Quantifying Light Absorption of Iron Oxides and Carbonaceous Aerosol in Seasonal Snow across Northern China
Abstract
:1. Introduction
2. Materials and Methods
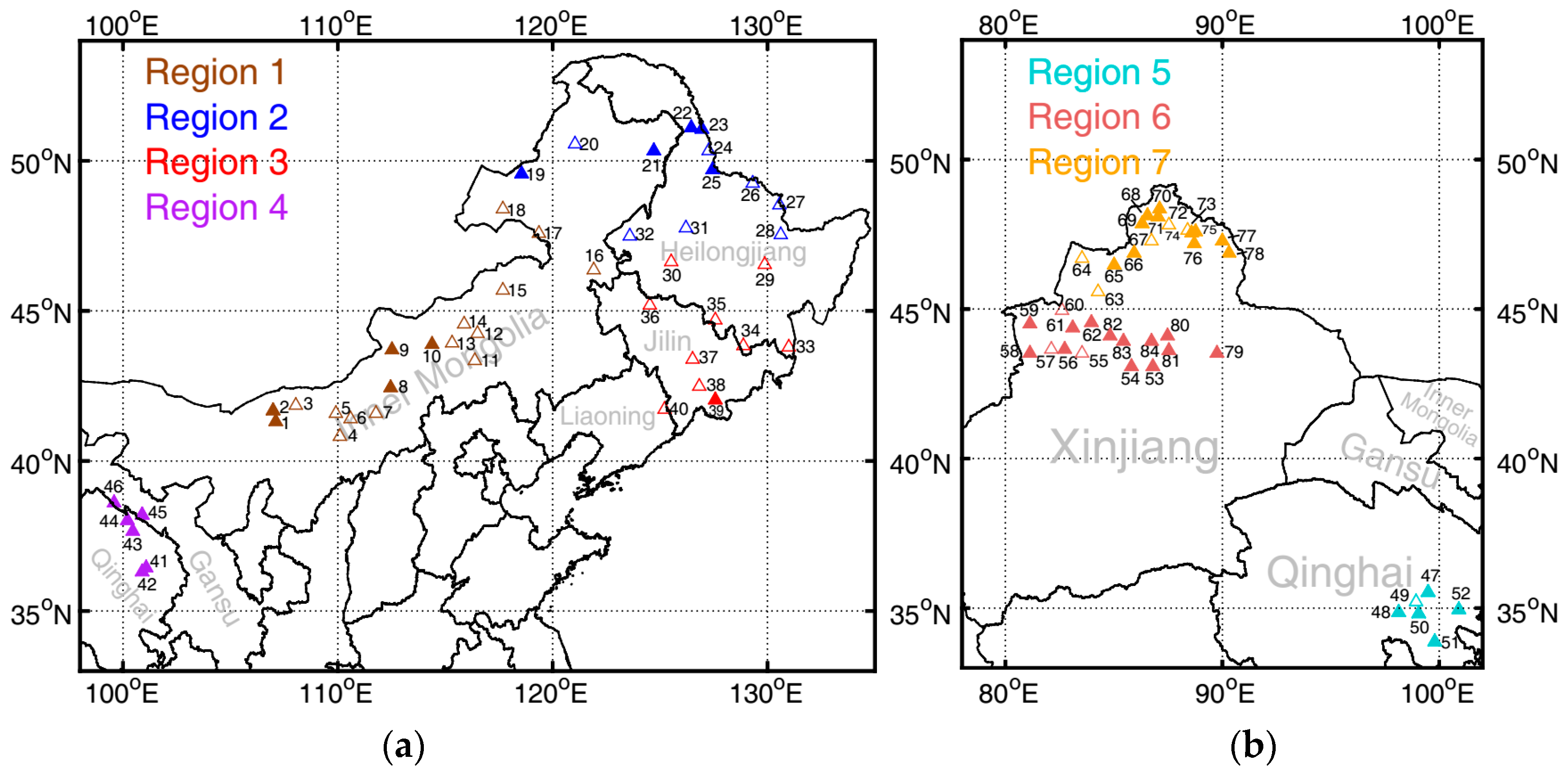
2.1. Sample Collections
2.2. Filtration Procedure
2.3. Spectrophotometric Analysis
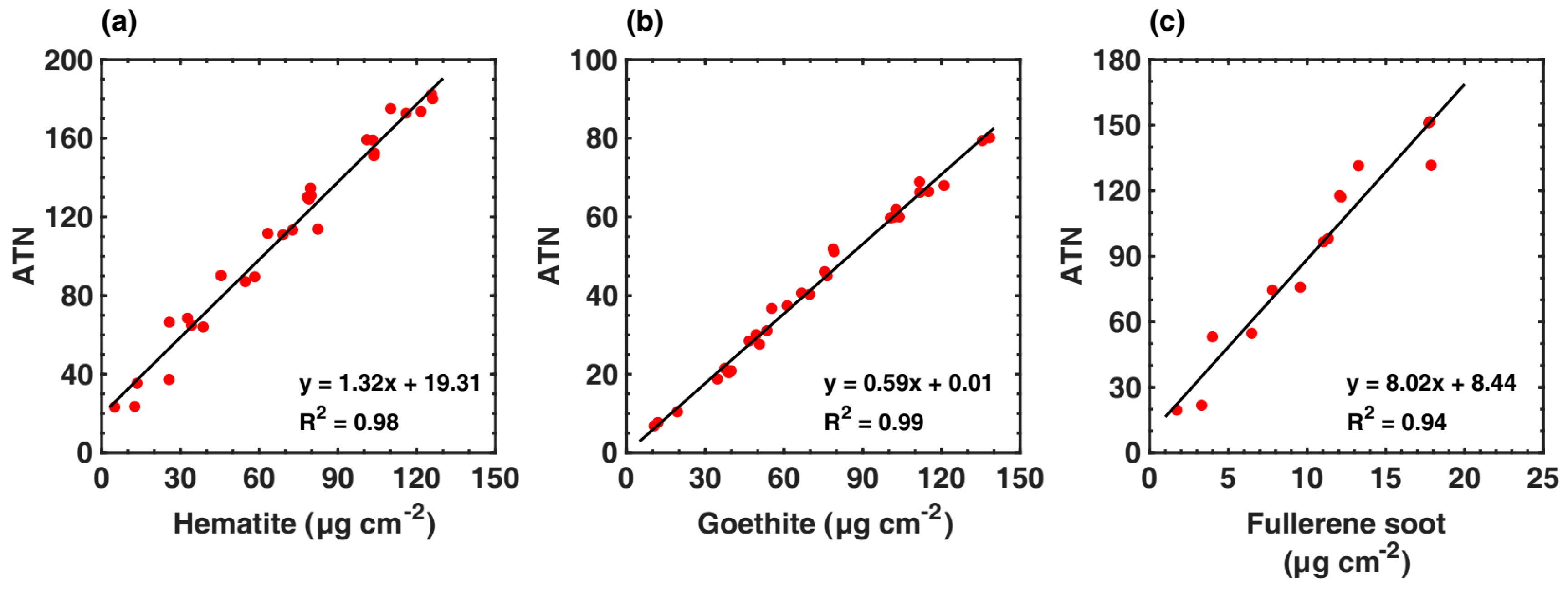
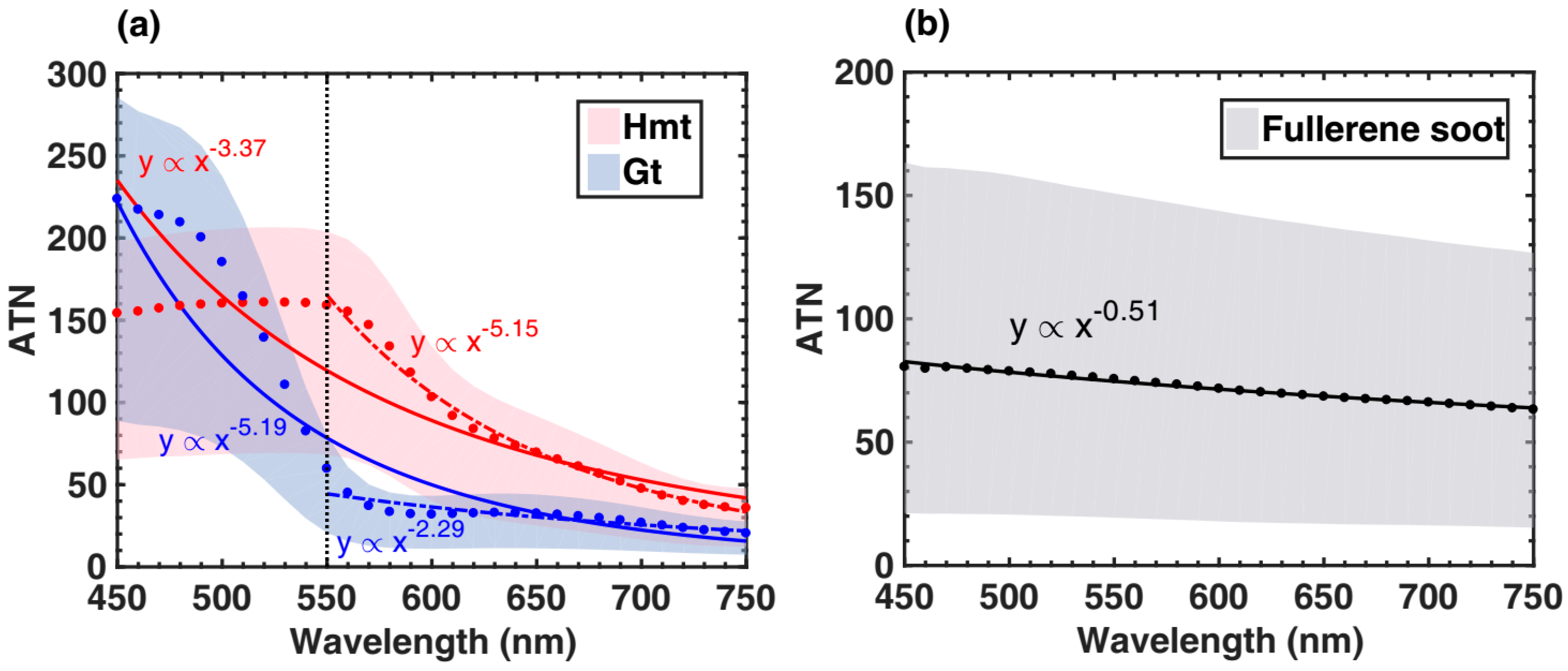
2.3.1. MAC and AAE Calculations for Goethite and Hematite
2.3.2. Re-Calculating the Light Absorption of BC, OC and Iron Oxides
3. Results and Discussion
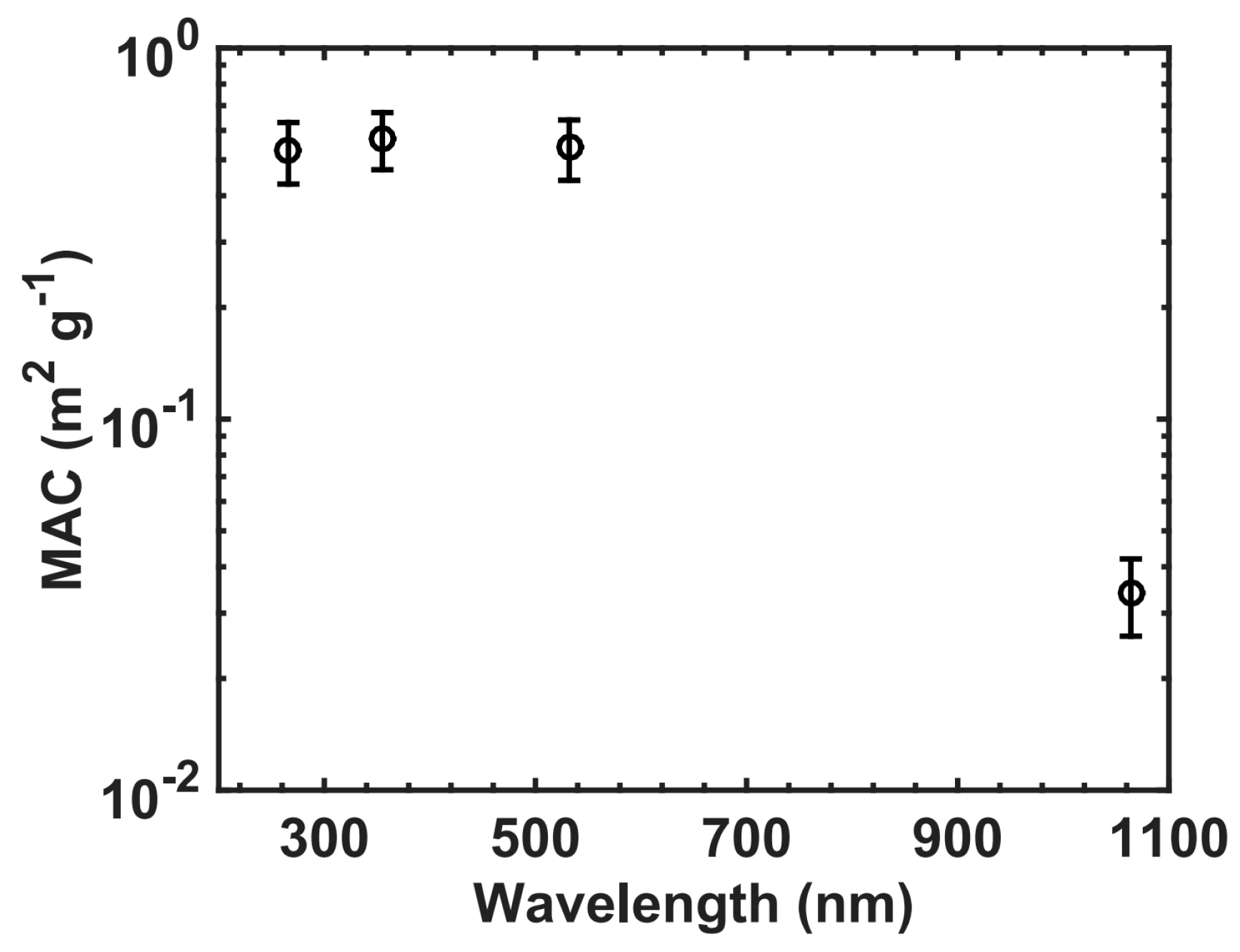
3.1. MACs of Fullerene Soot and Iron Oxides
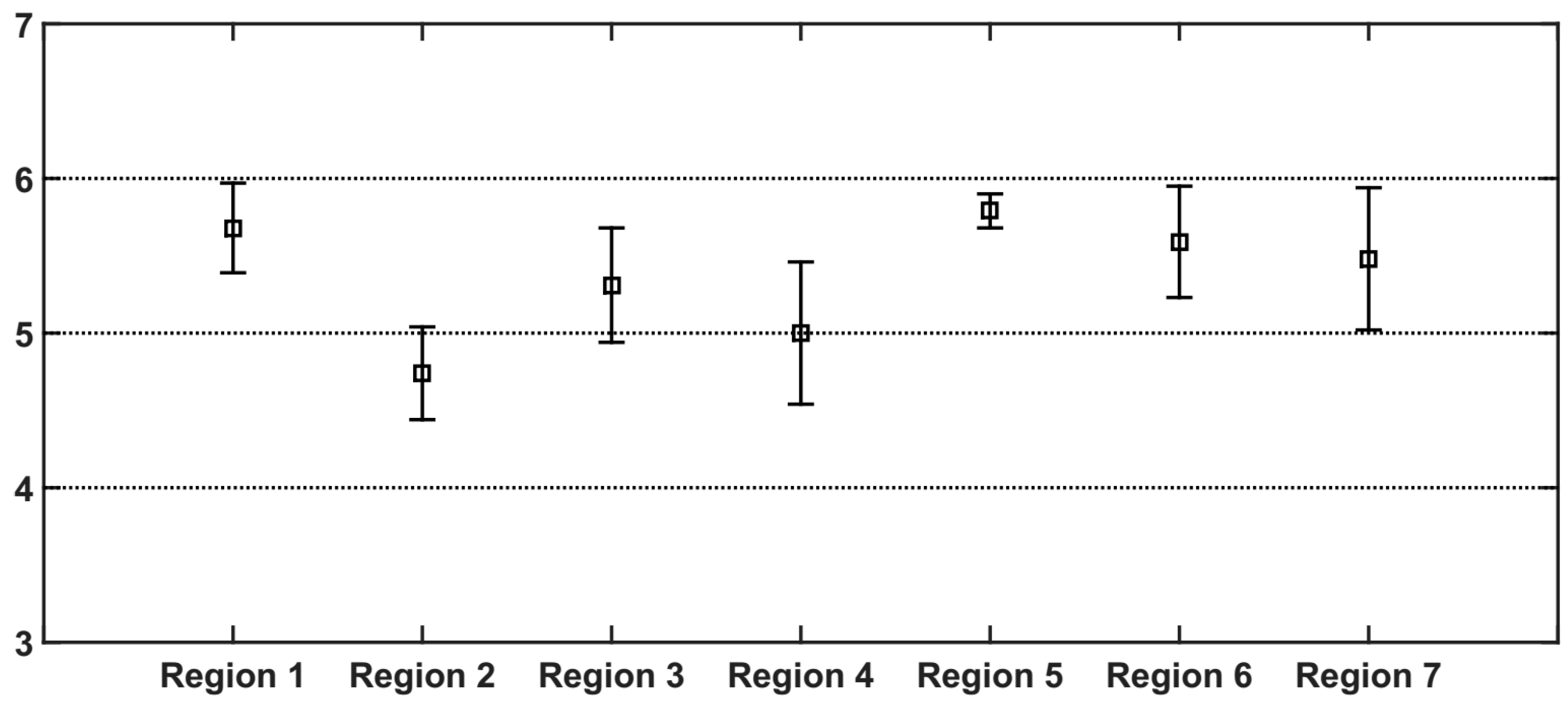
3.2. AAEs of Fullerene Soot and Iron Oxides
3.3. Comparison of Loading and Light Absorption Fraction of ILAPs
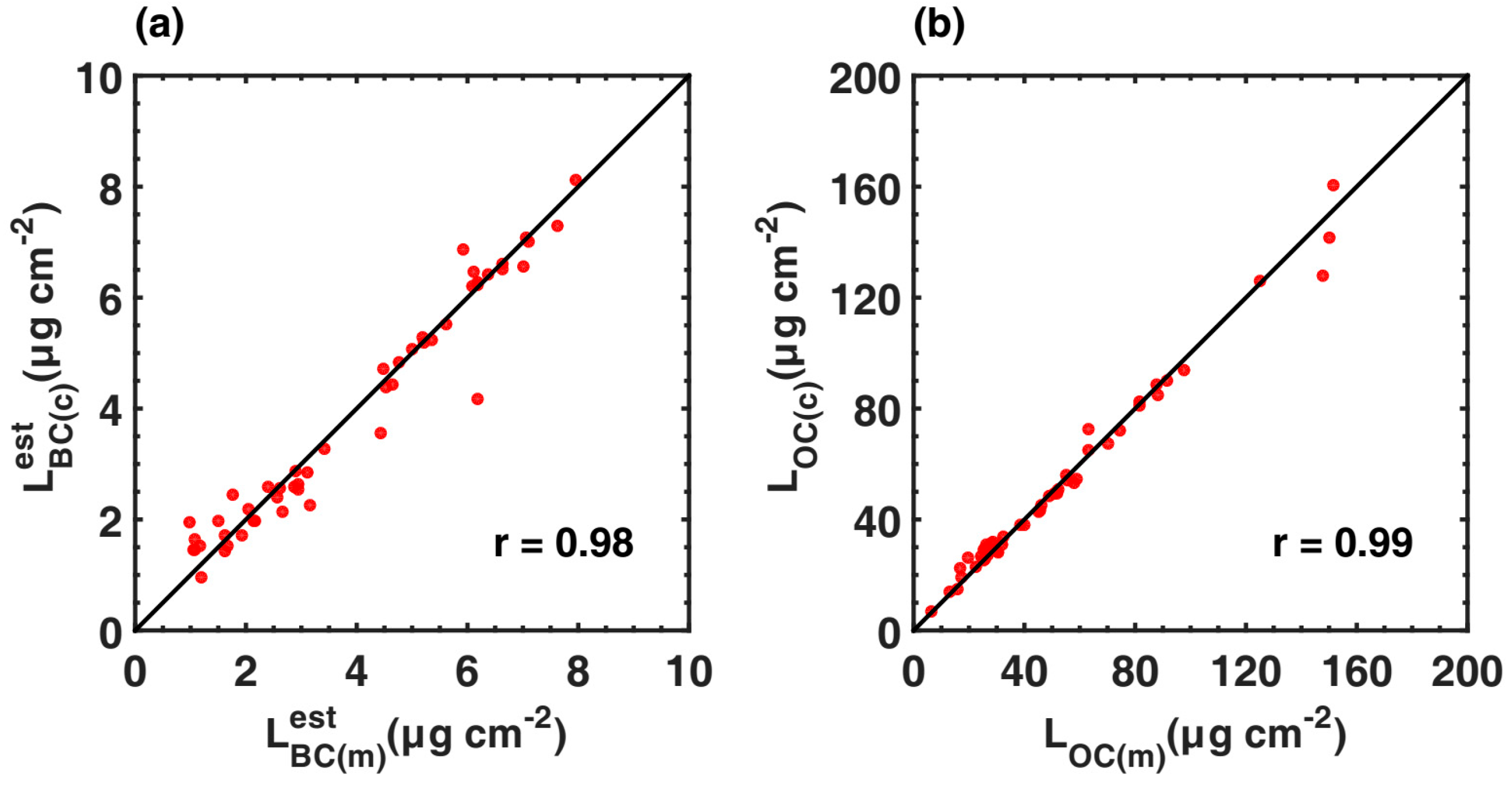
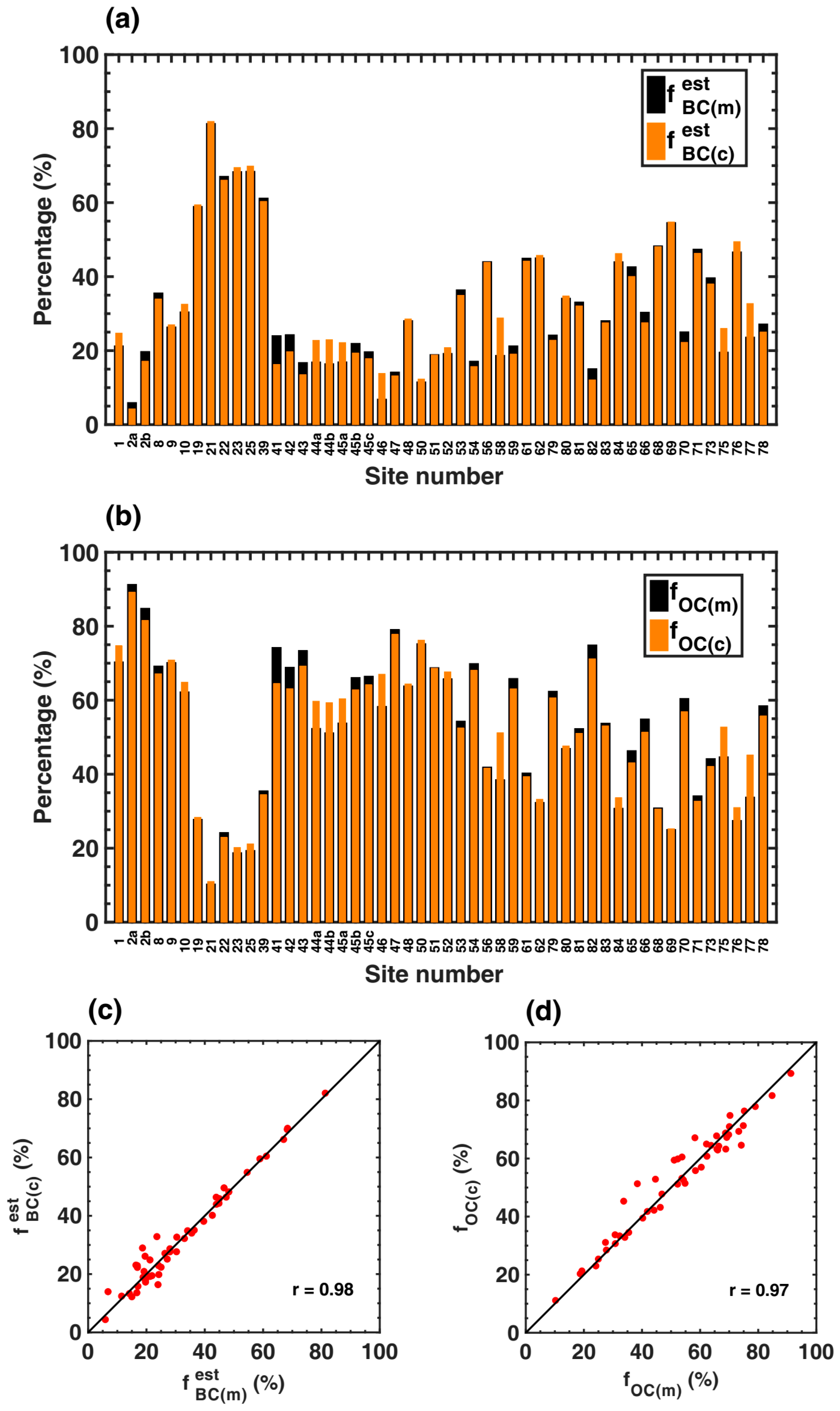
3.3.1. Variations in the Loadings and Relative Absorption Fractions of BC and OC
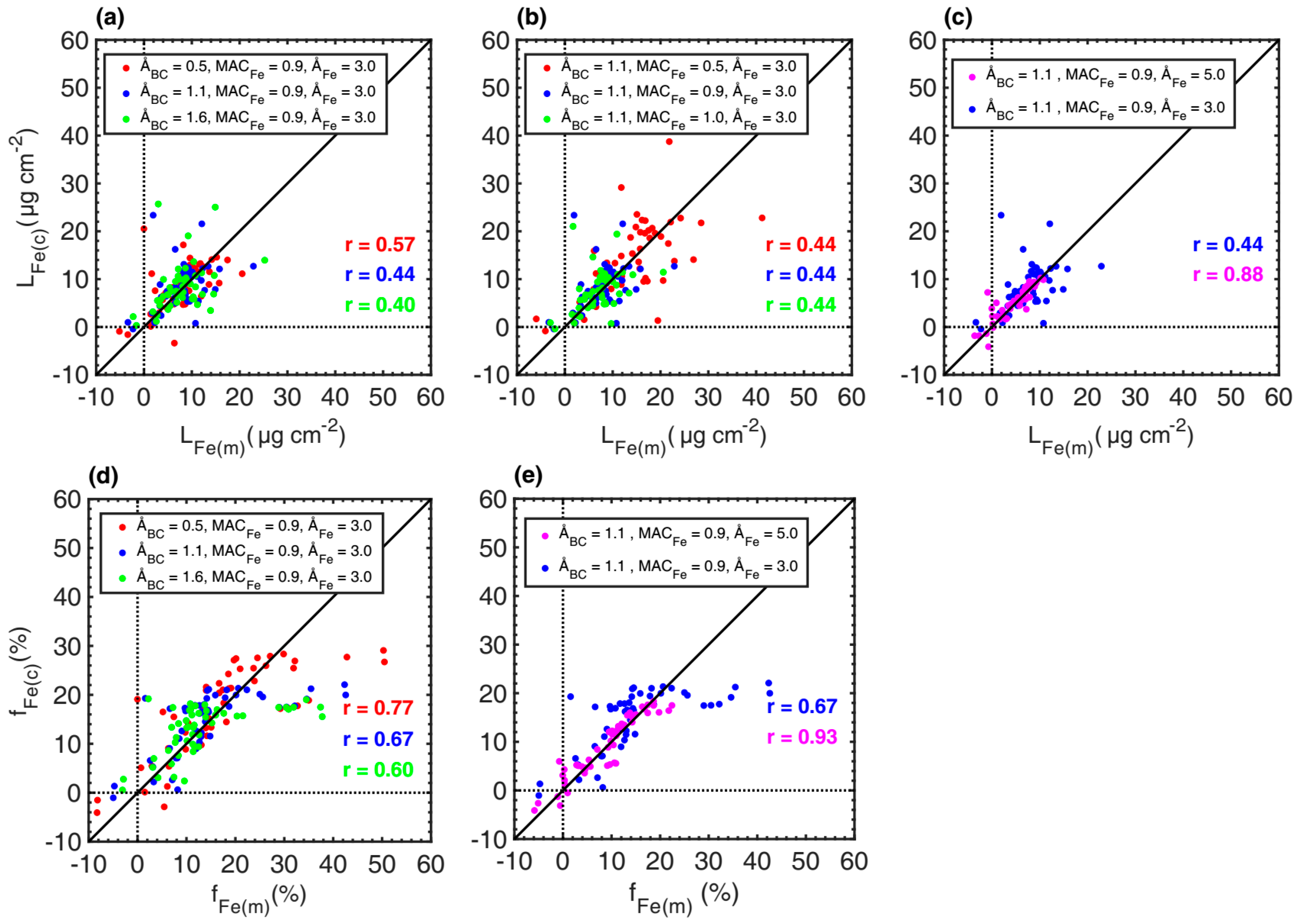
3.3.2. Sensitivities of the Loading and Relative Absorption Fraction of Iron
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Huang, J.P.; Minnis, P.; Lin, B.; Wang, T.H.; Yi, Y.H.; Hu, Y.X.; Sun-Mack, S.; Ayers, K. Possible influences of Asian dust aerosols on cloud properties and radiative forcing observed from MODIS and CERES. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Karcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Yin, Y.; Wurzler, S.; Levin, Z.; Reisin, T.G. Interactions of mineral dust particles and clouds: Effects on precipitation and cloud optical properties. J. Geophys. Res. Atmos. 2002, 107. [Google Scholar] [CrossRef]
- Rosenfeld, D.; Rudich, Y.; Lahav, R. Desert dust suppressing precipitation: A possible desertification feedback loop. Proc. Nat. Acad. Sci. USA 2001, 98, 5975–5980. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, D.; Lohmann, U.; Raga, G.B.; O’Dowd, C.D.; Kulmala, M.; Fuzzi, S.; Reissell, A.; Andreae, M.O. Flood or drought: How do aerosols affect precipitation? Science 2008, 321, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.G.; Wiscombe, W.J. A Model for the Spectral Albedo of Snow. 2. Snow Containing Atmospheric Aerosols. J. Atmos. Sci. 1980, 37, 2734–2745. [Google Scholar]
- Painter, T.H.; Barrett, A.P.; Landry, C.C.; Neff, J.C.; Cassidy, M.P.; Lawrence, C.R.; McBride, K.E.; Farmer, G.L. Impact of disturbed desert soils on duration of mountain snow cover. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Painter, T.H.; Bryant, A.C.; Skiles, S.M. Radiative forcing by light absorbing impurities in snow from MODIS surface reflectance data. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Yasunari, T.J.; Koster, R.D.; Lau, W.K.M.; Kim, K.M. Impact of snow darkening via dust, black carbon, and organic carbon on boreal spring climate in the Earth system. J. Geophys. Res. Atmos. 2015, 120, 5485–5503. [Google Scholar] [CrossRef]
- Flanner, M.G.; Zender, C.S.; Randerson, J.T.; Rasch, P.J. Present-day climate forcing and response from black carbon in snow. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Flanner, M.G.; Zender, C.S.; Hess, P.G.; Mahowald, N.M.; Painter, T.H.; Ramanathan, V.; Rasch, P.J. Springtime warming and reduced snow cover from carbonaceous particles. Atmos. Chem. Phys. 2009, 9, 2481–2497. [Google Scholar] [CrossRef]
- Aoki, T.; Kuchiki, K.; Niwano, M.; Kodama, Y.; Hosaka, M.; Tanaka, T. Physically based snow albedo model for calculating broadband albedos and the solar heating profile in snowpack for general circulation models. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Clarke, A.; McNaughton, C.; Kapustin, V.; Shinozuka, Y.; Howell, S.; Dibb, J.; Zhou, J.; Anderson, B.; Brekhovskikh, V.; Turner, H.; et al. Biomass burning and pollution aerosol over North America: Organic components and their influence on spectral optical properties and humidification response. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Doherty, S.J.; Dang, C.; Hegg, D.A.; Zhang, R.D.; Warren, S.G. Black carbon and other light-absorbing particles in snow of central North America. J. Geophys. Res. Atmos. 2014, 119, 12807–12831. [Google Scholar] [CrossRef]
- Wang, X.; Doherty, S.J.; Huang, J. Black carbon and other light-absorbing impurities in snow across Northern China. J. Geophys. Res. Atmos. 2013, 118, 1471–1492. [Google Scholar] [CrossRef]
- Painter, T.H.; Flanner, M.G.; Kaser, G.; Marzeion, B.; VanCuren, R.A.; Abdalati, W. End of the Little Ice Age in the Alps forced by industrial black carbon. Proc. Nat. Acad. Sci. USA 2013, 110, 15216–15221. [Google Scholar] [CrossRef] [PubMed]
- Hadley, O.L.; Kirchstetter, T.W. Black-carbon reduction of snow albedo. Nat. Clim. Chang. 2012, 2, 437–440. [Google Scholar] [CrossRef]
- Doherty, S.J.; Warren, S.G.; Grenfell, T.C.; Clarke, A.D.; Brandt, R.E. Light-absorbing impurities in Arctic snow. Atmos. Chem. Phys. 2010, 10, 11647–11680. [Google Scholar] [CrossRef]
- Xu, B.Q.; Cao, J.J.; Hansen, J.; Yao, T.D.; Joswia, D.R.; Wang, N.L.; Wu, G.J.; Wang, M.; Zhao, H.B.; Yang, W.; et al. Black soot and the survival of Tibetan glaciers. Proc. Nat. Acad. Sci. USA 2009, 106, 22114–22118. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Nazarenko, L. Soot climate forcing via snow and ice albedos. Proc. Nat. Acad. Sci. USA 2004, 101, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.D.; Noone, K.J. Soot in the Arctic Snowpack—A Cause for Perturbations in Radiative-Transfer. Atmos. Environ. 1985, 19, 2045–2053. [Google Scholar] [CrossRef]
- Dang, C.; Hegg, D.A. Quantifying light absorption by organic carbon in Western North American snow by serial chemical extractions. J. Geophys. Res. Atmos. 2014, 119. [Google Scholar] [CrossRef]
- Ginot, P.; Dumont, M.; Lim, S.; Patris, N.; Taupin, J.D.; Wagnon, P.; Gilbert, A.; Arnaud, Y.; Marinoni, A.; Bonasoni, P.; et al. A 10 year record of black carbon and dust from a Mera Peak ice core (Nepal): Variability and potential impact on melting of Himalayan glaciers. Cryosphere 2014, 8, 1479–1496. [Google Scholar] [CrossRef]
- Kaspari, S.; Painter, T.H.; Gysel, M.; Skiles, S.M.; Schwikowski, M. Seasonal and elevational variations of black carbon and dust in snow and ice in the Solu-Khumbu, Nepal and estimated radiative forcings. Atmos. Chem. Phys. 2014, 14, 8089–8103. [Google Scholar] [CrossRef]
- Wu, G.; Xu, T.; Zhang, X.; Zhang, C.; Yan, N. The visible spectroscopy of iron oxide minerals in dust particles from ice cores on the Tibetan Plateau. Tellus B 2016, 68, 29191. [Google Scholar] [CrossRef]
- Lafon, S.; Sokolik, I.N.; Rajot, J.L.; Caquineau, S.; Gaudichet, A. Characterization of iron oxides in mineral dust aerosols: Implications for light absorption. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Formenti, P.; Rajot, J.L.; Desboeufs, K.; Caquineau, S.; Chevaillier, S.; Nava, S.; Gaudichet, A.; Journet, E.; Triquet, S.; Alfaro, S.; et al. Regional variability of the composition of mineral dust from western Africa: Results from the AMMA SOP0/DABEX and DODO field campaigns. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wu, G.J.; Zhang, C.L.; Xu, T.L.; Zhou, Q.Q. What is the real role of iron oxides in the optical properties of dust aerosols? Atmos. Chem. Phys. 2015, 15, 12159–12177. [Google Scholar] [CrossRef]
- Moosmüller, H.; Engelbrecht, J.P.; Skiba, M.; Frey, G.; Chakrabarty, R.K.; Arnott, W.P. Single scattering albedo of fine mineral dust aerosols controlled by iron concentration. J. Geophys. Res. Atmos. 2012, 117, 90–100. [Google Scholar] [CrossRef]
- Sokolik, I.N.; Toon, O.B. Incorporation of mineralogical composition into models of the radiative properties of mineral aerosol from UV to IR wavelengths. J. Geophys. Res. Atmos. 1999, 104, 9423–9444. [Google Scholar] [CrossRef]
- Wang, X.; Xu, B.Q.; Ming, J. An Overview of the Studies on Black Carbon and Mineral Dust Deposition in Snow and Ice Cores in East Asia. J. Meteorol. Res. 2014, 28, 354–370. [Google Scholar] [CrossRef]
- Doherty, S.J.; Grenfell, T.C.; Forsstrom, S.; Hegg, D.L.; Brandt, R.E.; Warren, S.G. Observed vertical redistribution of black carbon and other insoluble light-absorbing particles in melting snow. J. Geophys. Res. Atmos. 2013, 118, 5553–5569. [Google Scholar] [CrossRef]
- Wendl, I.A.; Menking, J.A.; Farber, R.; Gysel, M.; Kaspari, S.D.; Laborde, M.J.G.; Schwikowski, M. Optimized method for black carbon analysis in ice and snow using the Single Particle Soot Photometer. Atmos. Meas. Tech. 2014, 7, 2667–2681. [Google Scholar] [CrossRef]
- Doherty, S.J.; Hegg, D.A.; Johnson, J.E.; Quinn, P.K.; Schwarz, J.P.; Dang, C.; Warren, S.G. Causes of variability in light absorption by particles in snow at sites in Idaho and Utah. J. Geophys. Res. Atmos. 2016, 121, 4751–4768. [Google Scholar] [CrossRef]
- Sterle, K.M.; McConnell, J.R.; Dozier, J.; Edwards, R.; Flanner, M.G. Retention and radiative forcing of black carbon in eastern Sierra Nevada snow. Cryosphere 2013, 7, 365–374. [Google Scholar] [CrossRef]
- Ming, J.; Cachier, H.; Xiao, C.; Qin, D.; Kang, S.; Hou, S.; Xu, J. Black carbon record based on a shallow Himalayan ice core and its climatic implications. Atmos. Chem. Phys. 2008, 8, 1343–1352. [Google Scholar] [CrossRef]
- Li, C.L.; Bosch, C.; Kang, S.C.; Andersson, A.; Chen, P.F.; Zhang, Q.G.; Cong, Z.Y.; Chen, B.; Qin, D.H.; Gustafsson, O. Sources of black carbon to the Himalayan-Tibetan Plateau glaciers. Nat. Commun. 2016, 7, 12574. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Fain, X.; Zanatta, M.; Cozic, J.; Jaffrezo, J.L.; Ginot, P.; Laj, P. Refractory black carbon mass concentrations in snow and ice: Method evaluation and inter-comparison with elemental carbon measurement. Atmos. Meas. Tech. 2014, 7, 3307–3324. [Google Scholar] [CrossRef]
- Grenfell, T.C.; Doherty, S.J.; Clarke, A.D.; Warren, S.G. Light absorption from particulate impurities in snow and ice determined by spectrophotometric analysis of filters. Appl. Opt. 2011, 50, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.C.; Anderson, T.L.; Campbell, D. Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols. Aerosol Sci. Technol. 1999, 30, 582–600. [Google Scholar] [CrossRef]
- Huang, J.P.; Fu, Q.A.; Zhang, W.; Wang, X.; Zhang, R.D.; Ye, H.; Warren, S.G. Dust And Black Carbon in Seasonal Snow across Northern China. Bull. Am. Meteorol. Soc. 2011, 92, 175–181. [Google Scholar]
- Ye, H.; Zhang, R.D.; Shi, J.S.; Huang, J.P.; Warren, S.G.; Fu, Q. Black carbon in seasonal snow across northern Xinjiang in northwestern China. Environ. Res. Lett. 2012, 7, 044002. [Google Scholar] [CrossRef]
- Cheng, Y.; He, K.B.; Zheng, M.; Duan, F.K.; Du, Z.Y.; Ma, Y.L.; Tan, J.H.; Yang, F.M.; Liu, J.M.; Zhang, X.L.; et al. Mass absorption efficiency of elemental carbon and water-soluble organic carbon in Beijing, China. Atmos. Chem. Phys. 2011, 11, 11497–11510. [Google Scholar] [CrossRef]
- Weingartner, E.; Saathoff, H.; Schnaiter, M.; Streit, N.; Bitnar, B.; Baltensperger, U. Absorption of light by soot particles: Determination of the absorption coefficient by means of aethalometers. J. Aerosol Sci. 2003, 34, 1445–1463. [Google Scholar]
- Alfaro, S.C.; Lafon, S.; Rajot, J.L.; Formenti, P.; Gaudichet, A.; Maille, M. Iron oxides and light absorption by pure desert dust: An experimental study. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Clarke, A.D. Effects of Filter Internal-Reflection Coefficient on Light-Absorption Measurements Made Using the Integrating Plate Method. Appl. Opt. 1982, 21, 3021–3031. [Google Scholar] [PubMed]
- Clarke, A.D. Integrating Sandwich—A New Method of Measurement of the Light-Absorption Coefficient for Atmospheric Particles. Appl. Opt. 1982, 21, 3011–3020. [Google Scholar] [PubMed]
- Bedidi, A.; Cervelle, B. Light scattering by spherical particles with hematite and goethitelike optical properties: Effect of water impregnation. J. Geophys. Res. Solid Earth 1993, 98, 11941–11952. [Google Scholar]
- Wonaschutz, A.; Hitzenberger, R.; Bauer, H.; Pouresmaeil, P.; Klatzer, B.; Caseiro, A.; Puxbaum, H. Application of the Integrating Sphere Method to Separate the Contributions of Brown and Black Carbon in Atmospheric Aerosols. Environ. Sci. Technol. 2009, 43, 1141–1146. [Google Scholar] [PubMed]
- Chakrabarty, R.K.; Moosmuller, H.; Chen, L.W.A.; Lewis, K.; Arnott, W.P.; Mazzoleni, C.; Dubey, M.K.; Wold, C.E.; Hao, W.M.; Kreidenweis, S.M. Brown carbon in tar balls from smoldering biomass combustion. Atmos. Chem. Phys. 2010, 10, 6363–6370. [Google Scholar] [CrossRef]
- Yang, M.; Howell, S.; Zhuang, J.; Huebert, B. Attribution of aerosol light absorption to black carbon, brown carbon, and dust in China–interpretations of atmospheric measurements during EAST-AIRE. Atmos. Chem. Phys. 2009, 9, 2035–2050. [Google Scholar]
- Barnard, J.C.; Volkamer, R.; Kassianov, E.I. Estimation of the mass absorption cross section of the organic carbon component of aerosols in the Mexico City Metropolitan Area. Atmos. Chem. Phys. 2008, 8, 6665–6679. [Google Scholar] [CrossRef]
- Hoffer, A.; Gelencser, A.; Guyon, P.; Kiss, G.; Schmid, O.; Frank, G.P.; Artaxo, P.; Andreae, M.O. Optical properties of humic-like substances (HULIS) in biomass-burning aerosols. Atmos. Chem. Phys. 2006, 6, 3563–3570. [Google Scholar] [CrossRef]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Szkarlat, A.C.; Japar, S.M. Optical and Chemical-Properties of Particle Emissions from on-Road Vehicles. J. Air Pollut. Control Assoc. 1983, 33, 592–597. [Google Scholar] [CrossRef]
- Scherrer, H.C.; Kittelson, D.B.; Dolan, D.F. Light Absorption Measurements of Diesel Particulate Matter; SAE Technical Paper 810181; SAE Technical Paper: Warrendale, PA, USA, 1981. [Google Scholar]
- Japar, S.M.; Szkarlat, A.C.; Gorse, R.A. Optical-Properties of Particulate-Emissions from on-Road Vehicles. Atmos. Environ. 1981, 15, 2063–2070. [Google Scholar]
- Ballach, J.; Hitzenberger, R.; Schultz, E.; Jaeschke, W. Development of an improved optical transmission technique for black carbon (BC) analysis. Atmos. Environ. 2001, 35, 2089–2100. [Google Scholar] [CrossRef]
- Utry, N.; Ajtai, T.; Pintér, M.; Tombácz, E.; Illés, E.; Bozóki, Z.; Szabó, G. Mass-specific optical absorption coefficients and imaginary part of the complex refractive indices of mineral dust components measured by a multi-wavelength photoacoustic spectrometer. Atmos. Meas. Tech. 2015, 8, 401–410. [Google Scholar] [CrossRef]
- Bond, T.C.; Bergstrom, R.W. Light absorption by carbonaceous particles: An investigative review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar]
- Lack, D.A.; Cappa, C.D. Impact of brown and clear carbon on light absorption enhancement, single scatter albedo and absorption wavelength dependence of black carbon. Atmos. Chem. Phys. 2010, 10, 4207–4220. [Google Scholar]
- Russell, P.B.; Bergstrom, R.W.; Shinozuka, Y.; Clarke, A.D.; DeCarlo, P.F.; Jimenez, J.L.; Livingston, J.M.; Redemann, J.; Dubovik, O.; Strawa, A. Absorption Angstrom Exponent in AERONET and related data as an indicator of aerosol composition. Atmos. Chem. Phys. 2010, 10, 1155–1169. [Google Scholar] [CrossRef]
- Bahadur, R.; Praveen, P.S.; Xu, Y.Y.; Ramanathan, V. Solar absorption by elemental and brown carbon determined from spectral observations. Proc. Nat. Acad. Sci. USA 2012, 109, 17366–17371. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, M.; Arnott, W.P.; Lewis, K.; Moosmuller, H. In situ aerosol optics in Reno, NV, USA during and after the summer 2008 California wildfires and the influence of absorbing and non-absorbing organic coatings on spectral light absorption. Atmos. Chem. Phys. 2009, 9, 8007–8015. [Google Scholar] [CrossRef]
- Linke, C.; Mohler, O.; Veres, A.; Mohácsi, Á. Optical properties and mineralogical composition of different Saharan mineral dust samples: A laboratory study. Atmos. Chem. Phys. 2006, 6, 3315–3323. [Google Scholar] [CrossRef]
- Pu, W.; Wang, X.; Wei, H.; Zhou, Y.; Shi, J.; Hu, Z.; Jin, H.; Chen, Q. Properties of black carbon and other insoluble light-absorbing particles in seasonal snow of northwest China. Cryosphere Discuss. 2016, 2016. [Google Scholar] [CrossRef]
| Particle Types | K1* | (m2·g−1) | (m2·g−1) | AAE (450–750 nm) | AAE (550–750 nm) |
|---|---|---|---|---|---|
| Hematite | 0.135 | 1.32 ± 0.03 | 0.97 ± 0.02 | 3.67 ± 0.36 | 5.53 ± 0.47 |
| Goethite | 0.135 | 0.59 ± 0.02 | 0.43 ± 0.01 | 5.30 ± 0.20 | 2.18 ± 0.16 |
| Fullerene soot | 0.023 | 8.02 ± 0.53 | 6.40 ± 0.42 | 0.54 ± 0.06 | – |
| Relative Biases | 10th Percentile | 25th Percentile | 50th Percentile | 75th Percentile | 90th Percentile |
|---|---|---|---|---|---|
| 0.74% | 1.76% | 6.29% | 16.11% | 33.41% | |
| 1.04% | 2.47% | 4.27% | 7.29% | 14.46% | |
| 6.60% | 14.47% | 33.01% | 46.65% | 73.93% |
| Cases | 10th Percentile | 25th Percentile | 50th Percentile | 75th Percentile | 90th Percentile |
|---|---|---|---|---|---|
| Base case | 6.60% | 14.47% | 33.01% | 46.65% | 73.93% |
| 5.19% | 12.38% | 29.66% | 46.32% | 93.64% | |
| 7.26% | 17.29% | 30.56% | 54.34% | 73.17% | |
| 6.60% | 14.47% | 33.01% | 46.65% | 73.93% | |
| 6.60% | 14.47% | 33.01% | 46.65% | 73.93% | |
| 2.31% | 5.80% | 14.40% | 25.06% | 83.15% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, X.; Wu, X.; Cong, Z.; Wu, G.; Ji, M. Quantifying Light Absorption of Iron Oxides and Carbonaceous Aerosol in Seasonal Snow across Northern China. Atmosphere 2017, 8, 63. https://doi.org/10.3390/atmos8040063
Zhou Y, Wang X, Wu X, Cong Z, Wu G, Ji M. Quantifying Light Absorption of Iron Oxides and Carbonaceous Aerosol in Seasonal Snow across Northern China. Atmosphere. 2017; 8(4):63. https://doi.org/10.3390/atmos8040063
Chicago/Turabian StyleZhou, Yue, Xin Wang, Xueqin Wu, Zhiyuan Cong, Guangming Wu, and Mingxia Ji. 2017. "Quantifying Light Absorption of Iron Oxides and Carbonaceous Aerosol in Seasonal Snow across Northern China" Atmosphere 8, no. 4: 63. https://doi.org/10.3390/atmos8040063





