Characteristics and Source Analysis of Water-Soluble Inorganic Ions in PM10 in a Typical Mining City, Central China
Abstract
:1. Introduction
2. Methods
2.1. Sample Collection
2.2. Chemical Analysis
3. Results and Discussion
3.1. Chemical Characterization
3.2. Temporal Variation
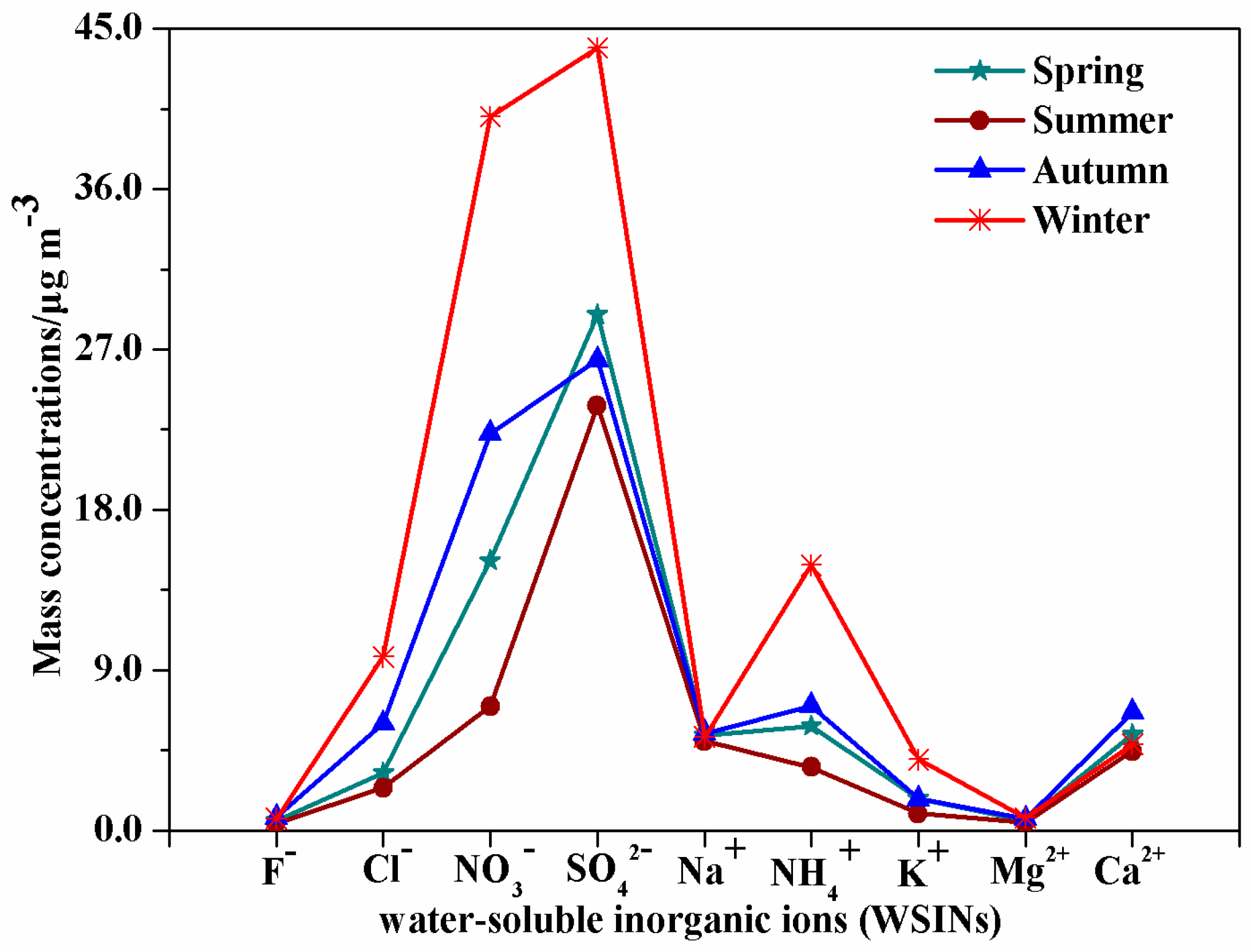
3.2.1. Seasonal Variation
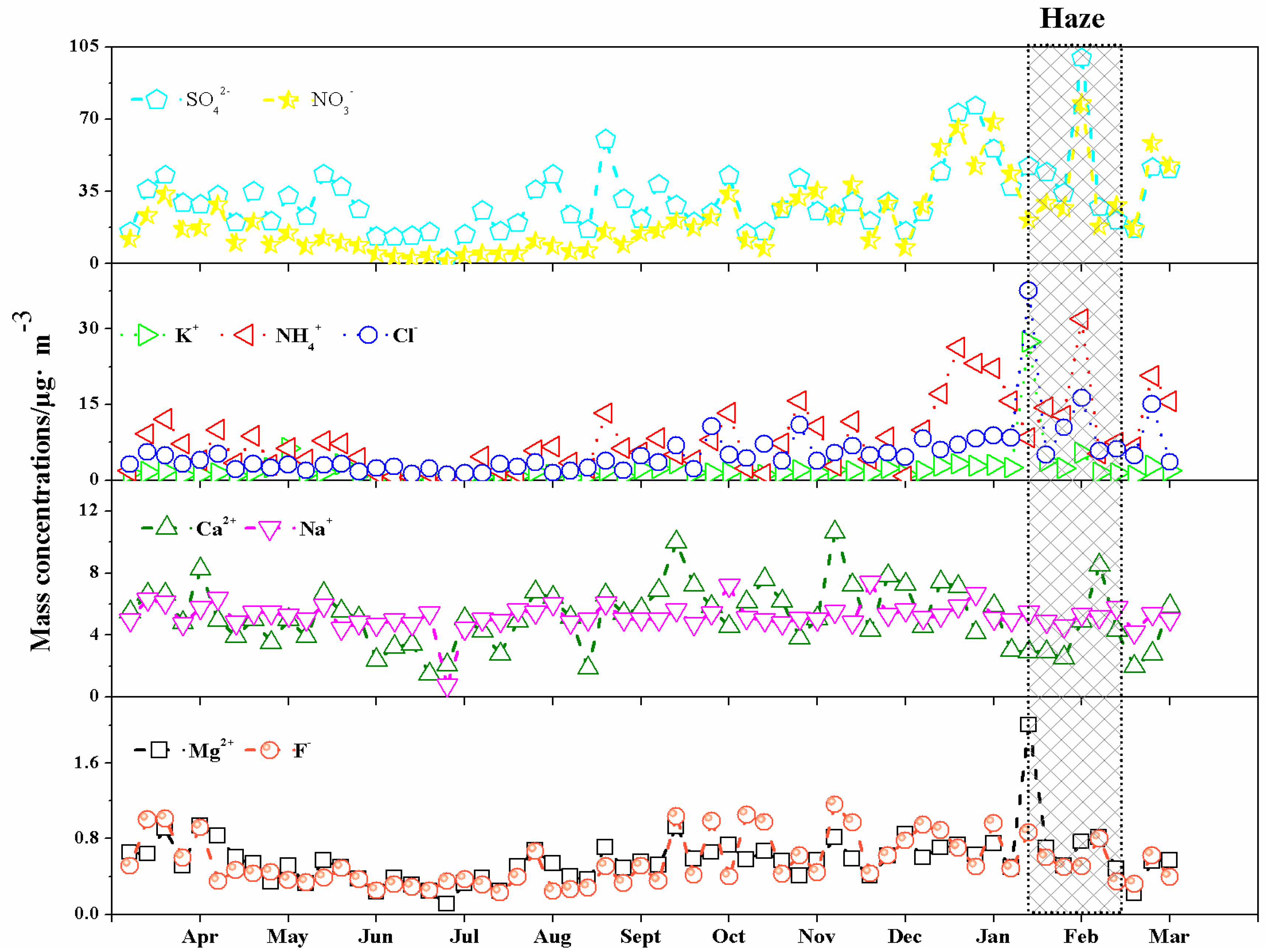
3.2.2. Monthly Variation
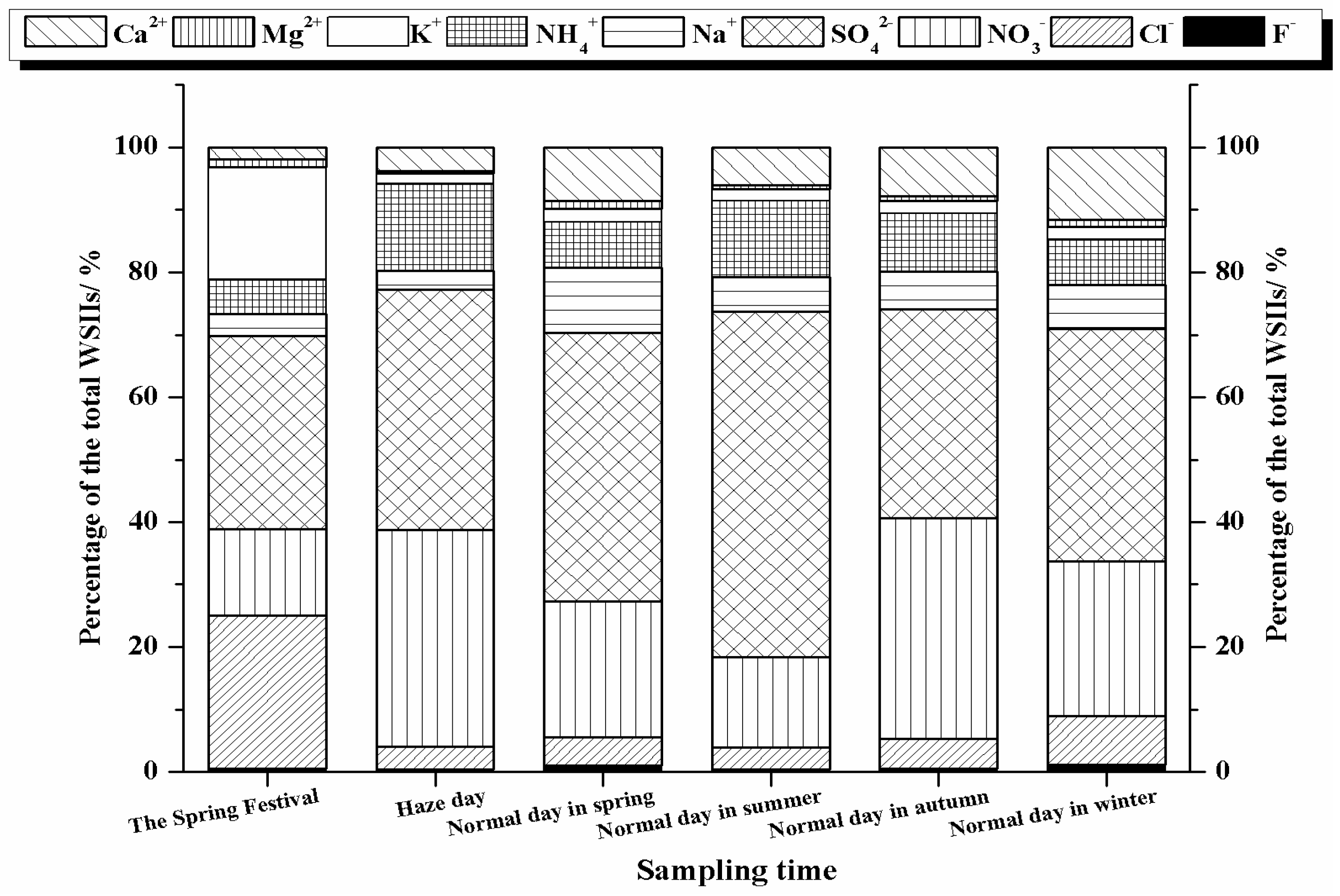
3.3. The Pollution Episode in February 2013
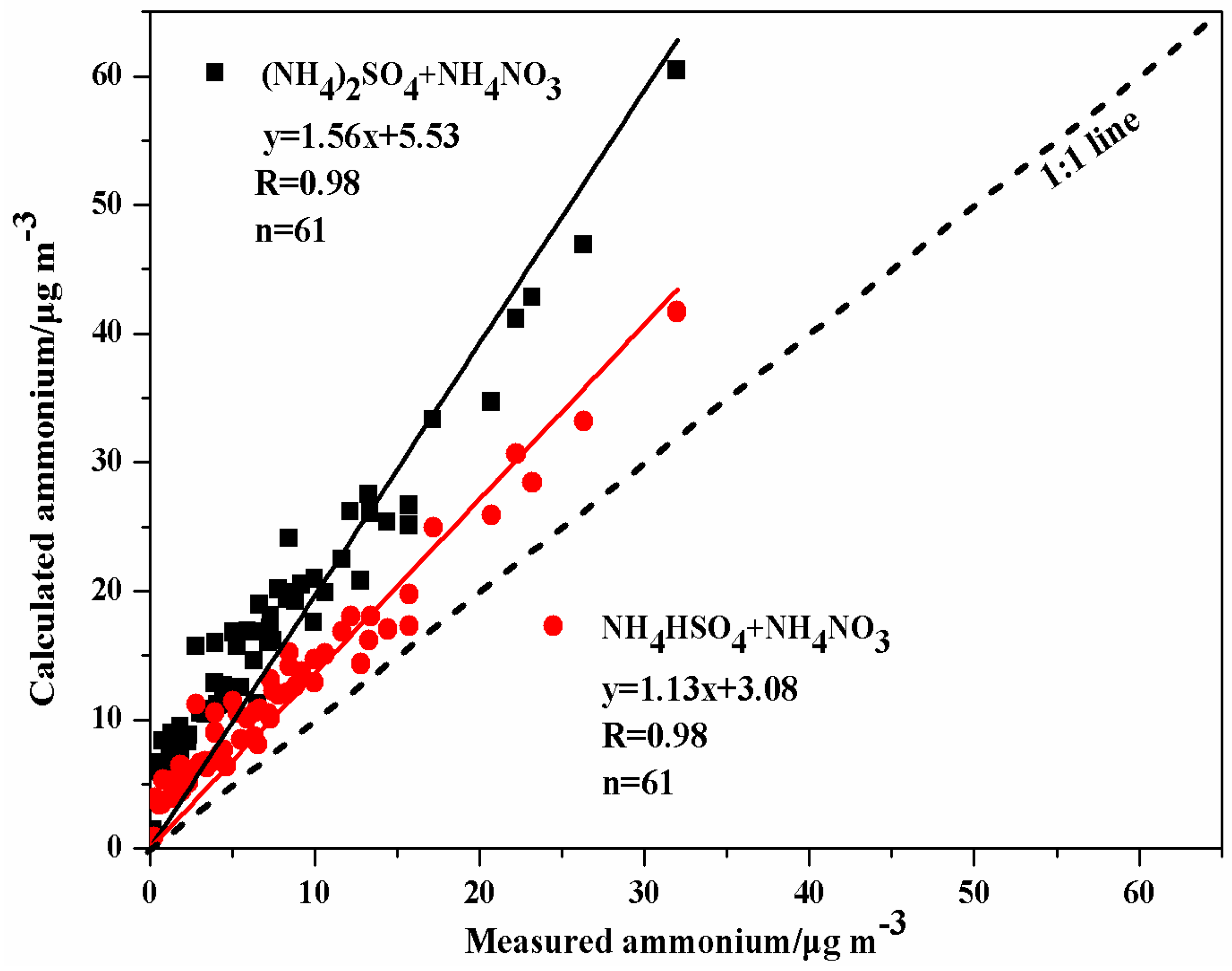
3.4. Charge Balance Analysis of WSIIs
3.5. Source Identification
3.5.1. The Ratio of NO3−/SO42−
3.5.2. Correlation Analysis
3.5.3. Principal Component Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kunwar, B.; Torii, K.; Zhu, C.M.; Fu, P.Q.; Kawamura, K. Springtime variations of organic and inorganic constituents in submicron aerosols (PM1.0) from Cape Hedo, Okinawa. Atmos. Environ. 2016, 130, 84–94. [Google Scholar] [CrossRef]
- Han, J.B.; Han, B.; Li, P.H.; Kong, S.F.; Bai, Z.P.; Han, D.H.; Dou, X.Y.; Zhao, X.D. Chemical characterizations of PM10 profiles for major emission sources in Xining, northwestern China. Aerosol Air Qual. Res. 2014, 14, 1017–1027. [Google Scholar] [CrossRef]
- Mkoma, S.L.; da Rocha, G.O.; Regis, A.C.D.; Domingos, J.S.S.; Santos, J.V.S.; de Andrade, S.J.; Carvalho, L.S.; de Andrade, J.B. Major ions in PM2.5 and PM10 released from buses: The use of diesel/biodiesel fuels under real conditions. Fuel 2014, 115, 109–117. [Google Scholar] [CrossRef]
- Huang, T.; Chen, J.; Zhao, W.T.; Cheng, J.X.; Cheng, S.G. Seasonal variations and correlation analysis of water-soluble inorganic ions in PM2.5 in Wuhan, 2013. Atmosphere 2016, 7, 49. [Google Scholar] [CrossRef]
- Kawamura, K.; Kasukabe, H.; Barrie, L.A. Secondary formation of water-soluble organic acids and α-dicarbonyls and their contributions to total carbon and water-soluble organic carbon: Photochemical aging of organic aerosols in the Arctic spring. J. Geophys. Res. Atmos. 2010, 115, 6–7. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, Q.Y.; Chow, J.C.; Watson, J.G.; Tie, X.X.; Shen, Z.X.; Wang, P.; An, Z.S. Impacts of aerosol compositions on visibility impairment in Xi’an, China. Atmos. Environ. 2012, 59, 559–566. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.D.; Deng, J.; Wang, H.Y. Stable sulfur isotope ratios and water-soluble inorganic compositions of PM10 in Yichang City, central China. Environ. Sci. Pollut. Res. 2015, 22, 13564–13572. [Google Scholar]
- Dao, X.; Wang, Z.; Lv, Y.B.; Teng, E.J.; Zhang, L.L.; Wang, C. Chemical characteristics of water-soluble ions in particulate matter in three metropolitan areas in the North China Plain. PLoS ONE 2014, 9, e113831. [Google Scholar] [CrossRef] [PubMed]
- Du, H.H.; Kong, L.D.; Cheng, T.T.; Chen, J.M.; Du, J.F.; Li, L.; Xia, X.G.; Leng, C.P.; Huang, G.H. Insights into summertime haze pollution events over Shanghai based on online water-soluble ionic composition of aerosols. Atmos. Environ. 2011, 45, 5131–5137. [Google Scholar] [CrossRef]
- Tan, J.H.; Duan, J.C.; Chen, D.H.; Wang, X.H.; Guo, S.J.; Bi, X.H.; Sheng, G.Y.; He, K.B.; Fu, J.M. Chemical characteristics of haze during summer and winter in Guangzhou. Atmos. Res. 2009, 94, 238–245. [Google Scholar] [CrossRef]
- Cao, J.J.; Zhu, C.S.; Tie, X.X.; Geng, F.H.; Xu, H.M.; Ho, S.; Wang, G.H.; Han, Y.M.; Ho, K.F. Characteristics and sources of carbonaceous aerosols from Shanghai, China. Atmos. Chem. Phys. 2013, 13, 803–817. [Google Scholar] [CrossRef]
- Zhan, C.L.; Zhang, J.Q.; Cao, J.J.; Han, Y.M.; Wang, P.; Zheng, J.R.; Yao, R.Z.; Liu, H.X.; Xiao, W.S. Characteristics and sources of black carbon in atmospheric dustfall particles from HS, China. Aerosol Air Qual. Res. 2016, 16, 2096–2016. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Qu, C.K.; Qi, S.H.; Cao, J.J.; Zhan, C.L.; Xing, X.L.; Xiao, Y.L.; Zheng, J.R.; Xiao, W.S. Polycyclic aromatic hydrocarbons (PAHs) in atmospheric dustfall from the industrial corridor in Hubei Province, Central China. Environ. Geochem. Health 2015, 37, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.K.; Kawamura, K.; Lazaar, M.; Kunwar, B.; Boreddy, S.K.R. Dicarboxylic acids, oxoacids, benzoic acid, α-dicarbonyls, WSOC, OC, and ions in spring aerosols from Okinawa Island in the western North Pacific Rim: Size distributions and formation processes. Atmos. Chem. Phys. Discuss. 2016, 16, 5263–5282. [Google Scholar] [CrossRef]
- Gugamsetty, B.; Wei, H.; Liu, C.N.; Awasthi, A.; Hsu, S.C.; Tsai, C.J.; Roam, G.D.; Wu, Y.C.; Chen, C.F. Source characterization and apportionment of PM10, PM2.5 and PM0.1 by using positive matrix factorization in Shinjung station Taiwan. Aerosol Air Qual. Res. 2012, 12, 476–491. [Google Scholar]
- Guo, Y.T.; Zhang, J.; Wang, S.G.; She, F.; Li, X. Long-tern characterization of major water-soluble inorganic ions in PM10 in coastal site on the Japan Sea. J. Atmos. Chem. 2012, 68, 299–316. [Google Scholar] [CrossRef]
- Nicolas, J.F.; Galindo, N.; Yubero, E.; Pastor, C.; Esclapez, R.; Crespo, J. Aerosol inorganic ions in a semiarid region on the southeastern Mediterranean coast. Water Air Soil Pollut. 2009, 201, 149–159. [Google Scholar] [CrossRef]
- Kuniyal, J.C.; Sharma, M.; Chand, K.; Mathela, C.S. Water soluble ionic components in particulate matter (PM10) during high pollution episode days at Mohal and Kothi in the north-western Himalaya, India. Aerosol Air Qual. Res. 2015, 15, 529–543. [Google Scholar] [CrossRef]
- Twigg, M.M.; Di Marco, C.F.; Leeson, S.; van Dijk, N.; Jones, M.R.; Leith, I.D.; Morrison, E.; Coyle, M.; Proost, R.; Peeters, A.N.M.; et al. Water soluble aerosols and gases at a UK background site-Part 1, Controls of PM2.5 and PM10 aerosol composition. Atmos. Chem. Phys. 2015, 15, 8131–8145. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, S.C.; Lee, S.C.; Chow, J.C.; Ho, K.F.; Watson, J.G.; Han, Y.M.; Zhang, R.J.; Zhang, F.; Yau, P.S.; et al. Characteristics and source apportionment of PM1 emissions at a roadside station. J. Hazard. Mater. 2011, 195, 82–91. [Google Scholar] [CrossRef] [PubMed]
- He, Q.S.; Yan, Y.L.; Guo, L.L.; Zhang, Y.L.; Zhang, G.X.; Wang, X.M. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Ho, K.F.; Ho, S.S.H.; Lee, S.C.; Kawamura, K.; Zou, S.C.; Cao, J.J.; Xu, H.M. Summer and winter variations of dicarboxylic acids, fatty acids and benzoic acid in PM2.5 in Pearl Delta River Region, China. Atmos. Chem. Phys. 2011, 11, 2197–2208. [Google Scholar] [CrossRef]
- Lin, C.C. A review of the impact of fireworks on particulate matter in ambient air. J. Air Waste Manag. Assoc. 2016, 66, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Chien, L.H.; Yuan, C.S.; Jen, Y.H.; Ie, I.R. Influences of fireworks on chemical characteristics of atmospheric fine and coarse particles during Taiwan’s Lantern Festival. Atmos. Environ. 2012, 62, 256–264. [Google Scholar] [CrossRef]
- Steinhauser, G.; Sterba, J.H.; Foster, M.; Grass, F.; Bichler, M. Heavy metals from pyrotechnics in New Years Eve snow. Atmos. Environ. 2008, 42, 8616–8622. [Google Scholar] [CrossRef]
- Kumar, S.; Raman, R.S. Inorganic ions in ambient fine particles over a National Park in central India: Seasonality, dependencies between SO42−, NO3−, and NH4+, and neutralization of aerosol acidity. Atmos. Environ. 2016, 143, 152–163. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, S. Seasonal variations in water soluble inorganic ions, OC and EC in PM10 and PM>10 aerosols over Delhi: Influence of sources and meteorological factors. Aerosol Air Qual. Res. 2016, 16, 1165–1178. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.J.; Tie, X.X.; Shen, Z.X.; Liu, S.X.; Ding, H.; Han, Y.M.; Wang, G.H.; Ho, K.F.; Qiang, J.; et al. Water-soluble ions in atmospheric aerosols measured in Xi’an, China, Seasonal variations and sources. Atmos. Res. 2011, 102, 110–119. [Google Scholar] [CrossRef]
- Xu, H.M.; Cao, J.J.; Chow, C.J.; Huang, R.J.; Shen, Z.X.; Chen, L.W.A.; Ho, K.F.; Watson, J.G. Inter-annual variability of wintertime PM2.5 chemical composition in Xi’an, China, Evidences of changing source emissions. Sci. Total Environ. 2016, 545–546, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Pavuluri, C.M.; Kawamura, K.; Mihalopoulos, N.; Fu, P.Q. Characteristics, seasonality and sources of inorganic ions and trace metals in North-east Asian aerosols. Environ. Chem. 2015, 12, 338–349. [Google Scholar] [CrossRef]

| Season | Sampling Time | Sampling Number | Temperature (°C) | Relative Humidity (%) | Remarks |
|---|---|---|---|---|---|
| Spring | 2012/4/3–2012/6/26 | 15 * | 23.5 ± 1.9 | 78.4 ± 11.1 | sunny with gentle breeze |
| Summer | 2012/7/2–2012/9/30 | 16 ** | 33.0 ± 2.5 | 87.0 ± 15.0 | moderate rain to showers with light breeze |
| Autumn | 2012/10/6–2012/12/29 | 15 * | 19.1 ± 3.0 | 85.4 ± 6.3 | clear to cloudy with level 4 of the north wind |
| Winter | 2013/1/4–2013/3/29 | 15 * | 4.2 ± 2.0 | 88.3 ± 5.1 | Overcast with rain and snow, no sustained winds |
| Location | Period | F− | Cl− | NO3− | SO42− | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huangshi, China | 2012/4–2013/3 | 0.57 | 5.32 | 21.1 | 30.9 | 5.25 | 7.77 | 2.10 | 0.58 | 5.29 | This study |
| Yichang, China | 2012/12 | n.a. | 1.75 | 9.91 | 24.6 | 4.60 | 3.85 | n.a. | n.a. | 6.25 | [7] |
| Beijing, China | 2013/1–2013/12 | 0.35 | 6.44 | 31.1 | 23.2 | 1.92 | 13.5 | 2.27 | 0.52 | 4.38 | [8] |
| Tianjin, China | 2013/1–2013/12 | 0.38 | 10.6 | 37.8 | 36.7 | 1.92 | 18.5 | 2.63 | 0.57 | 5.07 | [8] |
| Shijiazhuang, China | 2013/1–2013/12 | 1.66 | 17.3 | 42.3 | 40.2 | 4.01 | 23.2 | 4.01 | 1.03 | 13.0 | [8] |
| Shanghai, China | 2009/5–2009/6 | n.a. | 3.53 | 32.9 | 28.7 | 0.60 | 19.3 | 3.00 | 0.23 | 2.97 | [9] |
| Taipei, Taiwan | 2011/5–2011/11 | n.a. | 0.54 | 1.32 | 6.30 | 0.65 | 1.49 | 0.22 | 0.20 | 0.37 | [15] |
| Ishikawa, Japan | 2001/1–2003/12 | n.a. | 2.77 | 1.77 | 4.49 | 1.86 | 1.31 | 0.12 | 0.23 | 0.25 | [16] |
| Southern Spain | 2004/12–2005/11 | n.a. | 0.60 | 3.76 | 4.36 | 0.99 | 1.10 | 0.28 | 0.16 | 2.29 | [17] |
| Mohal, India | 2009/9–2011/12 | 0.34 | 3.16 | 2.67 | 5.56 | 1.14 | 0.84 | 0.50 | 0.09 | 1.51 | [18] |
| Auchencorth Moss, UK | 2013/1–2013/12 | n.a. | 1.11 | 1.86 | 1.43 | 0.84 | 0.98 | 0.07 | 0.06 | 0.06 | [19] |
| Composition | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic ions | ||||||||||||
| F− | 0.84 | 0.41 | 0.36 | 0.31 | 0.41 | 0.38 | 0.70 | 0.70 | 0.79 | 0.84 | 0.59 | 0.55 |
| Cl− | 4.10 | 3.23 | 2.31 | 1.71 | 2.62 | 2.96 | 6.47 | 6.01 | 5.42 | 7.79 | 15.5 | 6.88 |
| NO3− | 20.1 | 16.6 | 8.97 | 3.19 | 7.61 | 10.5 | 22.2 | 22.8 | 21.9 | 49.2 | 39.7 | 31.3 |
| SO42− | 30.2 | 28.3 | 28.2 | 11.7 | 29.4 | 30.8 | 29.9 | 24.6 | 24.1 | 49.9 | 52.5 | 30.8 |
| Na+ | 5.61 | 5.47 | 4.95 | 4.07 | 5.43 | 5.20 | 5.57 | 5.01 | 5.75 | 5.51 | 5.05 | 5.12 |
| NH4+ | 6.41 | 6.33 | 4.92 | 0.65 | 4.40 | 6.20 | 7.76 | 7.46 | 5.59 | 18.1 | 16.7 | 10.2 |
| K+ | 1.38 | 2.56 | 1.51 | 0.44 | 1.23 | 1.30 | 1.93 | 1.54 | 1.78 | 2.65 | 8.30 | 1.69 |
| Mg2+ | 0.76 | 0.56 | 0.40 | 0.27 | 0.50 | 0.50 | 0.67 | 0.56 | 0.65 | 0.67 | 0.89 | 0.58 |
| Ca2+ | 6.66 | 4.48 | 4.83 | 3.05 | 5.29 | 4.93 | 6.66 | 5.76 | 7.47 | 5.59 | 3.26 | 5.37 |
| Total | 76.1 | 68.0 | 56.5 | 25.4 | 56.8 | 62.7 | 81.8 | 74.5 | 73.4 | 140.3 | 142.5 | 92.4 |
| Cl−/Na+ | 0.73 | 0.59 | 0.47 | 0.42 | 0.48 | 0.57 | 1.16 | 1.20 | 0.94 | 1.41 | 3.07 | 1.34 |
| NO3−/SO42− | 0.66 | 0.59 | 0.32 | 0.27 | 0.26 | 0.34 | 0.74 | 0.92 | 0.91 | 0.99 | 0.76 | 1.01 |
| Selected elements | ||||||||||||
| K | 1.55 | 2.09 | 1.34 | 0.52 | 1.05 | 1.22 | 1.98 | 1.64 | 1.92 | 2.11 | 4.37 | 1.39 |
| Al | 0.71 | 0.39 | 0.37 | 0.34 | 0.42 | 0.34 | 0.64 | 0.55 | 0.72 | 0.45 | 0.57 | 0.49 |
| Mg | 1.90 | 0.72 | 0.72 | 0.50 | 0.63 | 0.77 | 1.18 | 1.45 | 1.87 | 1.10 | 1.34 | 1.30 |
| Pb | 0.31 | 0.22 | 0.25 | 0.13 | 0.44 | 0.19 | 0.40 | 0.19 | 0.28 | 0.42 | 0.41 | 0.23 |
| Ba | 0.11 | 0.08 | 0.09 | 0.08 | 0.13 | 0.09 | 0.13 | 0.11 | 0.10 | 0.11 | 0.37 | 0.09 |
| Sr | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.07 | 0.02 |
| Total | 4.61 | 3.50 | 2.79 | 1.58 | 2.68 | 2.63 | 4.36 | 3.95 | 4.92 | 4.22 | 7.12 | 3.53 |
| Spring | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F− | Cl− | NO3− | SO42− |
| element # | 0.36 (Na) | n.a. | 0.95 ** (K) | 0.74 ** (Mg) | 0.86 ** (Ca) | n.a. | 0.81 ** (Cl) | n.a. | 0.84 ** (S) |
| Na+ | 1.00 | ||||||||
| NH4+ | 0.62 * | 1.00 | |||||||
| K+ | 0.04 | 0.18 | 1.00 | ||||||
| Mg2+ | 0.65 * | 0.53 * | −0.04 | 1.00 | |||||
| Ca2+ | 0.54 * | 0.47 | 0.06 | 0.77 ** | 1.00 | ||||
| F− | 0.49 | 0.43 | −0.10 | 0.68 * | 0.72 ** | 1.00 | |||
| Cl− | 0.78 ** | 0.72 ** | 0.04 | 0.74 ** | 0.58 * | 0.68 * | 1.00 | ||
| NO3− | 0.77 ** | 0.85 ** | 0.03 | 0.79 ** | 0.53 * | 0.62 * | 0.87 ** | 1.00 | |
| SO42− | 0.55 * | 0.90 ** | 0.30 | 0.48 | 0.65 * | 0.40 | 0.57 * | 0.65 * | 1.00 |
| Autumn | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F− | Cl− | NO3− | SO42− |
| element # | −0.27 (Na) | n.a. | 0.45 (K) | 0.80 ** (Mg) | 0.85 ** (Ca) | n.a. | 0.20 (Cl) | n.a. | 0.87 ** (S) |
| Na+ | 1.00 | ||||||||
| NH4+ | 0.04 | 1.00 | |||||||
| K+ | −0.04 | 0.26 | 1.00 | ||||||
| Mg2+ | 0.06 | −0.40 | 0.26 | 1.00 | |||||
| Ca2+ | −0.30 | −0.53 | 0.44 | 0.73 ** | 1.00 | ||||
| F− | −0.21 | −0.42 | 0.16 | 0.53 | 0.65 * | 1.00 | |||
| Cl− | 0.03 | 0.39 | 0.27 | −0.07 | −0.10 | 0.38 | 1.00 | ||
| NO3− | −0.09 | 0.86 ** | 0.30 | −0.14 | −0.20 | −0.20 | 0.23 | 1.00 | |
| SO42− | 0.16 | 0.87 ** | 0.48 | −0.19 | −0.30 | −0.41 | 0.34 | 0.67 * | 1.00 |
| Summer | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F− | Cl− | NO3− | SO42− |
| element # | 0.25 (Na) | n.a. | 0.88 ** (K) | 0.24 (Mg) | 0.68 * (Ca) | n.a. | 0.74 ** (Cl) | n.a. | 0.88 ** (S) |
| Na+ | 1.00 | ||||||||
| NH4+ | 0.45 | 1.00 | |||||||
| K+ | 0.44 | 0.71 ** | 1.00 | ||||||
| Mg2+ | 0.66 * | 0.81 ** | 0.74 ** | 1.00 | |||||
| Ca2+ | 0.46 | 0.69 * | 0.54 * | 0.86 ** | 1.00 | ||||
| F− | 0.09 | 0.47 | 0.68 * | 0.68 * | 0.60 * | 1.00 | |||
| Cl− | 0.36 | 0.47 | 0.90 ** | 0.59 * | 0.30 | 0.61 * | 1.00 | ||
| NO3− | 0.49 | 0.88 ** | 0.91 ** | 0.86 ** | 0.71 ** | 0.64 * | 0.75 ** | 1.00 | |
| SO42− | 0.60 * | 0.95 ** | 0.62 * | 0.85 ** | 0.76 ** | 0.41 | 0.36 | 0.80 ** | 1.00 |
| Winter | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F− | Cl− | NO3− | SO42− |
| element # | 0.16 (Na) | n.a. | 0.99 ** (K) | 0.83 ** (Mg) | 0.95 ** (Ca) | n.a. | 0.97 ** (Cl) | n.a. | 0.85 ** (S) |
| Na+ | 1.00 | ||||||||
| NH4+ | 0.40 | 1.00 | |||||||
| K+ | 0.12 | −0.12 | 1.00 | ||||||
| Mg2+ | 0.23 | −0.06 | 0.95 ** | 1.00 | |||||
| Ca2+ | 0.31 | 0.17 | −0.20 | 0.07 | 1.00 | ||||
| F− | 0.05 | 0.02 | 0.30 | 0.50 | 0.54 * | 1.00 | |||
| Cl− | 0.12 | 0.07 | 0.94 ** | 0.87 ** | −0.28 | 0.24 | 1.00 | ||
| NO3− | 0.34 | 0.95 ** | −0.16 | −0.08 | 0.32 | 0.16 | −0.03 | 1.00 | |
| SO42− | 0.52 * | 0.92 ** | 0.15 | 0.23 | 0.21 | 0.05 | 0.23 | 0.79 ** | 1.00 |
| Factors | F1 | F2 | F3 | Communalities |
|---|---|---|---|---|
| Cl− | 0.86 | 0.04 | −0.42 | 0.92 |
| NO3− | 0.66 | −0.56 | 0.27 | 0.82 |
| SO42− | 0.72 | −0.58 | 0.26 | 0.91 |
| NH4+ | 0.66 | −0.70 | 0.21 | 0.97 |
| K+ | 0.79 | 0.24 | −0.50 | 0.93 |
| Mg2+ | 0.87 | 0.38 | 0.03 | 0.90 |
| Ca2+ | 0.25 | 0.40 | 0.82 | 0.89 |
| K | 0.79 | 0.39 | −0.33 | 0.89 |
| Mg | 0.48 | 0.76 | 0.20 | 0.85 |
| Cl | 0.85 | 0.12 | −0.24 | 0.80 |
| Ca | 0.23 | 0.57 | 0.75 | 0.93 |
| S | 0.65 | −0.48 | 0.37 | 0.78 |
| Characteristic value | 5.62 | 2.80 | 2.18 | |
| Contribution rate/% | 46.9 | 23.3 | 18.1 | |
| Cumulative contribution rate/% | 46.9 | 70.2 | 88.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zheng, J.; Qu, C.; Zhang, J.; Wang, Y.; Zhan, C.; Yao, R.; Cao, J. Characteristics and Source Analysis of Water-Soluble Inorganic Ions in PM10 in a Typical Mining City, Central China. Atmosphere 2017, 8, 74. https://doi.org/10.3390/atmos8040074
Liu H, Zheng J, Qu C, Zhang J, Wang Y, Zhan C, Yao R, Cao J. Characteristics and Source Analysis of Water-Soluble Inorganic Ions in PM10 in a Typical Mining City, Central China. Atmosphere. 2017; 8(4):74. https://doi.org/10.3390/atmos8040074
Chicago/Turabian StyleLiu, Hongxia, Jingru Zheng, Chengkai Qu, Jiaquan Zhang, Yongkui Wang, Changlin Zhan, Ruizhen Yao, and Junji Cao. 2017. "Characteristics and Source Analysis of Water-Soluble Inorganic Ions in PM10 in a Typical Mining City, Central China" Atmosphere 8, no. 4: 74. https://doi.org/10.3390/atmos8040074





