; Chaudhuri, J. Non-coding RNA Generated following Lariat Debranching Mediates Targeting of AID to DNA. Cell 2015, 161, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature 1988, 334, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Murat, P.; Balasubramanian, S. Existence and consequences of G-quadruplex structures in DNA. Curr. Opin. Genet. Dev. 2014, 25, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Besnard, E.; Babled, A.; Lapasset, L.; Milhavet, O.; Parrinello, H.; Dantec, C.; Marin, J.M.; Lemaitre, J.M. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat. Struct. Mol. Biol. 2012, 19, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Cadoret, J.C.; Meisch, F.; Hassan-Zadeh, V.; Luyten, I.; Guillet, C.; Duret, L.; Quesneville, H.; Prioleau, M.N. Genome-wide studies highlight indirect links between human replication origins and gene regulation. Proc. Natl. Acad. Sci. USA 2008, 105, 15837–15842. [Google Scholar] [CrossRef] [PubMed]
- Cayrou, C.; Ballester, B.; Peiffer, I.; Fenouil, R.; Coulombe, P.; Andrau, J.C.; van Helden, J.; Mechali, M. The chromatin environment shapes DNA replication origin organization and defines origin classes. Genome Res. 2015, 25, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, F.; Schlumpf, T.; Schmid, V.; Rohs, R.; Beisel, C.; Paro, R. High-resolution profiling of Drosophila replication start sites reveals a DNA shape and chromatin signature of metazoan origins. Cell Rep. 2015, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, S.; Yura, K.; Teranishi, H.; Kiyasu, N.; Tominaga, A.; Kadoma, H.; Nakatsuka, A.; Kunichika, T.; Obuse, C.; Waga, S. Human origin recognition complex binds preferentially to G-quadruplex-preferable RNA and single-stranded DNA. J. Biol. Chem. 2013, 288, 30161–30171. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavri, R. R Loops in the Regulation of Antibody Gene Diversification. Genes 2017, 8, 154. https://doi.org/10.3390/genes8060154
Pavri R. R Loops in the Regulation of Antibody Gene Diversification. Genes. 2017; 8(6):154. https://doi.org/10.3390/genes8060154
Chicago/Turabian StylePavri, Rushad. 2017. "R Loops in the Regulation of Antibody Gene Diversification" Genes 8, no. 6: 154. https://doi.org/10.3390/genes8060154




