Atmospheric Volatile Organic Compounds in a Typical Urban Area of Beijing: Pollution Characterization, Health Risk Assessment and Source Apportionment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monitoring Site and Period
2.2. Monitoring Instruments
2.3. Quality Assurance and Control
2.4. Data Processing
2.4.1. Calculation of Ozone Formation Potential of VOCs
2.4.2. Calculation of Secondary Organic Aerosol Formation Potential of VOCs
2.4.3. Calculation of SOAs from VOCs
2.4.4. Health Risk Assessment of VOCs
2.4.5. VOCs Positive Matrix Factorization (PMF) Source Analysis
3. Results and Discussion
3.1. Concentration of VOCs and Inorganic Pollutants
3.2. Variation Characteristics and Influential Factors
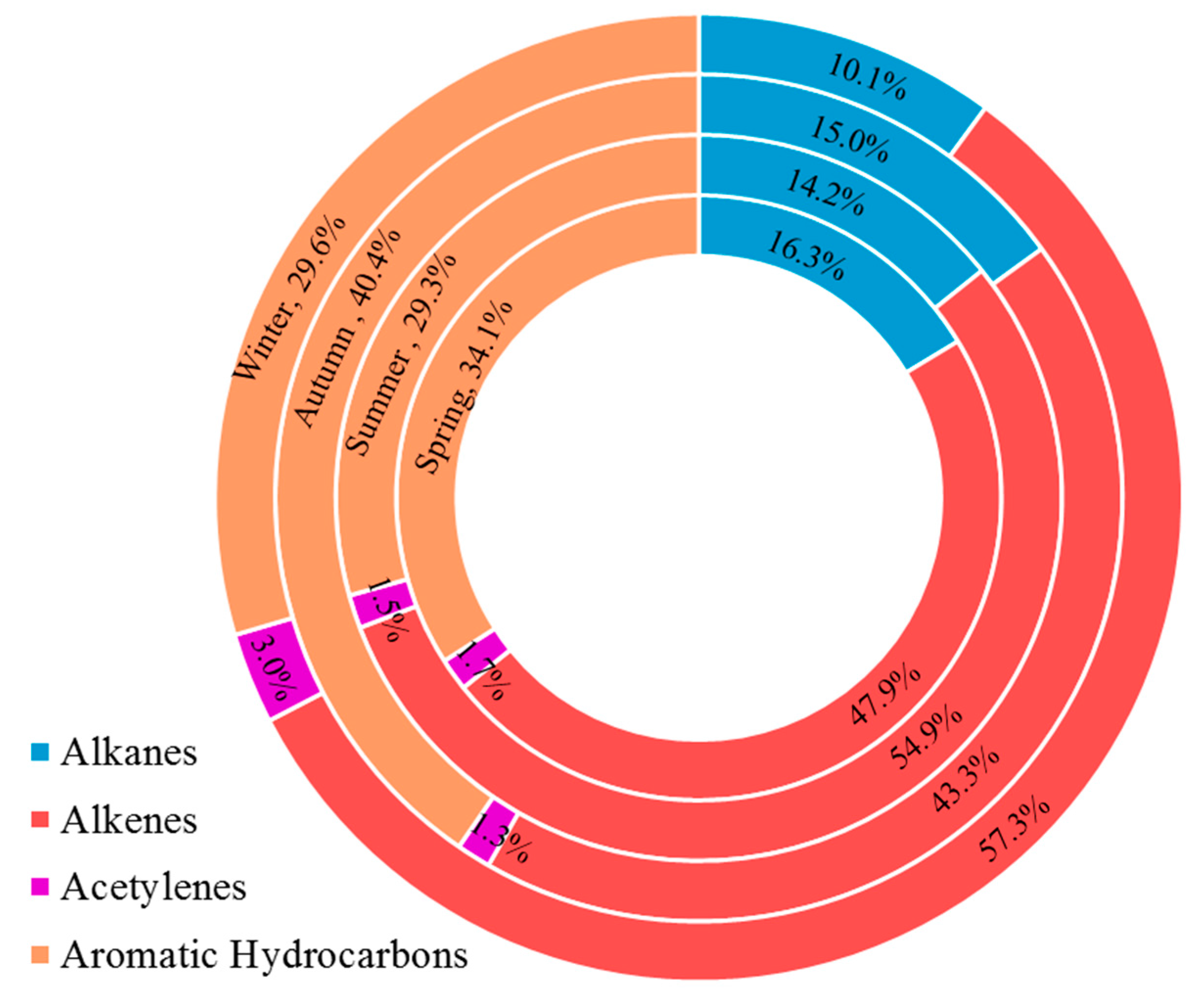
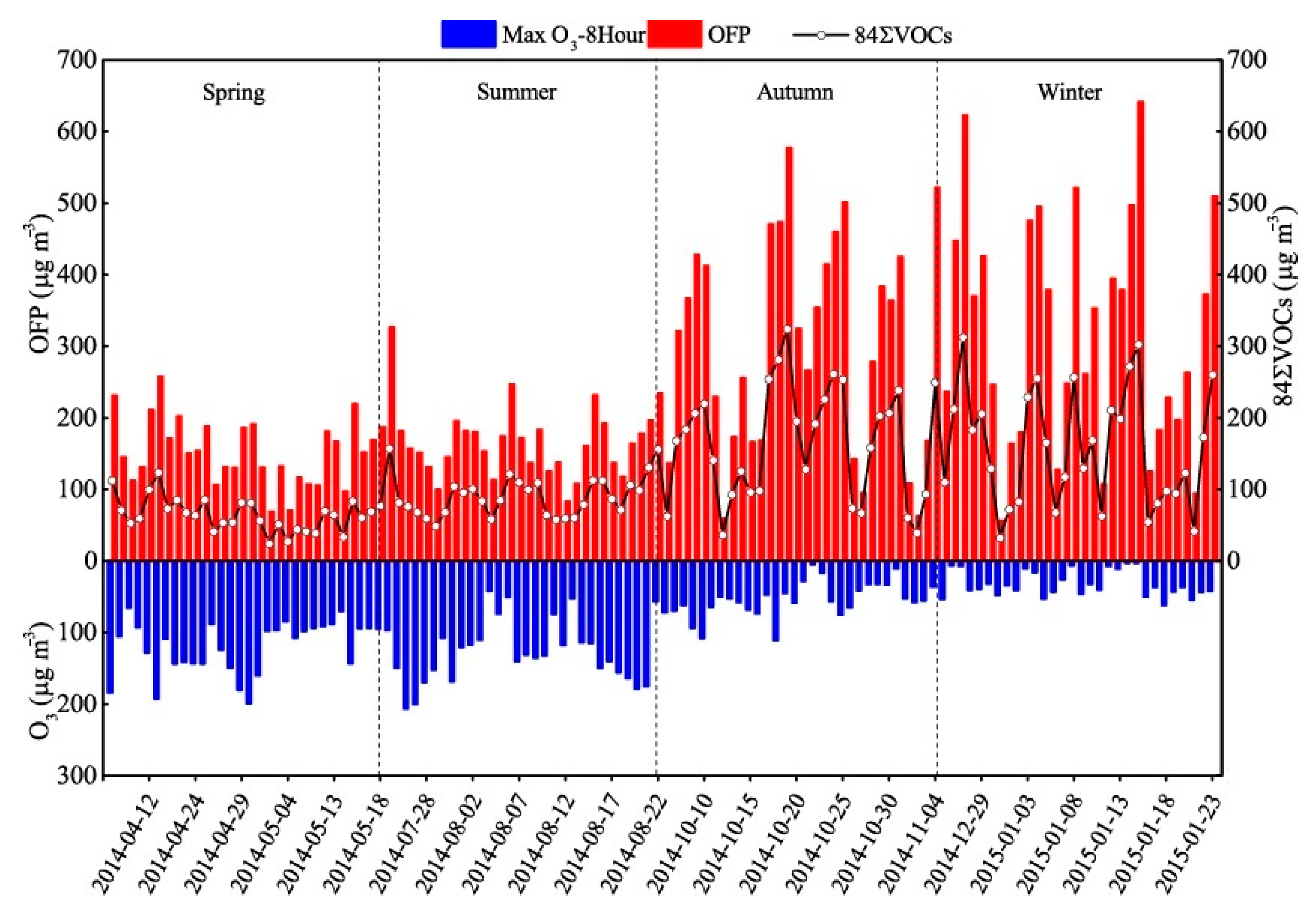
3.3. Ozone Formation Potential
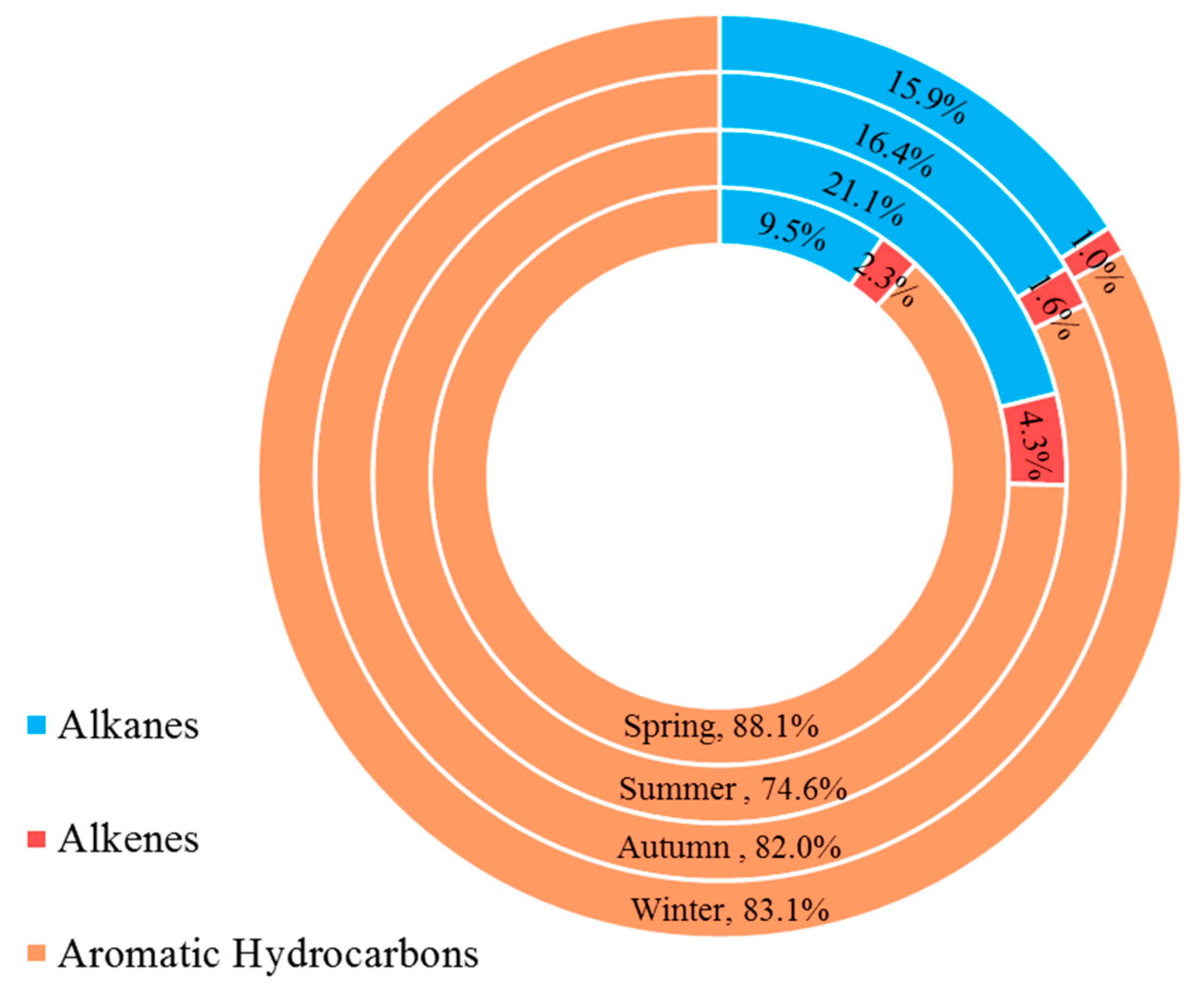
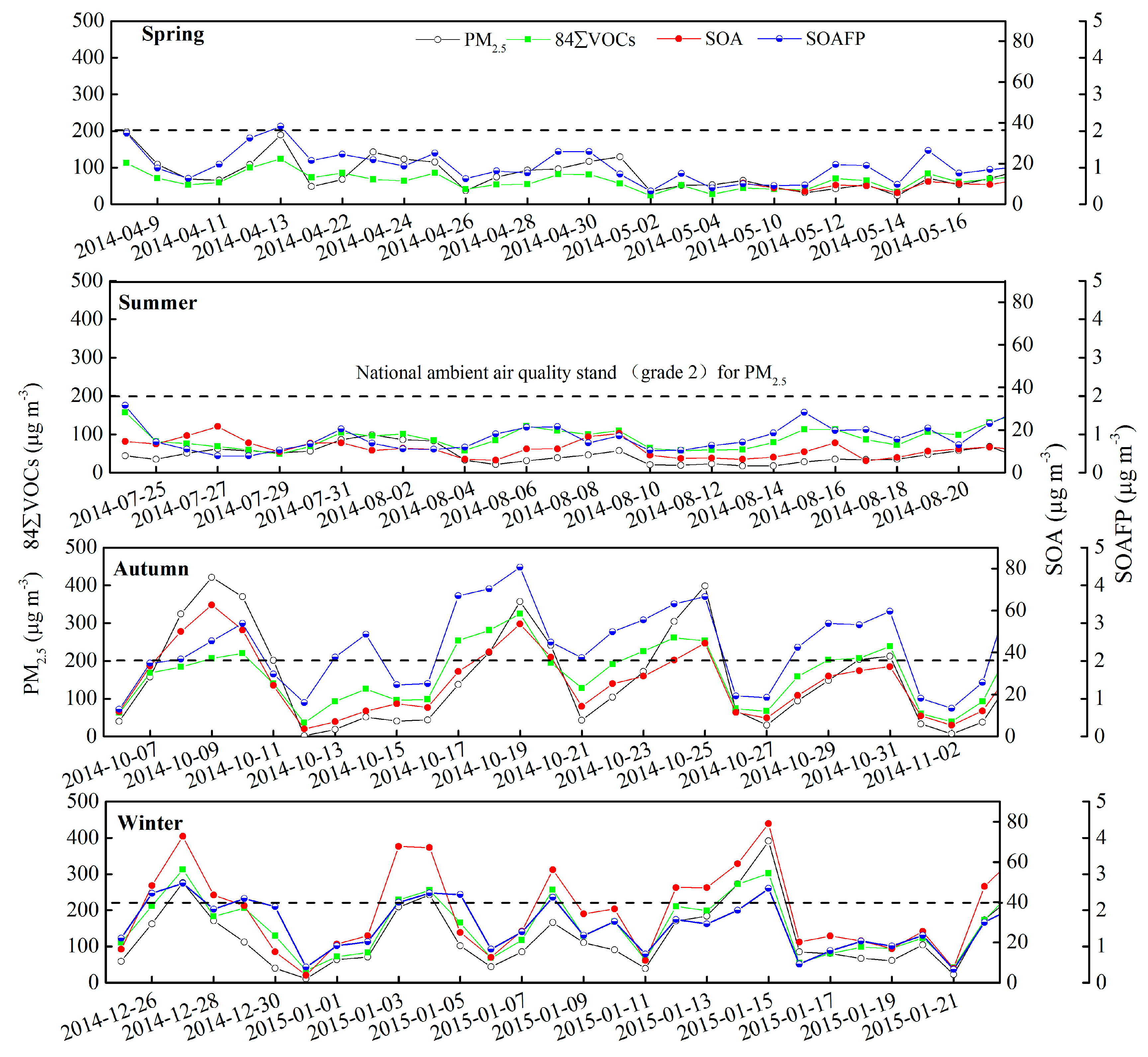
3.4. Secondary Organic Aerosol Formation Potential
3.5. Health Risk Assessment
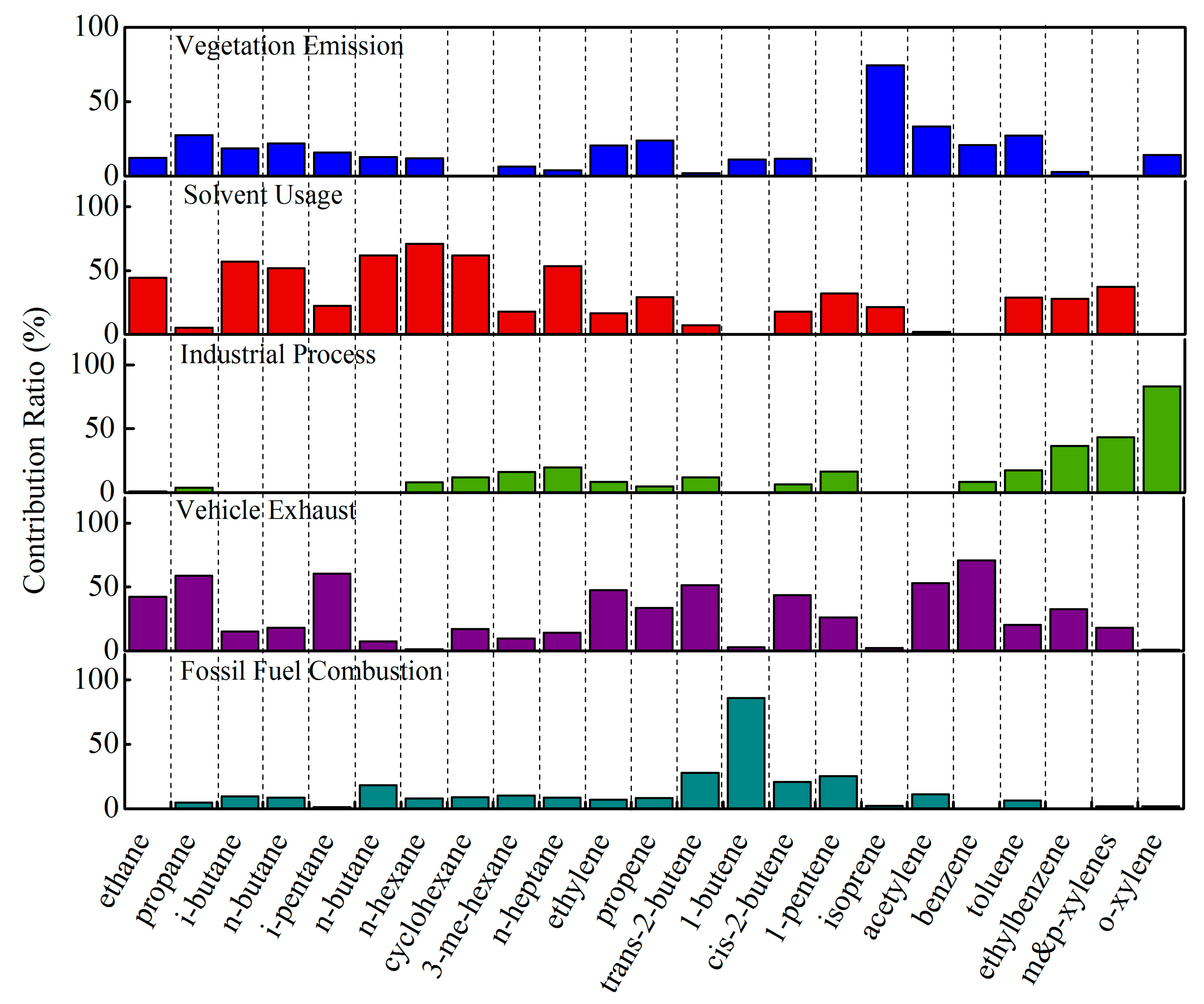
3.6. Source Apportionment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maroni, M.; Seifert, B.; Lindvall, T. Indoor Air Quality: A Comprehensive Reference Book; Elsevier Science: Amsterdam, The Netherlands, 1995. [Google Scholar]
- World Health Organization (WHO). Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications: Rome, Italy; World Health Organisation Regional Office for Europe: Copenhagen, Denmark, 1987. [Google Scholar]
- Molhave, L.; Clausen, G.; Berglund, B.; Deaurriz, J.; Kettrup, A.; Lindvall, T.; Maroni, M.; Pickering, A.C.; Risse, U.; Rothweiler, H.; et al. Total Volatile Organic Compounds (TVOC) in Indoors Air Quality Investigations. Indoor Air J. 1997, 7, 225–240. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. 2015 Report on the State of the Environment in China; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2016.
- Beijing Municipal Environmental Protection Bureau. 2015 Report on the State of the Environment in Beijing; Beijing Municipal Environmental Protection Bureau: Beijing, China, 2015. Available online: http://www.bjepb.gov.cn/bjhrb/xxgk/ywdt/hjjc/qtjcgzdtxx/513514/index.html (accessed on 13 March 2016).
- An, J.L. Ozone production efficiency in Beijing area with high NOx emissions. Acta Sci. Cirunmstantiae 2006, 26, 652–657. [Google Scholar]
- Wang, X.S.; Li, J.L. The contribution of anthropogenic hydrocarbons to zone formation in Beijing areas. China Environ. Sci. 2002, 22, 501–505. [Google Scholar]
- Wang, S.L.; Chai, F.H. Provincial characteristics of ozone pollution in Beijing. Sci. Geogr. Sin. 2002, 22, 360–364. [Google Scholar]
- Luo, S.J.; Zhu, B.; Liao, H. Impacts of O3 precursor on surface O3 concentration over China. Trans. Atmos. Sci. 2010, 33, 451–459. [Google Scholar]
- Li, L.Y.; Xie, S.D.; Zeng, L.M.; Wu, R.R.; Li, J. Characteristics of volatile organic compounds and their role in ground-level ozone formation in the Beijing-Tianjin-Hebei region, China. Atmos. Environ. 2015, 113, 247–254. [Google Scholar] [CrossRef]
- Li, Y.; Shao, M.; Lu, S.H. Review on Technologies of Ambient Volatile Organic Compounds Measurement. Environ. Monit. China 2015, 31, 1–7. [Google Scholar]
- Gao, Y.G.; Zhang, K.; Wang, T.J.; Chen, Z.M.; Geng, H.; Meng, F. Concentration Characteristics and Influencing Factors of Atmospheric Particulate Matters in Spring on Weizhou Island, Beihai, Guangxi Province. Environ. Sci. 2017, 38, 21–27. [Google Scholar]
- Weather Underground. Available online: http://www.wunderground.com (accessed on 15 April 2016).
- United States Environmental Agency. Technical Assistance Document for Sampling and Analysis of Ozone Precursors; United States Environmental Agency: Washington, DC, USA, 1998.
- United States Environmental Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air (Second Edition); United States Environmental Agency: Washington, DC, USA, 1999.
- Yao, Q.; Sun, M.L.; Cai, Z.Y.; Huang, H. Seasonal Variation and Anaylsis of the relationship between NO, NO2 and O3 Concentrations in Tianjin in 2009. Environ. Chem. 2011, 30, 1650–1656. [Google Scholar]
- Xia, F.M.; Li, H.; Li, J.J.; Chai, F.H.; Li, H.J.; Zhang, Y.J.; Wang, X.Z.; Zhang, W.Q.; Bi, F. Characteristics and Health Risk Assessment of Atmospheric Benzene Homologues in Summer in the Northeaster Urban Area of Beijing, China. Asian J. Ecotoxicol. 2014, 9, 1041–1052. [Google Scholar]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.P.L. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Grosjean, D. Parameterization of the Formation Potential of Secondary Organic Aerosols. Atmos. Environ. 1989, 23, 1733–1747. [Google Scholar] [CrossRef]
- Grosjean, D. In Situ Organic Aerosol Formation During A Smog Episode: Estimated Production and Chemical Functionality. Atoms. Environ. 1992, 26, 953–963. [Google Scholar] [CrossRef]
- Grosjean, E.; Rasmussen, R.A.; Grosjean, D. Toxic Air Contaminants in Porto Alegre, Brazil. Environ. Sci. Technol. 1999, 33, 1970–1978. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Lu, Z.Q.; Lowenthal, D.H.; Frazier, C.A.; Solomon, P.A.; Thuillier, R.H.; Magliana, K. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos. Environ. 1996, 30, 2079–2112. [Google Scholar] [CrossRef]
- Chan, T.W.; Huang, L.; Leaitch, W.R.; Sharma, S.; Brook, J.R.; Slowik, J.G.; Abbatt, J.P.D.; Brickell, P.C.; Liggio, J.; Li, S.M.; et al. Observations of OM/OC and specific attenuation coefficients (SAC) in ambient fine PM at a rural site in central Ontario, Canada. Atmos. Chem. Phys. 2010, 10, 2393–2411. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.J. Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumption for Estimating Organic Mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Carlo, P.D.; Brune, W.H.; Martinez, M.; Harder, H.; Lesher, R.; Ren, X.R.; Thornberry, T.; Carroll, M.A.; Young, V.; Shepson, P.B.; et al. Missing OH Reactivity in a Forest: Evidence for Unknown Reactive Biogenic VOCs. Science 2004, 304, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Ma, C. Health Hazard assessment of Volatile Organic Compounds in Ambient Atmosphere of Typical Urban in Liaoning Province. Environ. Prot. Circ. Econ. 2012, 32, 42–45. [Google Scholar]
- Li, L.; Li, H.; Zhang, X.M.; Wang, L.; Xu, L.H.; Wang, X.Z.; Yu, Y.T.; Zhang, Y.J.; Cao, G. Pollution Characteristics and Health Risk Assessment of Benzene Homologues in Ambient Air in the Northeastern Urban Area of Beijing, China. J. Environ. Sci. 2014, 26, 214–223. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Exposure Factors Handbook of Chinese Population; Chinese Environmental Science Press: Beijing, China, 2013; pp. 798–801.
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Yakovleva, E.; Hopke, P.K. Receptor Modeling Assessment of Particle Total Exposure Assessment Methodology Data. Environ. Sci. Technol. 1999, 33, 3645–3652. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source Apportionment of Atmospheric Polycyclic Aromatic Hydrocarbons Collected from an Urban Location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Song, Y.; Dai, W.; Shao, M.; Liu, Y.; Lu, S.H.; Kuster, W.; Goldan, P. Comparison of Receptor Models for Source Apportionment of Volatile Organic Compounds in Beijing, China. Environ. Pollut. 2008, 156, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.C.; Tan, J.H.; Yang, L.; Wu, S.; Hao, J.M. Concentration, Sources and Ozone Formation Potential of Volatile Organic Compounds (VOCs) during Ozone Episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar]
- Wang, B.; Shao, M.; Lu, S.H.; Yuan, B.; Zhao, Y.; Wang, M.; Zhang, S.Q.; Wu, D. Variation of Ambient Non-methane Hydrocarbons in Beijing City in Summer 2008. Atmos. Chem. Phys. Discuss. 2010, 10, 5565–5597. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. 2005 Report on the State of the Environment in China; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2006.
- Zhang, J.K.; Sun, Y.; Wu, F.K.; Sun, J.; Wang, Y.S. The characteristics, seasonal variation and source apportionment of VOCs at Gongga Mountain, China. Atmos. Environ. 2014, 88, 297–305. [Google Scholar] [CrossRef]
- Derwent, R.G.; Davies, T.J.; Delaney, M.; Dollard, G.J.; Field, R.A.; Dumitrean, P.; Nason, P.D.; Jones, B.M.R.; Pepler, S.A. Analysis and Interpretation of the Continuous Hourly Monitoring Data for 26 C2-C8 Hydrocarbons at 12 United Kingdom Sites during 1996. Atmos. Environ. 2000, 34, 297–312. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B.; Wheeler, A.J.; Héroux, M.-È.; Wallace, L.A. Source Apportionment of Indoor and Outdoor Volatile Organic Compounds at Homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Kuo, C.P.; Liao, H.T.; Chou, C.C.; Wu, C.F. Source Apportionment of Particulate Matter and Selected Volatile Organic Compounds with Multiple Time Resolution Data. Sci. Total Environ. 2014, 472, 880–887. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H.; Cheng, H.R.; Yu, Y.F. Sources of Ambient Volatile Organic Compounds and Their Contributions to Photochemical Ozone Formation at a Site in the Pearl River Delta, Southern China. Environ. Pollut. 2011, 159, 2310–2319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Zeng, L.; Shao, M.; Xie, S.; Chen, W.; Lu, S.H.; Wu, Y.S.; Cao, W. Biomass Burning Contribution to Ambient Volatile Organic Compounds (VOCs) in the Chengdu–Chongqing Region (CCR), China. Atmos. Environ. 2014, 99, 403–410. [Google Scholar] [CrossRef]
- Guo, H.; Wang, T.; Blake, D.R.; Simpson, I.J.; Kwok, Y.H.; Li, Y.S. Regional and Local Contributions to Ambient Non-methane Volatile Organic Compounds at a Polluted Rural/Coastal Site in Pearl River Delta, China. Atmos. Environ. 2006, 40, 2345–2359. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, Y.; Wu, F.; Lin, H.; Wang, W. Nonmethane Hydrocarbon Measurements at a Suburban Site in Changsha City, China. Sci. Total Environ. 2009, 408, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.H.; Mao, X.X.; Huang, T.; Liang, X.X.; Wang, Y.N.; Shen, Y.J.; Jiang, W.Y.H.; Wang, H.Q.; Bai, Z.L.; Ma, M.Q.; et al. Non-methane Hydrocarbons (NMHCs) and Their Contribution to Ozone Formation Potential in a Petrochemical Industrialized City, Northwest China. Atmos. Res. 2016, 169, 225–236. [Google Scholar] [CrossRef]
- Saito, S.; Nagao, I.; Kanzawa, H. Characteristics of Ambient C2-C11 Non-methane Hydrocarbons in Metropolitan Nagoya, Japan. Atmos. Environ. 2009, 43, 4384–4395. [Google Scholar] [CrossRef]
- Ren, H.L.; Hu, F.; Zhou, D.G.; Wang, W. Observation Study of Ozone Vertical and Its Impact on Environment in Summer in Beijing. J. Grad. Sch. Chin. Acad. Sci. 2005, 22, 429–435. [Google Scholar]
- Fu, L.X.; He, K.B.; He, D.Q.; Tang, Z.Z.; Hao, J.M. A Study on Models of Mobile Source Emission Factors. Acta Sci. Circumstantiae 1997, 17, 474–479. [Google Scholar]
- Mackeen, S.A.; Liu, S.C. Hydrocarbon Ratios and Photochemical History of Air Mass. Geopyhs. Res. Lett. 1993, 20, 2363–2366. [Google Scholar] [CrossRef]
- Wang, T.; Guo, H.; Blake, D.R.; Blake, D.R.; Kwok, Y.H.; Simpson, I.J.; Li, Y.S. Measurements of Trace Gases in the Inflow of South China Sea Background Air and Outflow of Regional Pollution at Tai O, Southern China. J. Atmos. Chem. 2005, 52, 295–317. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.L.; Chen, C.H.; Huang, C.; Li, L.; Cheng, Z.; Liu, J. The Composition and Chemical Reactivity of Volatile Organic Compounds from Vehicle Exhaust in Shanghai, China. Environ. Pollut. Control 2010, 32, 19–26. [Google Scholar]
- Kleindienst, T.E.; Corse, E.W.; Li, W.; Mclver, C.D.; Conver, T.S.; Edney, E.O.; Driscoll, R.E.; Speer, R.E.; Weather, W.S.; Tejada, S.B. Secondary Organic Aerosol Formation from the Irradiation of Simulated Automobile Exhaust. J. Air Waste Manag. Assoc. 2002, 52, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Shao, M.; Liu, Y.; Lu, S.H.; Tang, D.G. Tunnel Experimental Study of The Emission Factors of Volatile Organic Compounds from Vehicles. Acta Sci. Circunstantiae 2005, 25, 879–885. [Google Scholar]
- Chen, W. Study on Spatial-Temporal Distribution of Atmospheric Volatile Halocarbons and Their Sources Apportionment in Guangzhou City; Jinan University: Jilin, China, 2010. [Google Scholar]
- Fishbein, L. An Overview of Environmental and Toxicological Aspects of Aromatic Hydrocarbons. I. Benzene. Sci. Total Environ. 1984, 40, 189–218. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Mu, Y.J.; Liu, J.F.; Mellouki, A. Levels, Sources and Health risks of Carbonyls and BTEX in the Ambient Air of Beijing, China. J. Environ. Sci. 2012, 24, 124–130. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Hao, Z.P.; Wang, H.L. Health Risk Assessment of Atmospheric Volatile Organic Compounds in Urban-Rural Juncture Belt Area. Environ. Sci. 2011, 32, 3566–3570. [Google Scholar]
- Cai, C.J.; Geng, F.H.; Tie, X.X.; Yu, Q.; An, J.L. Characteristics and Source Apportionment of VOCs Measured in Shanghai, China. Atmos. Environ. 2010, 44, 5005–5014. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Wang, X.; Zhang, X.M.; Wen, C. Pollution Characteristics and Health Risk Assessment of Atmospheric VOCs in the Downtown Area of Guangzhou, China. Environ. Sci. 2013, 34, 4558–4564. [Google Scholar]
- Zhou, J.; You, Y.; Bai, Z.; Hu, Y.D.; Zhang, J.F.; Zhang, N. Health Risk Assessment of Personal Inhalation Exposure to Volatile Organic Compounds in Tianjin, China. Sci. Total Environ. 2011, 409, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z. Aromatic Hydrocarbons in the Air in Hangzhou, China: Characteristics, Sources and Risks; Zhejiang University: Hangzhou, China, 2007. [Google Scholar]
- Guenther, A.; Baugh, W.; Davis, K.; Hampton, G.; Harley, P.; Klinger, L. Isoprene fluxes measured by enclosure, relaxed eddy accumulation, surface layer gradient, mixed layer gradient, and mixed layer mass balance techniques. J. Geophys. Res. Atmos. 1996, 101, 18555–18567. [Google Scholar] [CrossRef]
- Jing, C. Analysis on Volatile Organic Compounds of Four Species of Trees and Effects on SOA; Beijing Forestry University: Beijing, China, 2016. [Google Scholar]
- William, H.S. Air Pollution and the Forestry; China Meteorological Press: Beijing, China, 1986.
- Zhang, J.G.; Wang, Y.S.; Wang, S.; Mao, T. Source Apportionment of Non-methane Hydrocarbon in Beijing Using Positive Matrix Factorization. Environ. Sci. Technol. 2009, 32, 35–39. [Google Scholar]
- Barletta, B.; Meinardi, S.; Rowland, F.S.; Chan, C.Y.; Wang, X.M.; Zou, S.C.; Chan, L.Y.; Blake, D.R. Volatile Organic Compounds in 43 Chinese Cities. Atmos. Environ. 2005, 39, 5979–5990. [Google Scholar] [CrossRef]
- Gee, I.L.; Sollars, C.J. Ambient Air Levels of Volatile Organic Compounds in Latin American and Asian Cities. Chemsphere 1998, 36, 2497–2506. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Guo, H.; Tsai, W.Y. Seasonal and Diurnal Variations of Volatile Organic Compounds (VOCs) in the Atmosphere of Hong Kong. Sci. Total Environ. 2004, 322, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shao, M.; Liu, Y.; Lu, S.H.; Kuster, W.; Goldan, P.; Xie, S.D. Source Apportionment of Ambient Volatile Organic Compounds in Beijing. Environ. Sci. Technol. 2007, 41, 4348–4353. [Google Scholar] [CrossRef] [PubMed]

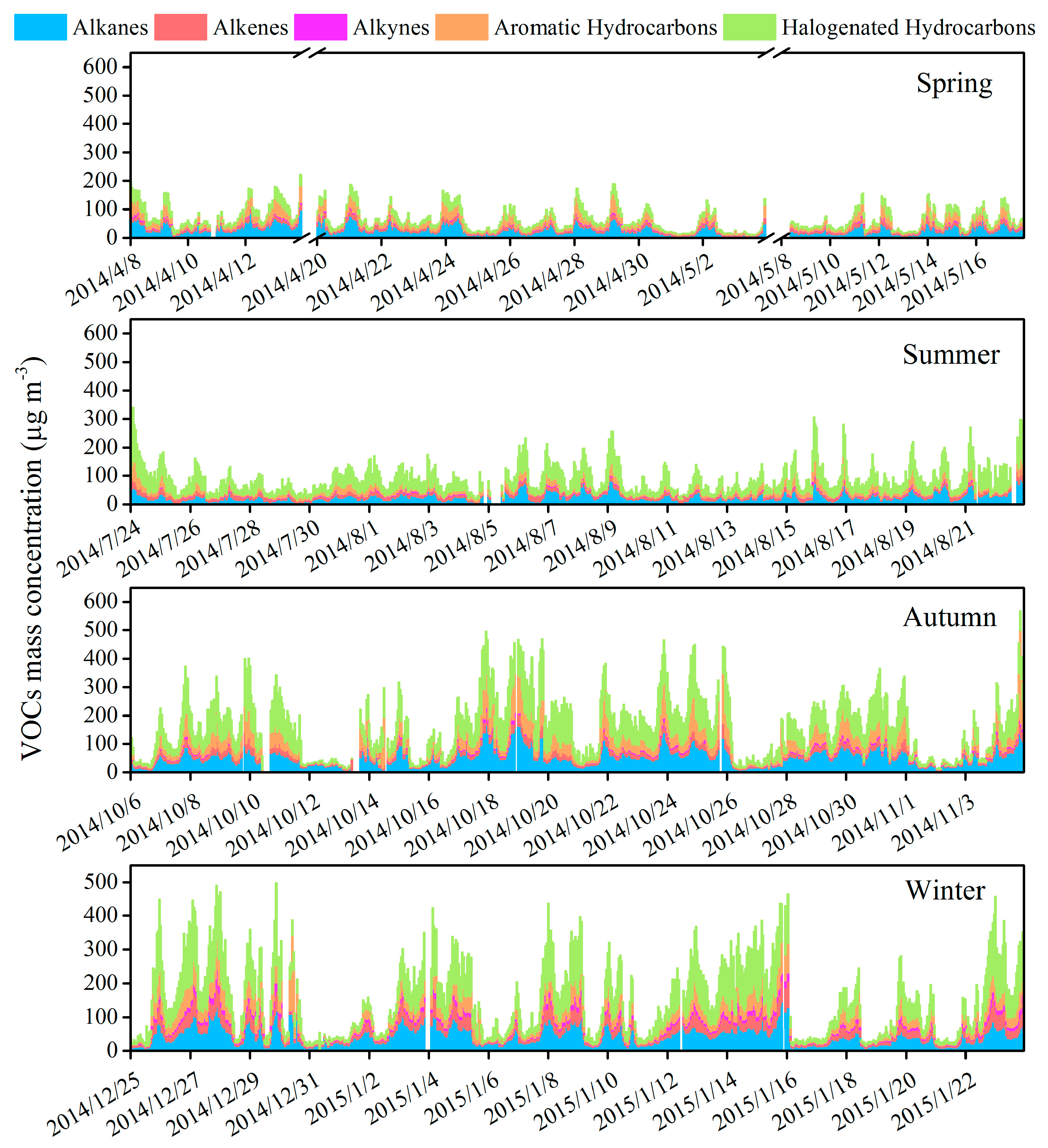
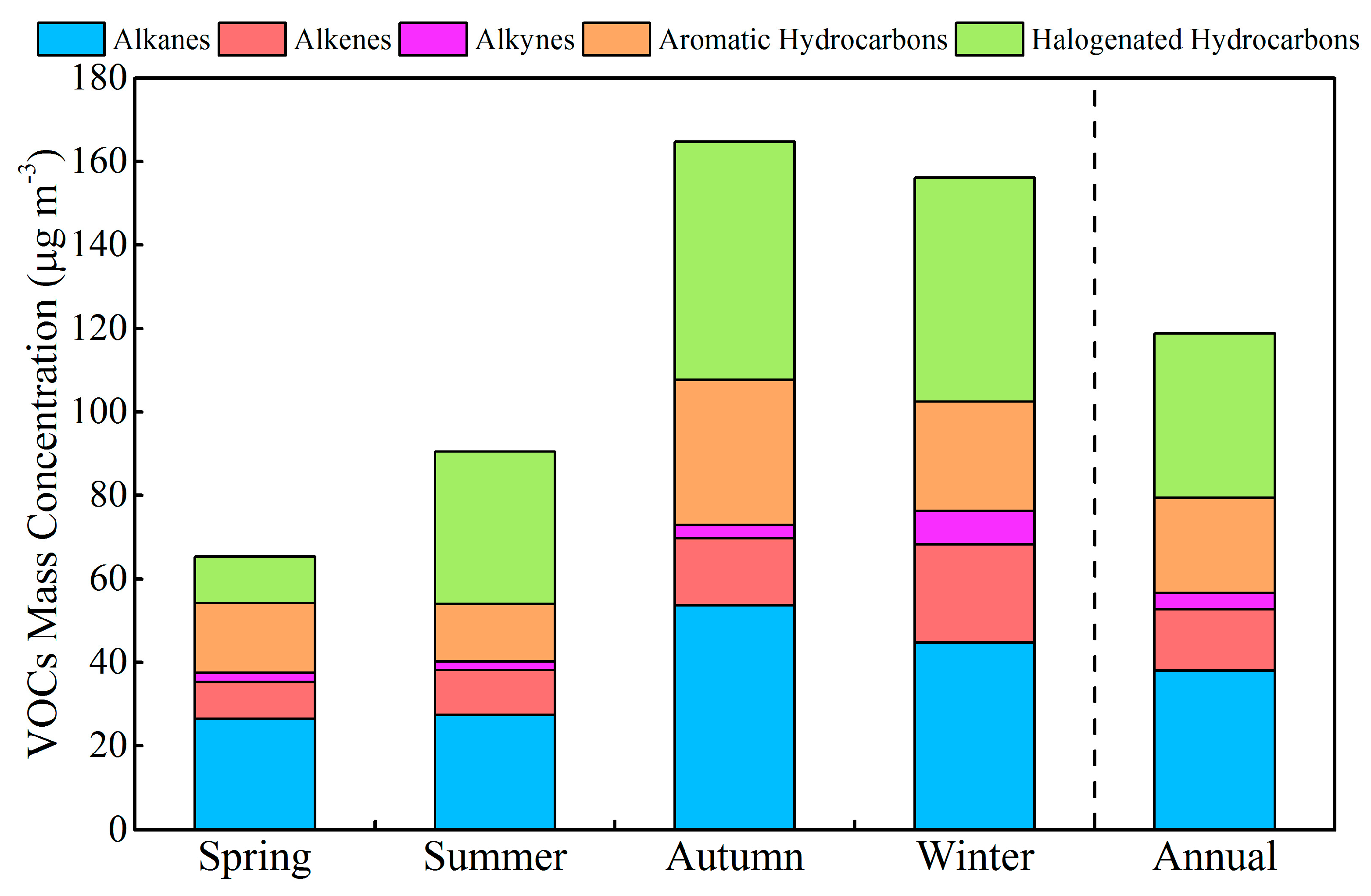
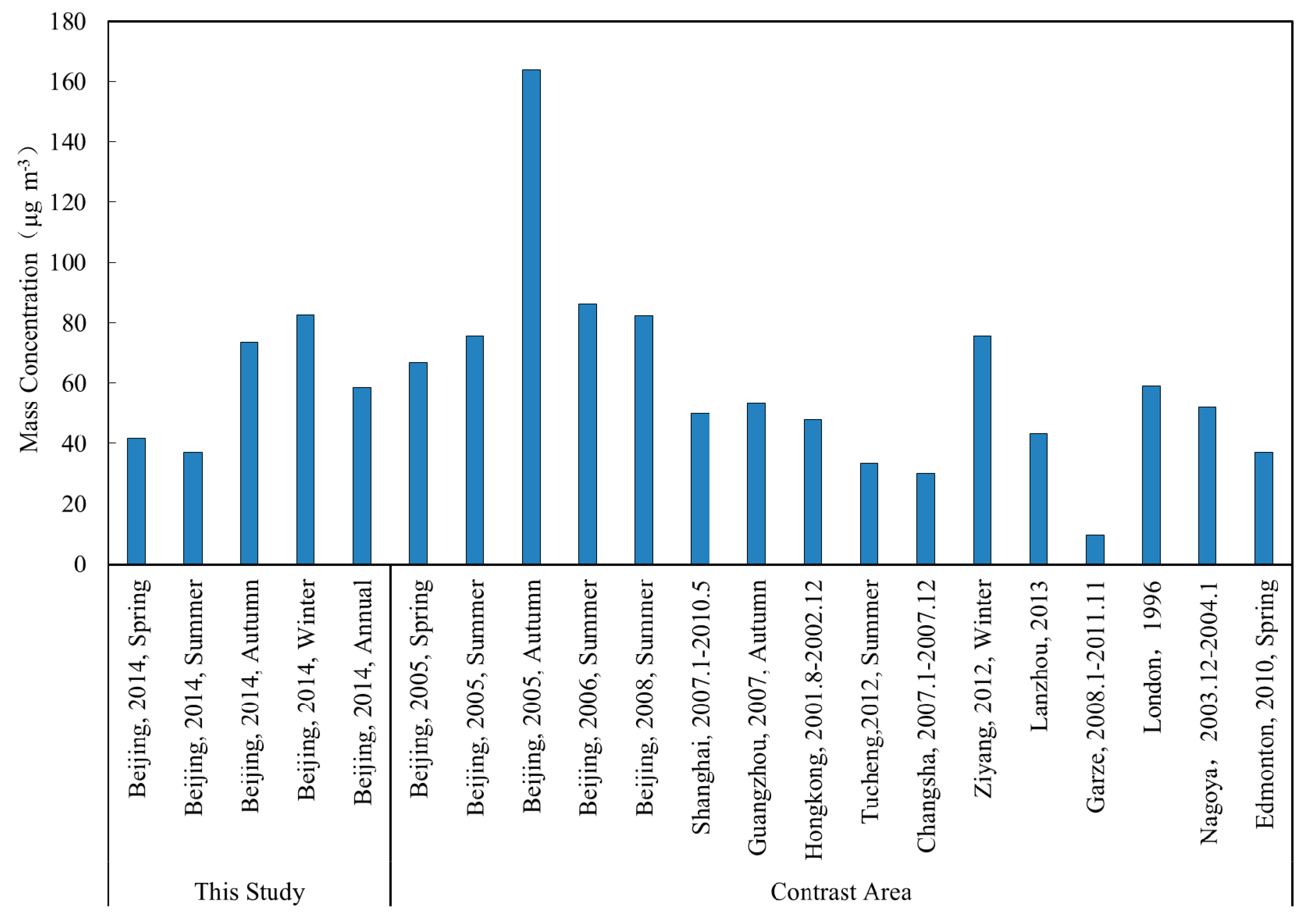

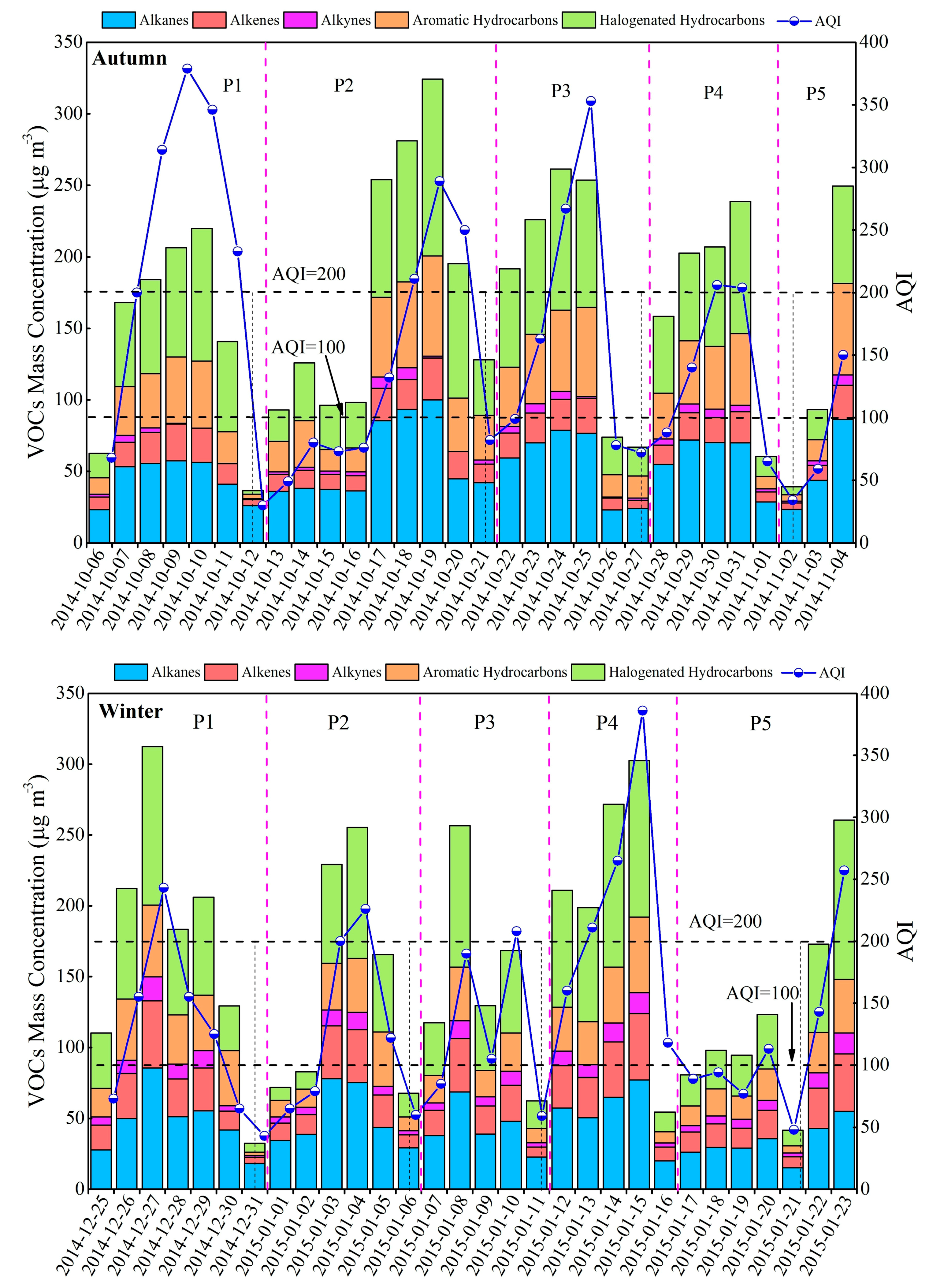
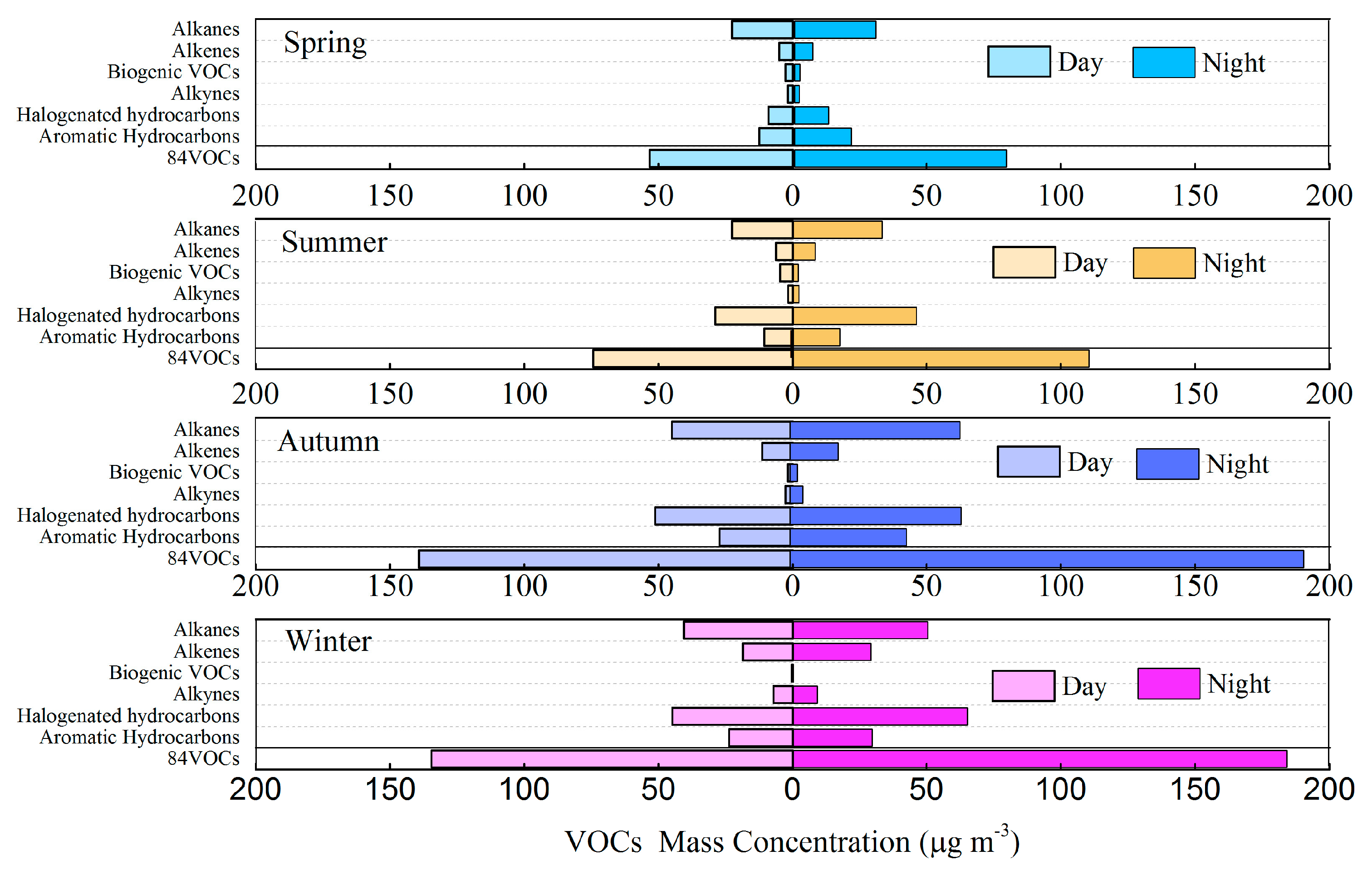
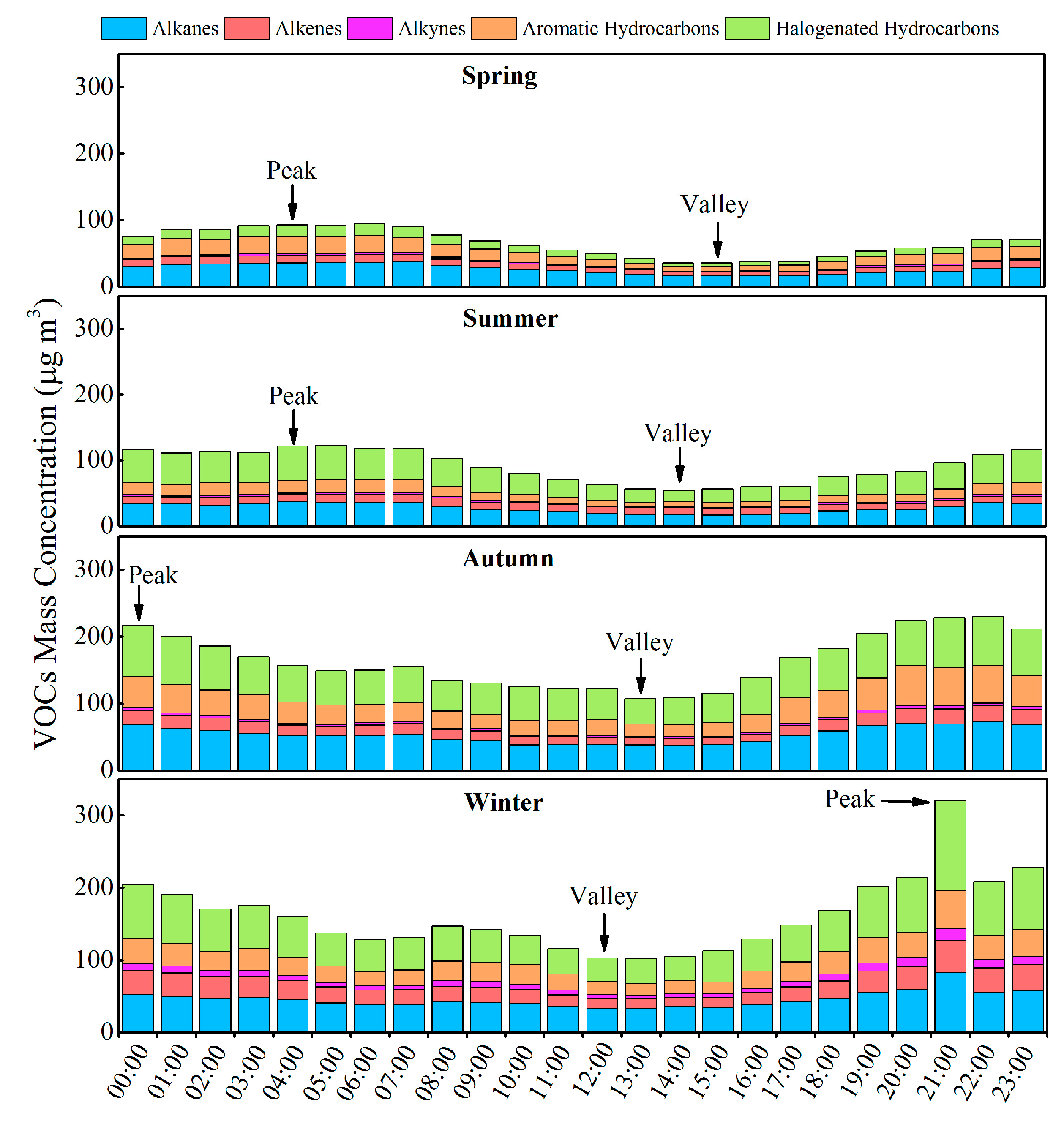
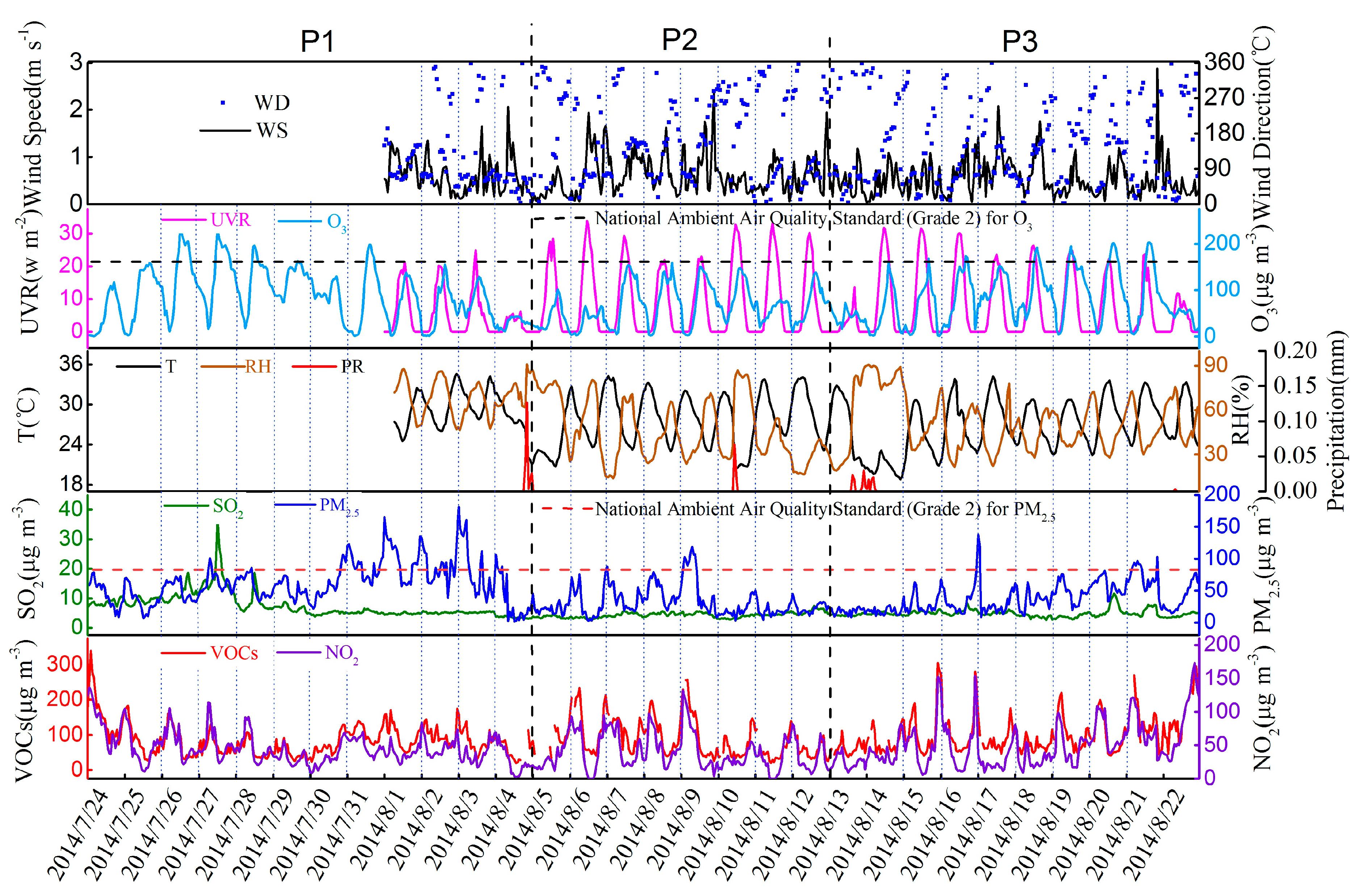
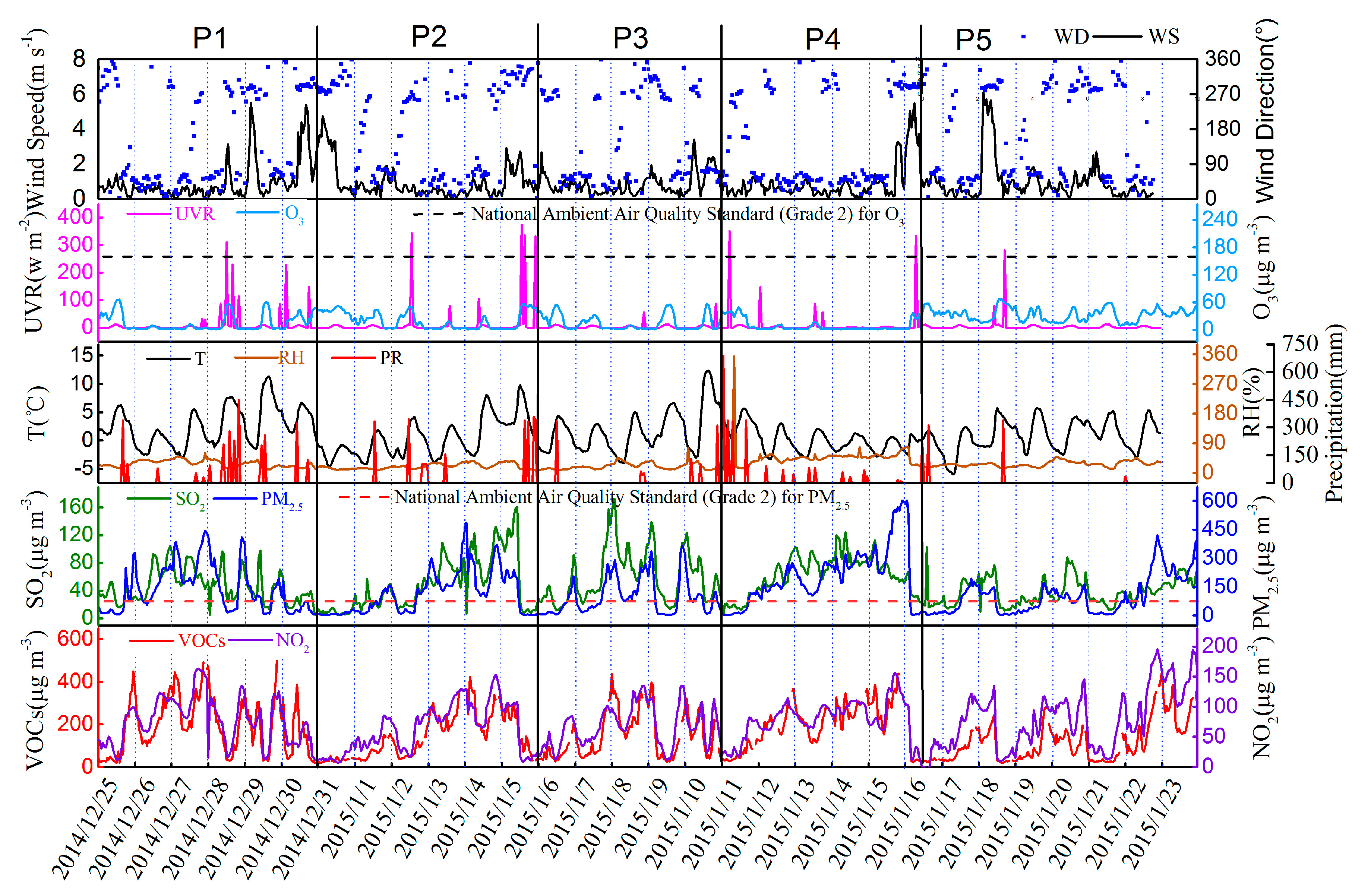

| Species | Spring (n = 30) | Summer (n = 30) | Autumn (n = 30) | Winter (n = 30) | Annual |
|---|---|---|---|---|---|
| Alkanes | |||||
| ethane * | 3.68 ± 1.05 | 2.97 ± 0.84 | 6.36 ± 3.00 | 8.60 ± 4.33 | 5.40 ± 3.50 |
| propane * | 4.96 ± 1.84 | 5.70 ± 2.08 | 10.8 ± 5.8 | 12.2 ± 6.2 | 8.42 ± 5.40 |
| i-butane * | 2.36 ± 0.84 | 2.68 ± 0.66 | 4.06 ± 2.13 | 3.30 ± 1.85 | 3.10 ± 1.62 |
| n-butane * | 3.81 ± 1.28 | 3.64 ± 1.41 | 5.62 ± 3.56 | 5.58 ± 3.00 | 4.66 ± 2.66 |
| cyclopentane * | 3.22 ± 1.37 | 2.29 ± 1.72 | 4.35 ± 3.32 | 2.08 ± 1.96 | 2.99 ± 2.37 |
| i-pentane * | 0.05 ± 0.23 | 0.25 ± 0.44 | 0.21 ± 0.50 | 0.24 ± 0.38 | 0.19 ± 0.40 |
| n-pentane * | 1.93 ± 1.19 | 1.10 ± 1.06 | 2.64 ± 2.32 | 0.12 ± 0.07 | 1.45 ± 1.68 |
| 2,2-dime-butane * | 0.26 ± 0.19 | 0.31 ± 0.18 | 0.65 ± 0.49 | 0.75 ± 0.49 | 0.49 ± 0.42 |
| me-cyclopentane *,# | 0.20 ± 0.17 | 0.15 ± 0.17 | 0.36 ± 0.44 | 0.49 ± 0.28 | 0.30 ± 0.31 |
| 2,3-dime-butane * | - | - | 1.32 ± 1.24 | 0.77 ± 0.51 | 0.52 ± 0.87 |
| 2&3-me-pentane * | - | - | - | - | - |
| n-hexane * | 1.33 ± 0.46 | 1.05 ± 0.63 | 2.53 ± 1.34 | 1.53 ± 1.12 | 1.61 ± 1.10 |
| cyclohexane *,# | 0.18 ± 0.09 | 0.19 ± 0.09 | 0.50 ± 0.31 | 0.20 ± 0.30 | 0.27 ± 0.26 |
| 2,3-dimec5+2mec6 * | 0.35 ± 0.17 | 0.38 ± 0.25 | 0.52 ± 0.59 | 0.63 ± 0.43 | 0.47 ± 0.41 |
| 3-me-hexane * | 0.28 ± 0.11 | 0.32 ± 0.15 | 0.58 ± 0.32 | 0.40 ± 0.27 | 0.40 ± 0.25 |
| 2,2,4-tme-pentane *,# | 0.17 ± 0.15 | - | - | - | 0.04 ± 0.11 |
| n-heptane *,# | 0.42 ± 0.15 | 0.34 ± 0.17 | 0.77 ± 0.34 | 0.82 ± 0.39 | 0.59 ± 0.35 |
| me-cyclohexane *,# | 0.12 ± 0.12 | - | 0.01 ± 0.01 | - | 0.03 ± 0.08 |
| 2,3,4-tme-pentane * | 0.42 ± 0.38 | 0.81 ± 1.19 | 1.32 ± 1.00 | 0.47 ± 0.33 | 0.75 ± 0.88 |
| 2-me-heptane *,# | 0.10 ± 0.05 | 0.10 ± 0.06 | 0.26 ± 0.15 | 0.22 ± 0.16 | 0.17 ± 0.14 |
| 3-me-heptane *,# | 0.05 ± 0.03 | 0.05 ± 0.03 | 0.18 ± 0.10 | 0.12 ± 0.09 | 0.10 ± 0.09 |
| n-octane *,# | 0.07 ± 0.11 | 0.29 ± 0.38 | 0.79 ± 1.03 | - | 0.29 ± 0.63 |
| n-nonane *,# | 0.15 ± 0.08 | 0.17 ± 0.08 | 0.36 ± 0.19 | 0.24 ± 0.14 | 0.23 ± 0.16 |
| n-decane *,# | 0.09 ± 0.12 | 0.17 ± 0.19 | 0.03 ± 0.05 | 0.03 ± 0.03 | 0.08 ± 0.13 |
| n-undecane *,# | 0.07 ± 0.06 | 0.24 ± 0.15 | 0.45 ± 0.72 | 0.40 ± 0.27 | 0.29 ± 0.42 |
| n-dodecane *,# | 2.06 ± 2.61 | 4.43 ± 3.96 | 8.91 ± 3.85 | 5.76 ± 3.41 | 5.29 ± 4.26 |
| Total | 26.3 ± 12.8 | 27.6 ± 15.9 | 53.6 ± 32.8 | 45.0 ± 26.0 | 38.1 ± 28.5 |
| Alkenes | |||||
| ethylene * | 2.59 ± 0.77 | 2.33 ± 0.68 | 6.38 ± 3.37 | 13.28 ± 7.82 | 6.15 ± 6.14 |
| propene * | 1.46 ± 0.52 | 1.16 ± 0.34 | 2.69 ± 1.08 | 7.43 ± 2.97 | 3.18 ± 2.99 |
| trans-2-butene * | 0.19 ± 0.12 | 0.38 ± 0.24 | 0.41 ± 0.31 | 0.43 ± 0.26 | 0.35 ± 0.26 |
| 1-butene * | 0.25 ± 0.23 | 0.13 ± 0.15 | 0.46 ± 0.32 | 0.91 ± 0.57 | 0.44 ± 0.46 |
| cis-2-butene * | 0.54 ± 0.67 | 2.05 ± 1.90 | 2.06 ± 2.53 | 0.03 ± 0.07 | 1.17 ± 1.84 |
| 1,3-butadiene * | 0.29 ± 0.15 | 0.23 ± 0.08 | 0.58 ± 0.29 | 0.11 ± 0.09 | 0.30 ± 0.24 |
| trans-2-pentene * | 0.08 ± 0.05 | 0.09 ± 0.07 | 0.27 ± 0.13 | 0.27 ± 0.16 | 0.18 ± 0.14 |
| 2-me-2-butene * | - | - | - | - | - |
| 1-pentene * | 0.08 ± 0.04 | 0.08 ± 0.04 | 0.23 ± 0.10 | 0.36 ± 0.22 | 0.19 ± 0.17 |
| cis-2-pentene * | 0.46 ± 0.28 | 0.49 ± 0.35 | 0.83 ± 0.81 | 0.14 ± 0.10 | 0.48 ± 0.52 |
| isoprene * | 2.65 ± 0.58 | 3.42 ± 1.96 | 1.68 ± 0.67 | 0.30 ± 0.18 | 2.01 ± 1.58 |
| 2-me-1-pentene | 0.16 ± 0.20 | 0.31 ± 0.87 | 0.35 ± 0.39 | 0.25 ± 0.16 | 0.26 ± 0.49 |
| α-pinene *,# | 0.05 ± 0.06 | 0.13 ± 0.09 | 0.12 ± 0.07 | 0.04 ± 0.04 | 0.08 ± 0.08 |
| β-pinene *,# | 0.03 ± 0.04 | 0 ± 0.01 | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.03 |
| limonene | - | - | - | - | - |
| Total | 8.83 ± 3.70 | 10.8 ± 6.8 | 16.1 ± 10.1 | 23.6 ± 12.7 | 14.8 ± 14.9 |
| Alkynes | |||||
| acetylene | 2.17 ± 0.93 | 1.99 ± 0.87 | 3.17 ± 2.44 | 8.01 ± 4.25 | 3.84 ± 3.51 |
| Aromatic Hydrocarbons | |||||
| benzene *,# | 2.47 ± 1.18 | 2.16 ± 0.72 | 4.74 ± 2.70 | 7.02 ± 4.27 | 4.09 ± 3.25 |
| toluene *,# | 7.20 ± 2.34 | 3.10 ± 2.74 | 9.31 ± 5.20 | 4.80 ± 3.44 | 6.10 ± 4.27 |
| ethylbenzene *,# | 1.67 ± 0.91 | 1.82 ± 0.92 | 4.54 ± 2.73 | 2.74 ± 1.90 | 2.69 ± 2.10 |
| m&p-xylenes *,# | 2.93 ± 1.49 | 3.55 ± 1.85 | 8.97 ± 5.43 | 6.82 ± 4.45 | 5.57 ± 4.41 |
| styrene * | 0.36 ± 0.55 | 1.27 ± 0.68 | 0.84 ± 0.90 | 0.43 ± 0.42 | 0.72 ± 0.75 |
| o-xylene *,# | 1.00 ± 0.76 | 0.15 ± 0.08 | 2.97 ± 2.47 | 0.85 ± 0.51 | 1.24 ± 1.67 |
| i-propylbenzene *,# | - | 0.03 ± 0.03 | 0.07 ± 0.09 | 0.04 ± 0.04 | 0.04 ± 0.05 |
| n-propylbenzene *,# | 0.02 ± 0.03 | 0.09 ± 0.08 | 0.14 ± 0.15 | 0.22 ± 0.22 | 0.12 ± 0.16 |
| m-ethyltoluene# | 0.06 ± 0.04 | 0.20 ± 0.33 | 0.38 ± 0.62 | 0.94 ± 0.68 | 0.40 ± 0.59 |
| p-ethyltoluene# | 0.35 ± 0.23 | 0.37 ± 0.19 | 1.07 ± 0.64 | 0.03 ± 0.02 | 0.46 ± 0.52 |
| 1,3,5-tmb *,# | 0.28 ± 0.21 | 0.30 ± 0.18 | 0.86 ± 0.60 | 0.52 ± 0.33 | 0.49 ± 0.43 |
| o-ethyltoluene# | 0.18 ± 0.11 | 0.30 ± 0.39 | 0.51 ± 0.27 | 0.06 ± 0.05 | 0.26 ± 0.29 |
| 1,2,4-tmb *,# | - | 0.31 ± 0.60 | 0.02 ± 0.07 | 1.31 ± 0.89 | 0.41 ± 0.75 |
| 1,2,3-tmb *,# | 0.03 ± 0.04 | - | 0.01 ± 0.03 | 0.37 ± 0.23 | 0.10 ± 0.19 |
| m-diethylbenzene# | 0.08 ± 0.07 | 0.03 ± 0.02 | 0.15 ± 0.22 | 0.07 ± 0.05 | 0.08 ± 0.12 |
| p-diethylbenzene# | 0.03 ± 0.03 | 0.09 ± 0.06 | 0.27 ± 0.36 | 0.11 ± 0.07 | 0.12 ± 0.21 |
| naphthalene *,# | - | - | - | - | - |
| Total | 16.7 ± 8.01 | 13.8 ± 8.9 | 34.8 ± 22.5 | 26.3 ± 17.6 | 22.9 ± 19.8 |
| Halogenated Hydrocarbons | |||||
| freon-12 | 0.16 ± 0.49 | - | - | 2.36 ± 1.41 | 0.63 ± 1.25 |
| chloromethane | - | - | - | 0.35 ± 0.74 | 0.09 ± 0.40 |
| vinylchloride | - | - | - | 3.52 ± 2.04 | 0.88 ± 1.83 |
| freon-11 | - | - | - | - | - |
| dichloromethane | - | - | - | 0.21 ± 0.32 | 0.05 ± 0.18 |
| freon-113 | - | - | - | - | - |
| 1,1-dichloroethane | 4.57 ± 1.67 | 6.32 ± 2.53 | 9.51 ± 5.16 | 6.54 ± 3.78 | 6.73 ± 3.92 |
| c-1,2-dicl-ethene | 0.25 ± 0.19 | - | 0.12 ± 0.13 | - | 0.09 ± 0.15 |
| chloroform | - | 13.1 ± 6.4 | 18.7 ± 13.6 | 19.7 ± 16.0 | 12.9 ± 13.4 |
| 1,2-dichloroethane | 1.58 ± 1.15 | 2.72 ± 1.21 | 4.83 ± 4.04 | 2.52 ± 2.60 | 2.91 ± 2.78 |
| 1,1,1-tricl-ethane | - | - | 0.01 ± 0.06 | 0.01 ± 0.04 | 0.01 ± 0.04 |
| CCl4 | - | - | - | - | - |
| 1,2-dicl-propane | 0.03 ± 0.08 | - | - | 2.05 ± 1.52 | 0.52 ± 1.16 |
| trichloroethene | 0.63 ± 0.49 | 1.26 ± 0.74 | 2.79 ± 1.37 | 2.68 ± 1.53 | 1.84 ± 1.44 |
| t-1,3-dicl-propen | 0.42 ± 0.28 | 0.75 ± 0.50 | 2.01 ± 1.32 | 1.69 ± 1.19 | 1.22 ± 1.13 |
| c-1,3-dicl-propen | - | - | - | 0.05 ± 0.14 | 0.01 ± 0.07 |
| 1,1,2-tricl-ethane | - | - | - | - | - |
| 1,2-dibromoethane | 0.02 ± 0.11 | 0.03 ± 0.08 | 0.01 ± 0.02 | - | 0.01 ± 0.07 |
| tetrachloroethene | 1.21 ± 1.51 | 0.58 ± 0.31 | 0.68 ± 0.60 | 4.80 ± 3.89 | 1.82 ± 2.72 |
| chlorobenzene | 0.09 ± 0.05 | 0.10 ± 0.06 | 0.31 ± 0.34 | 0.19 ± 0.16 | 0.17 ± 0.21 |
| 1,3-dicl-benzene | 1.24 ± 0.53 | 1.36 ± 0.81 | 3.11 ± 1.67 | 1.11 ± 0.91 | 1.71 ± 1.34 |
| 1,4-dicl-benzene | 0.34 ± 0.29 | 0.46 ± 0.42 | 1.06 ± 0.78 | 0.31 ± 0.35 | 0.54 ± 0.58 |
| 1,2-dicl-benzene | 0.30 ± 0.22 | 0.56 ± 0.21 | 1.11 ± 0.64 | 1.21 ± 0.85 | 0.79 ± 0.67 |
| 1,2,4-tricl-benzene | - | 9.44 ± 8.57 | 12.7 ± 9.8 | 4.55 ± 4.51 | 6.66 ± 8.34 |
| hexcl-1,3-butadien | 0.18 ± 0.55 | - | - | - | 0.04 ± 0.28 |
| Total | 11.0 ± 7.6 | 36.7 ± 21.8 | 56.9 ± 39.5 | 53.9 ± 42.0 | 39.6 ± 42.0 |
| ∑84 VOCs | 65.0 ± 33.1 | 90.9 ± 54.2 | 164 ± 107 | 157 ± 102 | 119 ± 109 |
| Conventional Pollutants | |||||
| NO | 15.3 ± 33.3 | 3.78 ± 7.20 | 33.7 ± 41.7 | 57.5 ± 64.8 | 30.2 ± 48.4 |
| NO2 | 45.8 ± 30.2 | 44.0 ± 25.7 | 65.0 ± 34.9 | 73.9 ± 40.8 | 58.2 ± 36.4 |
| NH3 | 11.7 ± 8.5 | 10.7 ± 11.3 | 10.5 ± 8.5 | 8.08 ± 8.41 | 10.3 ± 8.9 |
| SO2 | 21.4 ± 71.4 | 5.41 ± 6.71 | 5.7 ± 14.4 | 50.0 ± 31.3 | 23.2 ± 49.8 |
| CO | 1.38 ± 1.69 | 1.15 ± 0.32 | 1.66 ± 1.23 | 3.21 ± 1.83 | 1.92 ± 1.72 |
| O3 | 56.6 ± 54.8 | 113 ± 76 | 24.9 ± 29.9 | 20.1 ± 18.3 | 44.3 ± 53.2 |
| PM2.5 | 87.8 ± 61.0 | 84.6 ± 45.6 | 155 ± 141 | 130 ± 122 | 117 ± 108 |
| Factors | VOCs | NO2 | SO2 | PM2.5 | O3 | T | RH | UVR |
|---|---|---|---|---|---|---|---|---|
| NO2 (n = 720) | 0.71 | |||||||
| SO2 (n = 720) | −0.03 | 0.10 | ||||||
| PM2.5 (n = 720) | 0.46 | 0.46 | 0.14 | |||||
| O3 (n = 720) | −0.42 | −0.42 | 0.42 | −0.15 | ||||
| T (n = 720) | −0.01 | −0.12 | −0.56 | −0.16 | −0.10 | |||
| RH (n = 720) | 0.13 | 0.03 | −0.54 | 0.04 | −0.49 | 0.66 | ||
| UVR (n = 720) | −0.22 | −0.36 | −0.20 | −0.28 | 0.21 | 0.48 | 0.03 | |
| PR (n = 720) | −0.11 | −0.13 | −0.10 | −0.08 | −0.04 | −0.18 | 0.19 | −0.06 |
| Factors | VOCs | NO2 | SO2 | PM2.5 | O3 | T | RH | UVR |
|---|---|---|---|---|---|---|---|---|
| NO2 (n = 720) | 0.79 | |||||||
| SO2 (n = 720) | 0.66 | 0.56 | ||||||
| PM2.5 (n = 720) | 0.89 | 0.80 | 0.60 | |||||
| O3 (n = 720) | −0.63 | −0.57 | −0.61 | −0.56 | ||||
| T (n = 720) | −0.12 | −0.01 | −0.12 | −0.18 | 0.33 | |||
| RH (n = 720) | 0.41 | 0.35 | 0.34 | 0.48 | −0.42 | −0.33 | ||
| UVR (n = 720) | −0.12 | −0.15 | −0.11 | −0.13 | 0.17 | 0.16 | −0.09 | |
| PR (n = 720) | −0.10 | −0.16 | −0.12 | −0.13 | 0.14 | 0.16 | −0.11 | 0.13 |
| Species | RfC (mg·m−3) | HQ | |||
|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | ||
| benzene | 3.00 × 10−02 | 1.26 × 10−02 | 1.12 × 10−02 | 2.43 × 10−02 | 3.58 × 10−02 |
| 1,3-butadiene | 2.00 × 10−03 | 2.22 × 10−02 | 1.75 × 10−02 | 4.42 × 10−02 | 8.14 × 10−03 |
| vinylchloride | 1.00 × 10−01 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 5.39 × 10−03 |
| dichloromethane | 6.00 × 10−01 | 8.13 × 10−07 | 0.00 × 10+00 | 0.00 × 10+00 | 5.38 × 10−05 |
| tetrachloroethene | 4.00 × 10−02 | 4.68 × 10−03 | 2.18 × 10−03 | 2.62 × 10−03 | 1.84 × 10−02 |
| CCl4 | 1.00 × 10−01 | 8.23 × 10−07 | 2.16 × 10−06 | 0.00 × 10+00 | 0.00 × 10+00 |
| 1,2-dibromoethane | 9.00 × 10−03 | 3.67 × 10−04 | 4.63 × 10−04 | 8.86 × 10−05 | 4.92 × 10−05 |
| 1,3-dicl-propen | 2.00 × 10−02 | 3.24 × 10−03 | 5.81 × 10−03 | 1.55 × 10−02 | 1.33 × 10−02 |
| trichloroethene | 2.00 × 10−03 | 4.85 × 10−02 | 9.70 × 10−02 | 2.13 × 10−01 | 2.05 × 10−01 |
| 1,2-dicl-propane | 4.00 × 10−03 | 9.73 × 10−04 | 0.00 × 10+00 | 0.00 × 10+00 | 7.78 × 10−02 |
| ethylbenzene | 1.00 × 10+00 | 2.58 × 10−04 | 2.81 × 10−04 | 6.97 × 10−04 | 4.19 × 10−04 |
| styrene | 1.00 × 10+00 | 5.54 × 10−05 | 1.96 × 10−04 | 1.27 × 10−04 | 6.49 × 10−05 |
| cyclohexane | 6.00 × 10+00 | 4.73 × 10−06 | 4.95 × 10−06 | 1.28 × 10−05 | 5.15 × 10−06 |
| i-propylbenzene | 4.00 × 10−01 | 1.90 × 10−06 | 1.26 × 10−05 | 2.50 × 10−05 | 1.50 × 10−05 |
| 1,4-dicl-benzene | 8.00 × 10−01 | 6.67 × 10−05 | 8.67 × 10−05 | 1.99 × 10−04 | 5.91 × 10−05 |
| n-hexane | 7.00 × 10−01 | 2.93 × 10−04 | 2.27 × 10−04 | 5.57 × 10−04 | 3.36 × 10−04 |
| chloromethane | 9.00 × 10−02 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 5.85 × 10−04 |
| naphtalene | 3.00 × 10−03 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 |
| 1,1,1-tricl-ethane | 5.00 × 10+00 | 1.33 × 10−08 | 0.00 × 10+00 | 3.77 × 10−07 | 2.42 × 10−07 |
| toluene | 5.00 × 10+00 | 2.23 × 10−04 | 9.46 × 10−05 | 2.87 × 10−04 | 1.48 × 10−04 |
| m&p-xylenes | 1.00 × 10−01 | 1.55 × 10−03 | 2.19 × 10−04 | 4.58 × 10−03 | 1.32 × 10−03 |
| o-xylene | 1.00 × 10−01 | 4.52 × 10−03 | 5.47 × 10−03 | 1.38 × 10−02 | 1.04 × 10−02 |
| HI | 9.96 × 10−02 | 1.41 × 10−01 | 3.20 × 10−01 | 3.78 × 10−01 | |
| Species | IUR (m3·µg−1) | Risk | |||
|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | ||
| benzene | 7.80 × 10−06 | 2.94 × 10−06 | 2.61 × 10−06 | 5.68 × 10−06 | 8.37 × 10−06 |
| 1,3-butadiene | 3.00 × 10−05 | 1.33 × 10−06 | 1.05 × 10−06 | 2.65 × 10−06 | 4.89 × 10−07 |
| vinylchloride | 8.80 × 10−06 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 4.74 × 10−06 |
| dichloromethane | 1.00 × 10−08 | 4.88 × 10−12 | 0.00 × 10+00 | 0.00 × 10+00 | 3.23 × 10−10 |
| tetrachloroethene | 2.60 × 10−07 | 4.87 × 10−08 | 2.27 × 10−08 | 2.72 × 10−08 | 1.92 × 10−07 |
| CCl4 | 6.00 × 10−06 | 4.94 × 10−10 | 1.30 × 10−09 | 0.00 × 10+00 | 0.00 × 10+00 |
| 1,2-dibromoethane | 6.00 × 10−04 | 1.98 × 10−06 | 2.50 × 10−06 | 4.78 × 10−07 | 2.66 × 10−07 |
| 1,3-dicl-propen | 4.00 × 10−06 | 2.59 × 10−07 | 4.65 × 10−07 | 1.24 × 10−06 | 1.06 × 10−06 |
| trichloroethene | 4.10 × 10−06 | 3.98 × 10−07 | 7.95 × 10−07 | 1.74 × 10−06 | 1.68 × 10−06 |
| City | Benzene | Toluene | Ethylbenzene | m&p-Xylenes | O-Xylene | Styrene | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Carcinogenic Risk | HQ | HQ | HQ | HQ | HQ | HQ | HI | ||
| Beijing (2014–2015) | 4.90 × 10−06 | 2.09 × 10−02 | 1.88 × 10−04 | 4.13 × 10−04 | 1.92 × 10−03 | 8.55 × 10−03 | 1.11 × 10−04 | 3.21 × 10−02 | This work |
| Beijing (2013) | 8.80 × 10−06 | 3.76 × 10−02 | 5.84 × 10−04 | 1.15 × 10−03 | 1.53 × 10−02 | 7.19 × 10−03 | 8.70 × 10−05 | 6.19 × 10−02 | [17] |
| Beijing (2008–2010) | 1.34 × 10−05 | 5.73 × 10−02 | 1.08 × 10−03 | 1.19 × 10−03 | 2.09 × 10−02 | 5.32 × 10−03 | 1.60 × 10−04 | 8.60 × 10−02 | [56] |
| Beijing (2012) | 4.19 × 10−05 | 1.57 × 10−01 | 2.39 × 10−02 | 3.29 × 10−03 | 8.06 × 10−03 | 3.53 × 10−03 | - | 1.96 × 10−01 | [28] |
| Beijing (2011) | 2.21 × 10−05 | 1.58 × 10−01 | - | 1.69 × 10−02 | 7.76 × 10−03 * | 1.06 × 10−02 | 1.93 × 10−01 | [57] | |
| Shanghai (2007–2010) | 7.55 × 10−06 | 3.23 × 10−02 | 5.93 × 10−04 | 0.00 × 10+00 | 1.02 × 10−02 | 3.56 × 10−03 | 9.99 × 10−05 | 4.67 × 10−02 | [58] |
| Lanzhou (2013) | 8.09 × 10−06 | 3.46 × 10−02 | 1.27 × 10−04 | 4.07 × 10−04 | 6.18 × 10−03 | 2.76 × 10−03 | 1.28 × 10−04 | 4.42 × 10−02 | [45] |
| Ganzi (2008–2011) | 3.00 × 10−06 | 1.28 × 10−02 | 5.56 × 10−05 | 2.04 × 10−04 | 2.04 × 10−03 | 2.26 × 10−03 | 1.64 × 10−04 | 1.76 × 10−02 | [37] |
| Guangzhou (2005) | 9.97 × 10−06 | 4.26 × 10−02 | 8.85 × 10−04 | 8.44 × 10−04 | 1.06 × 10−02 | 5.17 × 10−03 | 1.57 × 10−04 | 6.03 × 10−02 | [33] |
| Guangzhou (2009) | 5.34 × 10−05 | 2.28 × 10−01 | 3.95 × 10−03 | 4.26 × 10−03 | 3.06 × 10−03 | 2.42 × 10−03 | - | 2.42 × 10−01 | [59] |
| Tianjin (2008) | 2.18 × 10−05 | - | - | - | - | - | - | - | [60] |
| Hangzhou (2006–2007) | 1.22 × 10−05 | 1.22 × 10−01 | 2.05 × 10−02 | 2.30 × 10−03 | 5.25 × 10−02 | 6.00 × 10−04 | 1.98 × 10−01 | [61] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, H.; Zhang, Q.; Zhang, Y.; Zhang, W.; Wang, X.; Bi, F.; Chai, F.; Gao, J.; Meng, L.; et al. Atmospheric Volatile Organic Compounds in a Typical Urban Area of Beijing: Pollution Characterization, Health Risk Assessment and Source Apportionment. Atmosphere 2017, 8, 61. https://doi.org/10.3390/atmos8030061
Zhang H, Li H, Zhang Q, Zhang Y, Zhang W, Wang X, Bi F, Chai F, Gao J, Meng L, et al. Atmospheric Volatile Organic Compounds in a Typical Urban Area of Beijing: Pollution Characterization, Health Risk Assessment and Source Apportionment. Atmosphere. 2017; 8(3):61. https://doi.org/10.3390/atmos8030061
Chicago/Turabian StyleZhang, Hao, Hong Li, Qingzhu Zhang, Yujie Zhang, Weiqi Zhang, Xuezhong Wang, Fang Bi, Fahe Chai, Jian Gao, Lingshuo Meng, and et al. 2017. "Atmospheric Volatile Organic Compounds in a Typical Urban Area of Beijing: Pollution Characterization, Health Risk Assessment and Source Apportionment" Atmosphere 8, no. 3: 61. https://doi.org/10.3390/atmos8030061






