The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe
Abstract
1. Introduction
2. Methods
2.1. Selected Locations
2.2. Sampling and Processing Cores
3. Results
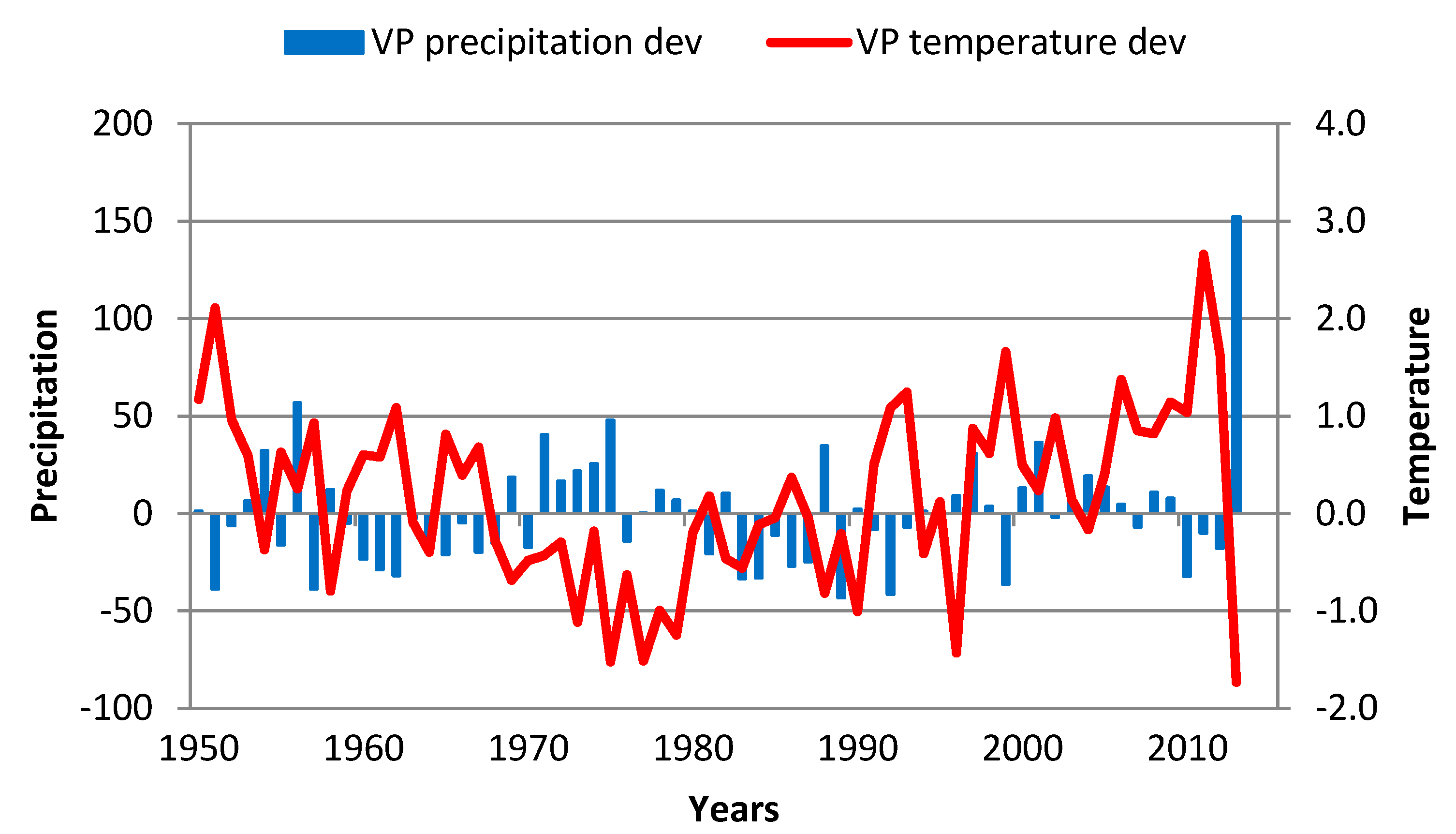
3.1. Observed Climate Change
3.2. Chronology of Beech Trees
3.3. Relationship between Climate and Radial Growth of Trees
4. Discussion
4.1. Kosmaj
4.2. Vrsacki Breg
- 1968: period 1966–1968 was very cold in Vrsacki Breg region with only a few months per year (February–June 1968, for example) with temperatures above normal. A cold winter and late summer and autumn were particularly important to the dramatic reduction in TRW (1.2) in this year;
- 1988: the decrease in TRW (0.7) was again caused by a cold period from March 1988 to the end of the year (except in July and August), when monthly temperatures were below normal and the precipitation was below average for the GS and over the year;
- 2001: the decrease in TRW (1.2) was the result of a very dry period in February 2000–March 2001, when monthly temperatures were permanently above normal and the precipitation was far below average in 2000.
4.3. Lopare
- 1987 (1988) had a very cold period from June 1986 to May 1987 when monthly average temperatures were permanently below Clim3. The 1988 winter (including November) was warmer than normal, and the cold spring (Mar–June) with temperatures again below normal resulted in TRW being far below the 1981–2010 average (3);
- 2003 had a cold period from August 2002 to April 2003, followed by a warm May–August period;
- 2012(2013) had very warm years 2011–2013 when (except February 2011 and 2012) temperatures were permanently above normal while precipitation was below normal inducing dry conditions.
4.4. Kukavica
- 1980 was quite a cold year (Figure 10) at this locality with temperatures below normal over the January–September period;
- 1988 had low temperatures during the March–December period (except in July and August) with GS precipitation (both in 1987and 1988) at approximately 25% below the GS average;
- 1994 had temperatures above normal during May 1993 to December 1994 (except November 93 and June 94), and 1993 GS precipitation was 40% below average;
- 2001 had dry conditions that continued from 2000 into 2001 in the form of reduced precipitation (monthly precipitation below average) in 7 out of the 10 first months.
4.5. Istocna Boranja
- 1988 had a warm autumn and winter of 1987 (September 1987–February 1988) and summer of 1988 (July–September 1988) and significantly (37%) reduced GS precipitation;
- 1994 had average GS precipitation, but from April 1993 to the end of 1994, the temperatures were above normal;
- 2001 had temperatures above normal from February 2000 to March 2001, and annual and GS precipitation levels in 2001 were 50% above average.
4.6. Mrkonjic Grad
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| CLIMATOLOGY 1 (1951–1980) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOCALITY | Months | Average | ||||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |||
| LOWER LIMIT | ||||||||||||||
| LOPARE (LO) | t (°C) | −1.0 | 1.4 | 5.3 | 10.2 | 14.6 | 18.2 | 19.5 | 19.1 | 15.3 | 10.3 | 5.7 | 1.1 | 10.0 |
| H(mm) | 49.0 | 49.0 | 45.0 | 61.0 | 81.0 | 89.0 | 79.0 | 67.0 | 56.0 | 51.0 | 65.0 | 67.0 | 63.3 | |
| SRBAC (SR) | t (°C) | −0.3 | 2.1 | 6.1 | 11.0 | 15.6 | 19.2 | 20.7 | 20.1 | 16.4 | 11.3 | 6.3 | 1.7 | 10.9 |
| H(mm) | 53.0 | 52.0 | 48.0 | 66.0 | 73.0 | 81.0 | 69.0 | 64.0 | 56.0 | 59.0 | 80.0 | 73.0 | 64.5 | |
| FRUSKA GORA (FG) | t (°C) | −1.2 | 1.1 | 5.4 | 10.8 | 15.7 | 19.2 | 20.6 | 19.9 | 16.0 | 10.8 | 5.8 | 1.1 | 10.4 |
| H (mm) | 38.0 | 41.0 | 37.0 | 47.0 | 63.0 | 82.0 | 64.0 | 55.0 | 39.0 | 40.0 | 53.0 | 54.0 | 51.1 | |
| KOSMAJ (KO) | t (°C) | −0.6 | 1.6 | 5.5 | 10.8 | 15.5 | 19.0 | 20.5 | 20.1 | 16.3 | 11.0 | 6.3 | 1.6 | 10.6 |
| H (mm) | 43.0 | 41.0 | 42.0 | 54.0 | 82.0 | 87.0 | 74.0 | 59.0 | 47.0 | 46.0 | 52.0 | 54.0 | 56.8 | |
| VRSACKI BREG (VB) | t (°C) | −0.5 | 1.5 | 5.8 | 11.3 | 16.2 | 19.6 | 21.1 | 20.8 | 17.0 | 11.6 | 6.6 | 1.8 | 11.1 |
| H (mm) | 40.0 | 42.0 | 35.0 | 52.0 | 74.0 | 83.0 | 64.0 | 52.0 | 40.0 | 39.0 | 50.0 | 54.0 | 52.1 | |
| OPTIMUM | ||||||||||||||
| VLASENICA (VL) | t (°C) | −2.9 | −0.9 | 2.6 | 7.0 | 11.6 | 15.1 | 16.6 | 16.5 | 13.0 | 8.2 | 3.7 | −0.9 | 7.5 |
| H (mm) | 54.0 | 53.0 | 50.0 | 65.0 | 87.0 | 88.0 | 81.0 | 68.0 | 66.0 | 68.0 | 76.0 | 71.0 | 68.9 | |
| MRKONJIC GRAD (MG) | t (°C) | −1.6 | 0.1 | 3.4 | 7.7 | 12.3 | 15.9 | 17.7 | 17.3 | 13.6 | 8.8 | 4.4 | 0.1 | 8.3 |
| H (mm) | 75.0 | 73.0 | 70.0 | 84.0 | 82.0 | 87.0 | 63.0 | 73.0 | 71.0 | 98.0 | 124.0 | 109.0 | 84.1 | |
| ISROCNA BORANJA (IB) | t (°C) | −1.5 | 0.8 | 4.5 | 9.4 | 13.9 | 17.5 | 18.8 | 18.5 | 14.8 | 9.8 | 5.2 | 0.6 | 9.4 |
| H (mm) | 48.0 | 47.0 | 46.0 | 61.0 | 87.0 | 92.0 | 83.0 | 71.0 | 59.0 | 53.0 | 64.0 | 64.0 | 64.6 | |
| ZELJIN (ZE) | t (°C) | −2.2 | 0.1 | 3.9 | 9.1 | 13.7 | 17.1 | 18.6 | 18.5 | 14.7 | 9.5 | 5.0 | 0.1 | 9.0 |
| H (mm) | 43.0 | 42.0 | 44.0 | 56.0 | 93.0 | 86.0 | 72.0 | 61.0 | 52.0 | 52.0 | 59.0 | 55.0 | 59.6 | |
| KUKAVICA (KU) | t (°C) | −1.3 | 1.1 | 4.8 | 10.0 | 14.6 | 18.1 | 19.8 | 19.6 | 15.7 | 10.4 | 5.7 | 0.8 | 9.9 |
| H (mm) | 37.0 | 42.0 | 45.0 | 51.0 | 70.0 | 69.0 | 50.0 | 46.0 | 44.0 | 51.0 | 60.0 | 53.0 | 51.5 | |
| UPPER LIMIT | ||||||||||||||
| JAHORINA (JA) | t (°C) | −2.9 | −1.3 | 1.9 | 5.9 | 10.6 | 14.1 | 15.8 | 15.9 | 12.4 | 7.7 | 3.5 | −1.0 | 6.9 |
| H (mm) | 64.0 | 61.0 | 56.0 | 64.0 | 78.0 | 79.0 | 68.0 | 62.0 | 70.0 | 86.0 | 90.0 | 80.0 | 71.5 | |
| ISTOCNI DRVAR (ID) | t (°C) | −2.9 | −1.5 | 1.6 | 5.7 | 10.4 | 14.0 | 15.8 | 15.5 | 11.9 | 7.2 | 3.0 | 1.2 | 6.6 |
| H (mm) | 80.0 | 77.0 | 79.0 | 93.0 | 87.0 | 90.0 | 64.0 | 81.0 | 79.0 | 111.0 | 139.0 | 116.0 | 91.3 | |
| GOLIJA (GO) | t (°C) | −5.0 | −2.9 | 0.6 | 5.5 | 10.2 | 13.5 | 15.0 | 14.9 | 11.4 | 6.7 | 2.5 | −2.5 | 5.8 |
| H (mm) | 44.0 | 39.0 | 41.0 | 50.0 | 81.0 | 77.0 | 69.0 | 62.0 | 55.0 | 64.0 | 68.0 | 56.0 | 58.8 | |
| VLASINA (V) | t (°C) | −4.8 | −2.9 | 0.3 | 5.3 | 10.0 | 13.5 | 15.4 | 15.4 | 11.6 | 6.5 | 2.0 | −2.7 | 5.8 |
| H (mm) | 35.0 | 38.0 | 44.0 | 53.0 | 83.0 | 83.0 | 60.0 | 53.0 | 42.0 | 51.0 | 54.0 | 49.0 | 53.8 | |
| BABIN ZUB (BZ) | t (°C) | −3.3 | −1.3 | 2.6 | 8.1 | 12.9 | 16.4 | 18.4 | 18.3 | 14.4 | 9.1 | 3.9 | −0.5 | 8.3 |
| H (mm) | 42.0 | 44.0 | 47.0 | 61.0 | 98.0 | 98.0 | 72.0 | 58.0 | 55.0 | 50.0 | 58.0 | 50.0 | 61.1 | |
| Lower limit | Optimum | Upper limit | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Lopare | Srbac | F Gora | Kosmaj | V Breg | Vlasenica | M Grad | I Boranja | Zeljin | Kukavica | Jahorina | I Drvar | Golija | Vlasina | B Zub |
| 1951 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| 1952 | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
| 1953 | * | * | * | * | * | * | * | * | * | * | * | * | |||
| 1954 | |||||||||||||||
| 1955 | |||||||||||||||
| 1956 | |||||||||||||||
| 1957 | * | * | * | * | * | * | * | * | * | * | |||||
| 1958 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 1959 | |||||||||||||||
| 1960 | * | * | * | * | * | * | * | * | * | * | |||||
| 1961 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 1962 | * | ||||||||||||||
| 1963 | * | * | * | ||||||||||||
| 1964 | |||||||||||||||
| 1965 | |||||||||||||||
| 1966 | * | * | |||||||||||||
| 1967 | * | * | * | * | * | * | * | ||||||||
| 1968 | * | * | * | * | * | * | * | ||||||||
| 1969 | |||||||||||||||
| 1970 | |||||||||||||||
| 1971 | * | * | * | ||||||||||||
| 1972 | * | * | * | * | * | * | |||||||||
| 1973 | |||||||||||||||
| 1974 | |||||||||||||||
| 1975 | * | * | * | * | |||||||||||
| 1976 | |||||||||||||||
| 1977 | * | ||||||||||||||
| 1978 | |||||||||||||||
| 1979 | * | * | * | * | |||||||||||
| 1980 | |||||||||||||||
| 1981 | |||||||||||||||
| 1982 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| 1983 | * | * | * | * | * | * | * | * | * | * | * | ||||
| 1984 | |||||||||||||||
| 1985 | * | ||||||||||||||
| 1986 | * | * | * | ||||||||||||
| 1987 | |||||||||||||||
| 1988 | * | * | * | * | * | * | * | * | * | * | * | * | |||
| 1989 | * | * | * | * | * | ||||||||||
| 1990 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 1991 | |||||||||||||||
| 1992 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| 1993 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| 1994 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 1995 | |||||||||||||||
| 1996 | |||||||||||||||
| 1997 | * | * | * | * | * | * | |||||||||
| 1998 | * | * | * | * | * | * | * | ||||||||
| 1999 | * | ||||||||||||||
| 2000 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 2001 | * | * | |||||||||||||
| 2002 | * | * | |||||||||||||
| 2003 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| 2004 | * | ||||||||||||||
| 2005 | |||||||||||||||
| 2006 | * | * | * | * | |||||||||||
| 2007 | * | * | |||||||||||||
| 2008 | * | * | * | * | * | * | * | * | * | * | * | * | |||
| 2009 | * | * | |||||||||||||
| 2010 | |||||||||||||||
| 2011 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| 2012 | * | * | * | * | * | * | * | * | * | * | * | ||||
| 2013 | * | * | * | * | * | * | * | * | |||||||
| 2014 | |||||||||||||||
| 2015 | * | * | * | * | * | ||||||||||
References
- Jovanović, B. Dendrologija sa Osnovama Fitocenologije; Naučna Knjiga: Beograd, Serbia, 1971. [Google Scholar]
- Lavadinović, V.; Isajev, V. Genetski potencijal semenskih objekata bukve u Srbiji- osnova za oplemenjivanje vrste. Zbornik radova Instituta za šumarstvo 2002, 46–47, 32–39. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Kompa, T. Thenorth-eastern distribution range of European beech–areview. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Mátyás, C.; Berki, I.; Czúcz, B.; Gálos, B.; Móricz, N.; Rasztovits, E. Future of beech in Southeast Europe from the perspective of evolutionary ecology. Acta Silvatica et Lignaria Hungarica 2010, 6, 91–110. [Google Scholar]
- Nabuurs, G.J.; Pussinen, A.; van Bruselen, J.; Schelhaas, M.J. Future harvesting pressure on European forests. Eur. J. For. Res. 2007, 126, 391–400. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Impacts of Europe’s Changing Climate; EEA Report No. 2; European Environmental Agency: Copenhagen, Denmark, 2004. [Google Scholar]
- Strengbom, J.; Dahlberg, A.; Larsson, A.; Lindelöw, A.; Sandström, J.; Widenfalk, O.; Gustafsson, L. Introducing Intensively Managed Spruce Plantations in Swedish Forest Landscapes will Impair Biodiversity Decline. Forests 2011, 2, 610–630. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Lexer, M.J. Climate change vulnerability of sustainable forest management in the Eastern Alps. Clim. Chang. 2011, 106, 225–254. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Maugeri, M.; Schirone, B.; Piovesan, G. Bioclimate and growth history affect beech lifespan in the Italian Alps and Apennines. Glob. Chang. Biol. 2012, 18, 960–972. [Google Scholar] [CrossRef]
- Seidling, W.; Ziche, D.; Beck, W. Climate responses and interrelations of stem increment and crown transparency in Norway spruce, Scots pine, and common beech. For. Ecol. Manag. 2012, 284, 196–204. [Google Scholar] [CrossRef]
- Van der Maaten, E. Climate sensitivity of radial growth in European beech (Fagus sylvatica L.) at different aspects in southwestern Germany. Trees 2012, 26, 777–788. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Latte, N.; Lebourgeois, F.; Claessens, H. Increased tree-growth synchronization of beech (Fagus sylvatica L.) in response to climate change in northwestern Europe. Dendrochronologia 2015, 33, 69–77. [Google Scholar]
- Müller-Haubold, H.; Hertel, D.; Seidel, D.; Knutzen, F.; Leuschner, C. Climate Responses of Aboveground Productivity and Allocation in Fagus sylvatica: A Transect Study in Mature Forests. Ecosystems 2013, 16, 1498–1516. [Google Scholar] [CrossRef]
- Pötzelsberger, E.; Wolfslehner, B.; Hasenauer, H. Climate change impacts on key forest functions of the Vienna Woods. Eur. J. For. Res. 2015, 134, 481–496. [Google Scholar] [CrossRef]
- Mette, T.; Dolos, K.; Meinardus, C.; Braüning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Pluess, A.R.; Walthert, L.; Rigling, A. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees 2013, 27, 171–181. [Google Scholar] [CrossRef]
- Matović, B.; Koprivica, M.; Kišin, B.; Stojanović, D.; Kneginjić, I.; Stjepanovć, S. Comparison of stand structure in managed and virgin european beech forests in Serbia. Šumarski List 2018, 142, 47–57. [Google Scholar] [CrossRef]
- Lakatos, F.; Molnar, M. Mass mortality of beech (Fagus sylvatica L.) in Suuth- West Hungary. Acta Silv. Lign. Hung. 2009, 5, 75–82. [Google Scholar]
- Stojanović, D. Uticaj klimatskih promena na vezivanje ugljenika, rast i biodiverzitet bukovih šuma u Srbiji. Doktorska disertacija, Prirodno-Matematički fakultet, Departman za Biologiju i ekologiju, Univerzitet u Novom Sadu, Novi Sad, Serbia, 2014. [Google Scholar]
- Stojanović, D.B.; Kržič, A.; Matović, B.; Orlović, S.; Duputie, A.; Djurdjević, V.; Galić, Z.; Stojnić, S. Prediction of the European beech (Fagus sylvatica L.) xeric limit using a regional climate model: An example from southeast Europe. Agric. For. Meteorol. 2013, 176, 94–103. [Google Scholar] [CrossRef]
- Garamvoelgyi, A.; Hufnagel, L. Impacts of climate change on vegetation distribution no.1. Climate change induced vegetation shifts in the Palearctic region. Appl. Ecol. Environ. Res. 2013, 11, 79–122. [Google Scholar] [CrossRef]
- Saltré, F.; Duputié, A.; Gaucherel, C.; Chuine, I. How climate, migration ability and habitat fragmentation affect the projected future distribution of European beech. Glob. Chang. Biol. 2015, 21, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Bugmann, H.; Klopcic, M.; Cailleret, M. Accurate modelling of harvesting is key for projecting future forest dynamics: A case study in the Slovenian mountains. Reg. Environ. Chang. 2017, 17, 49–64. [Google Scholar] [CrossRef]
- Hlásny, T.; Barcza, Z.; Fabrika, M.; Balázs, B.; Churkina, G.; Pajtík, J.; Sedmak, H.; Turcani, M. Climate change impacts on growth and carbon balance of forests in Central Europe. Clim. Res. 2011, 47, 219–236. [Google Scholar] [CrossRef]
- Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon? How trees may cope with more frequent climate change-type drought events. Glob. Chang. Biol. 2011, 17, 642–655. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Dursky, J. Modellierung der Absterbeprozesse in Rein–und Mischbeständen aus Fichte und Buche. Allgemeine Forst und Jagtzeitung 1997, 168, 131–134. [Google Scholar]
- Pretzsch, H.; Biber, P.; Dursky, J.; von Gadow, K.; Hasenauer, H.; Kändler, G.; Kenk, K.; Kublin, E.; Nage, J.; Pukkala, T.; et al. Recommendations for Standardized Documentation and Further Development of Forest Growth Simulators. Forstwissenschaftliches Centralblatt 2002, 121, 138–151. [Google Scholar] [CrossRef]
- Bošela, M.; Štefančík, I.; Petráš, R.; Vacek, S. The effects of climate warming on the growth of European beech forests depend critically on thinning strategy and site productivity. Agric. For. Meteorol. 2016, 222, 21–31. [Google Scholar] [CrossRef]
- Machar, I.; Vlckova, V.; Bucek, A.; Vozenilek, V.; Salek, L.; Jerabkova, L. Modelling of Climate Conditions in Forest Vegetation Zones as a Support Tool for Forest Management Strategy in European Beech Dominated Forests. Forests 2017, 8, 82. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, H.J.; Verbeeck, H. Drought-associated changes in climate and their relevance for ecosystem experiments and models. Biogeosciences 2011, 8, 1121–1130. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Anderegg, W.R.L.; Berry, J.A. Not all droughts are created equal: Translating meteorological drought into woody plant mortality. Tree Physiol. Rev. 2013, 33, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Budeanu, M.; Petritan, A.M.; Popescu, F.; Vasile, D.; Tudose, N.C. The resistance of European beech (Fagus sylvatica) from the eastern natural limit of species to climate change. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2016, 44, 625–633. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; pp. 107–113. ISBN 012268450-8. [Google Scholar]
- Lebourgeois, F.; Bréda, N.; Ulrich, E.; nd Granier, A. Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees 2005, 19, 385–401. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Bernabei, M.; Di Filippo, A.; Schirone, B. Spatial and altitudinal bioclimatic zones of the Italian Peninsula identified from a beech (Fagus sylvatica L.) tree-ring network. Acta Oecol. 2005, 27, 197–210. [Google Scholar] [CrossRef]
- DiFilippo, A.; Biondi, F.; Cufar, K.; deLuis, M.; Grabner, M.; Maugeri, M.; Saba, E.P.; Schirone, B.; Piovesan, G. Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: Spatial and altitudinal climatic signal sidentified through a tree-ring network. J. Biogeogr. 2007, 34, 1873–1892. [Google Scholar] [CrossRef]
- Biondi, F. Comparing tree-ring chronologies and repeated timber inventories as forest monitoring tools. Ecol. Appl. 1999, 9, 216–227. [Google Scholar] [CrossRef]
- Cook, E.R.; Glitzenstein, J.S.; Krusic, P.J.; Harcombe, P.A. Identifying functional groups of trees in west Gulf Coast forests (USA): A tree-ring approach. Ecol. Appl. 2001, 11, 883–903. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Araujo, M.B.; Jepson, P.; Ladle, R.J.; Watson, J.E.M.; Willis, K.J. Conservation biogeography: Assessment and prospect. Divers. Distrib. 2005, 11, 3–23. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Bolliger, J.; Gehrig-Fasel, J.; Guisan, A.; Kienast, F.; Lischeke, H.; Rickebusch, S.; Wohlgemuth, T. Wo wachsen die Baume in 100 Jahren? Wald und Klimalwandel. In Forum fur Wissen; Gassmann: Biel, Switzerland, 2006; pp. 63–71. [Google Scholar]
- Hacket-Pain, A.J.; Friend, A.D. Increased growth and reduced summer drought limitation at the southern limit of Fagus sylvatica L., despite regionally warmer and drier conditions. Dendrochronologia 2017, 44, 22–30. [Google Scholar] [CrossRef]
- Stojanović, B.D.; Levanic, T.; Matovic, B.; Stjepanovic, S.; Orlovic, S. Growth response of different tree species (oaks, beech and pine) from SE Europe to precipitation over time. Dendrobiology 2018, 79, 97–110. [Google Scholar] [CrossRef]
- Salamon-Albert, E.; Abaligeti, G.; Ortmann-Ajkai, A. Functional Response Trait Analysis Improves Climate Sensitivity Estimation in Beech Forests at a Trailing Edge. Forests 2017, 8, 324. [Google Scholar] [CrossRef]
- Cufar, K.; Prislan, P.; de Luis, M.; Gricar, J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica L.) from a representative site in Slovenia, SE Central Europe. Trees-Struct. Funct. 2008, 22, 749–758. [Google Scholar] [CrossRef]
- Garamszegi, B.; Kern, Z. Climate influence on radial growth of Fagus sylvatica growing near the edge of its distribution in Bükk Mts., Hungary. Dendrobiology 2014, 72, 93–102. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Fell, M.; Ogle, K. Legacy effects of drought in the southwestern United States: A multi-species synthesis. Ecol. Monogr. 2016, 86, 312–326. [Google Scholar] [CrossRef]
- Bhuyan, U.; Zang, C.; Menzel, A. Different responses of multispecies tree ring growth to various drought indices across Europe. Dendrochronologia 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Nahm, M.; Radoglou, K.; Rennenberg, H.; Halyvopoulos, G.; Matzarakis, A. Seasonal and interannual ecophysiological responses of beech (Fagus sylvatica) at its south-eastern distribution limit in Europe. For. Ecol. Manag. 2009, 257, 1157–1164. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change–range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Czúcz, B.; Galhidy, L.; Matyas, C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011, 68, 99–108. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe: A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schröder, C.; Wilmking, M. Droughtmatters–declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef]
- Tegel, W.; Seim, A.; Hakelberg, D.; Hoffmann, S.; Panev, M.; Westphal, T.; Büntgen, U. A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur. J. For. Res. 2014, 133, 61–71. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Oladi, R.; Pourtahmasi, R.; Eckstein, D.; Brauning, A. Seasonal dynamics of wood formation on Oriental beech (Fagus orientalis Lipsky) along an altitudinal gradient in the Hyrcanian forest, Iran. Trees 2011, 25, 425–433. [Google Scholar] [CrossRef]
- Radinovic, D.J. Weather and Climate of Yugoslavia; Civil Engineering Book: Belgrade, Serbia, 1979; pp. 1–283. [Google Scholar]
- European Climate Assessment Dataset. Available online: www.ecad.eu (accessed on 30 June 2018).
- Levanic, T. ATRICS—A new system for image acquisition in dendrochronology. Tree-Ring Res. 2007, 63, 117–122. [Google Scholar] [CrossRef]
- Larsson, L. CooRecorder and Cdendro programs of the CooRecorder/Cdendro package version 7.7. Available online: http://www.cybis.se/forfun/dendro (accessed on 3 January 2014).
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Pault, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; (Ted)Hog, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, S.J.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swtenam, T.W.; Rauscher, S.A.; Seager, R.; Gryssino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Engelbrecht, B.M.; Joswig, J.; Pereyra, G.; Schuldt, B.; Jansen, S.; Kattge, J.; Landhausser, S.M.; Levick, S.R.; Preisler, Y.; et al. A synthesis of tree functional traits related to drought-induced mortality in forests across climatic zones. J. Appl. Ecol. 2017, 54, 1669–1686. [Google Scholar] [CrossRef]
- Spiecker, H. Tree rings and forest management in Europe. Dendrochronologia 2002, 20, 191–202. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Springer-Science+Bussines Media, B.V.: Berlin/Heidelberg, Germany, 2013; pp. 289–339. ISBN 978-94-015-7879-0. (ebook). [Google Scholar]
- Beck, W. Growth patterns of forest stands-the response towards pollutants and climatic impact. iForest-Biogeosci. For. 2009, 2, 4–6. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur. J. For. Res. 2010, 129, 261–276. [Google Scholar] [CrossRef]
- Lalic, B.; Eitzinger, J.; Mihailovic, D.T.; Thaler, S.; Jancic, M. Climate change impacts on winter wheat yield change—which climatic parameters are crucial in Pannonian lowland? J. Agric. Sci. 2012, 151, 757–774. [Google Scholar] [CrossRef]
- Augustaitis, A.; Kliucius, A.; Marozas, V.; Pilkauskas, M.; Augustaitiene, I.; Vitas, A.; Staszewski, T.; Jansons, A.; Dreimanis, A. Sensitivity of European beech trees to unfavorable environmental factors on the edge and outside of their distribution range in north-eastern Europe. IForest 2015, 9, 259–269. [Google Scholar] [CrossRef]
- Casalegno, S.; Amatulli, G.; Bastrup-Birk, A.; Houston Durrant, T.; Pekkarinen, A. Modelling and mapping the suitability of European forest formations at 1-km resolution. Eur. J. For. Res. 2011, 130, 971–981. [Google Scholar] [CrossRef]
- Fang, J.; Lechowicz, M.L. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeography 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Bouriaud, O.; Breda, N.; Le Moguedec, G.; Nepveu, G. Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees 2004, 18, 264–276. [Google Scholar] [CrossRef]
- Čufar, K.; De Luis, M.; Eckstein, D.; Kajfez-Bogataj, L. Reconstructing dry and wet summers in SE Slovenia from oak tree rings series. Int. J. Biometeorol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Metyger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mucher, C.A.; Watkins, J.W. A climatic stratification of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Trnka, M.; Olesen, J.E.; Kersebaum, K.C.; Skjelvåg, A.O.; Eitzinger, J.; Seguin, B.; Peltonen-Sainio, P.; Rötter, R.; Iglesias, A.; Orlandini, S.; et al. Agroclimatic conditions in Europe under climate change. Glob. Chang. Biol. 2011, 17, 2298–2318. [Google Scholar] [CrossRef]











| Site | Altitude (m) | Coordinates | |
|---|---|---|---|
| Fruska Gora (FG) | 417 | N 45°08′09.0″ E 19°38′29.4″ | LOW |
| Kosmaj (KO) | 600 | N 44°27′57.4″ E 20°34′00.5″ | |
| Vrsacki Breg (VB) | 583 | N 45°07′55.4″ E 21°24′52.3″ | |
| Lopare (LO) | 353 | N 44°41′35.2″ E 18°48′28.2″ | |
| Srbac (SR) | 252 | N 45°05′23.2″ E 17°37′55.2″ | |
| Istocna Boranja (IB) | 823 | N 44°21′26.4″ E 19°16′43.4″ | OPT |
| Zeljin (ZE) | 1170 | N 43°30′00.2″ E 20°46′56.3″ | |
| Kukavica (KU) | 885 | N 42°48′35.1″ E 21°53′39.9″ | |
| Vlasenica (VL) | 1050 | N 44°10′06.0″ E 18°55′10.0″ | |
| Mrkonjic Grad (MG) | 1030 | N 44°23′53.5″ E 17°03′46.21″ | |
| Golija (GO) | 1580 | N 43°19′49.1″ E 20°14′29.0″ | UPP |
| Vlasina (V) | 1600 | N 42°35′38.4″ E 22°15′57.5″ | |
| Stara Planina- Babin zub (BZ) | 1640 | N 43°22′23.0″ E 22°36′41.4″ | |
| Jahorina (JA) | 1335 | N 43°41′36.0″ E 18°38′30.0″ | |
| Istocni Drvar (ID) | 1569 | N 44°23′36.0″ E 16°37′30.0″ |
| Location | Deviation for Clim2 | Deviation for Clim3 | Deviation for Clim4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | GS | Year | GS | Year | GS | |||||||
| Dt (°C) | DH (%) | Dt (°C) | DH (%) | Dt (°C) | DH (%) | Dt (°C) | DH (%) | Dt (°C) | DH (%) | Dt (°C) | DH (%) | |
| FG | 0.0 | −4.5 | 0.1 | −3.2 | 0.2 | −3.7 | 0.2 | 1.3 | 0.5 | 1.0 | 0.7 | 5.6 |
| KO | 0.0 | −2.3 | 0.0 | −3.8 | 0.2 | −1.7 | 0.1 | 0.2 | 0.6 | −2.5 | 0.7 | −3.3 |
| VB | −0.2 | −0.7 | −0.2 | 0.6 | 0.0 | −0.7 | −0.1 | 5.9 | 0.3 | −1.8 | 0.4 | 1.7 |
| LO | −0.1 | −0.4 | 0.0 | 0.7 | 0.1 | −2.1 | 0.1 | 0.4 | 0.4 | 2.7 | 0.6 | 4.5 |
| SR | −0.1 | −1.7 | 0.0 | −1.2 | 0.1 | −4.8 | 0.2 | −1.3 | 0.5 | −2.8 | 0.7 | 0.1 |
| IB | 0.0 | −0.9 | 0.0 | −1.0 | 0.2 | −0.6 | 0.1 | 0.7 | 0.6 | 2.7 | 0.7 | 2.2 |
| ZE | −0.1 | −1.8 | −0.1 | −3.9 | 0.1 | −1.8 | 0.0 | −1.7 | 0.5 | −1.6 | 0.6 | −3.7 |
| KU | −0.2 | −2.8 | −0.3 | −5.0 | −0.2 | −3.5 | −0.3 | −1.6 | 0.2 | −1.5 | 0.2 | −2.8 |
| VL | −0.1 | 2.9 | 0.0 | 2.3 | 0.1 | 3.5 | 0.1 | 4.4 | 0.5 | 7.3 | 0.7 | 6.3 |
| MG | −0.2 | 2.6 | −0.1 | 3.0 | 0.1 | 0.6 | 0.1 | 3.9 | 0.4 | 1.8 | 0.7 | 1.6 |
| GO | 0.0 | 0.8 | 0.0 | 0.3 | 0.2 | 5.8 | 0.1 | 7.1 | 0.6 | 7.6 | 0.7 | 4.6 |
| VS | −0.1 | −3.6 | −0.1 | −6.5 | 0.0 | −7.9 | 0.1 | −8.4 | 0.3 | −4.0 | 0.4 | −7.9 |
| BZ | 0.0 | −5.8 | 0.0 | −7.8 | 0.1 | −6.9 | 0.1 | −5.8 | 0.4 | −6.2 | 0.6 | −7.2 |
| JA | −0.1 | 3.1 | 0.0 | 5.9 | 0.2 | 3.1 | 0.2 | 5.9 | 0.5 | 6.6 | 0.7 | 8.4 |
| ID | −0.2 | 3.0 | −0.1 | 3.3 | 0.1 | 2.7 | 0.1 | 5.4 | 0.5 | 3.9 | 0.7 | 2.5 |
| Location | Clim1 | Clim2 | Clim3 | Clim4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | GS | Year | GS | Year | GS | Year | GS | |||||||||
| Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | Vt (%) | VH (%) | |
| FG | 6.2 | 18.2 | 4.3 | 26.8 | 5.0 | 16.6 | 3.7 | 24.6 | 6.3 | 19.1 | 4.8 | 24.4 | 6.8 | 23.8 | 4.1 | 28.0 |
| KO | 5.9 | 15.8 | 4.8 | 23.6 | 4.5 | 16.8 | 4.0 | 25.1 | 6.3 | 17.7 | 5.4 | 23.1 | 6.8 | 18.5 | 4.4 | 22.7 |
| VB | 5.9 | 17.6 | 4.9 | 27.5 | 4.6 | 18.7 | 3.8 | 27.2 | 6.0 | 22.0 | 5.0 | 26.5 | 6.7 | 23.9 | 4.4 | 29.0 |
| LO | 6.4 | 15.2 | 4.6 | 22.5 | 4.9 | 13.2 | 3.9 | 21.9 | 6.6 | 15.0 | 5.3 | 21.8 | 7.2 | 18.7 | 4.5 | 24.3 |
| SR | 5.9 | 16.1 | 4.2 | 23.5 | 4.9 | 15.3 | 3.6 | 23.6 | 6.2 | 16.0 | 4.8 | 23.9 | 6.4 | 19.5 | 4.0 | 24.0 |
| IB | 6.6 | 14.4 | 4.7 | 20.5 | 5.0 | 12.6 | 4.1 | 19.8 | 7.0 | 13.8 | 5.7 | 18.5 | 7.6 | 16.5 | 4.7 | 19.9 |
| ZE | 6.9 | 16.1 | 5.4 | 22.9 | 4.6 | 14.0 | 4.3 | 20.9 | 6.9 | 14.7 | 5.8 | 20.3 | 7.4 | 15.3 | 4.9 | 19.9 |
| KU | 7.0 | 14.1 | 5.7 | 24.8 | 4.6 | 13.0 | 4.4 | 25.2 | 6.0 | 16.0 | 5.4 | 24.9 | 6.9 | 17.2 | 4.7 | 22.3 |
| VL | 8.0 | 14.9 | 5.8 | 21.1 | 5.8 | 12.2 | 4.9 | 20.1 | 8.4 | 14.8 | 6.9 | 19.6 | 8.6 | 16.7 | 5.5 | 20.6 |
| MG | 8.4 | 17.7 | 5.8 | 25.0 | 5.5 | 13.9 | 4.6 | 24.4 | 8.5 | 14.3 | 6.7 | 23.3 | 8.0 | 16.8 | 5.2 | 19.4 |
| GO | 10.4 | 17.1 | 6.7 | 24.5 | 7.5 | 14.6 | 5.6 | 22.7 | 10.5 | 15.8 | 7.7 | 21.3 | 10.2 | 16.0 | 6.1 | 20.0 |
| VS | 10.3 | 13.3 | 7.0 | 22.6 | 7.4 | 15.0 | 5.7 | 26.3 | 10.6 | 19.0 | 7.8 | 28.6 | 9.7 | 23.2 | 6.1 | 26.5 |
| BZ | 6.5 | 13.1 | 4.5 | 21.1 | 4.9 | 12.0 | 3.8 | 22.0 | 6.4 | 15.8 | 4.9 | 22.8 | 7.5 | 17.6 | 4.5 | 21.8 |
| JA | 8.0 | 16.4 | 6.0 | 21.7 | 5.8 | 13.3 | 5.2 | 19.2 | 8.8 | 15.6 | 7.5 | 20.6 | 8.3 | 16.6 | 5.8 | 22.2 |
| ID | 10.2 | 18.0 | 6.6 | 25.5 | 7.0 | 14.2 | 5.4 | 25.2 | 10.8 | 14.3 | 7.9 | 23.3 | 9.8 | 15.6 | 6.0 | 18.9 |
| Locality | Period | Temperature (°C) | Precipitation (mm) | |
|---|---|---|---|---|
| Kosmaj | 1951–1980 | −0.4 | 0.4 | Lower limit |
| 1961–1990 | −0.4 | 0.5 | ||
| 1971–2000 | −0.5 | 0.7 | ||
| 1981–2010 | −0.3 | 0.7 | ||
| Vrsacki Breg | 1951–1980 | - | - | |
| 1961–1990 | −0.1 | 0.5 | ||
| 1971–2000 | −0.4 | 0.6 | ||
| 1981–2010 | −0.4 | 0.6 | ||
| Lopare | 1951–1980 | −0.2 | 0.2 | |
| 1961–1990 | −0.1 | 0.3 | ||
| 1971–2000 | −0.2 | 0.6 | ||
| 1981–2010 | −0.4 | 0.6 | ||
| Kukavica | 1951–1980 | 0.2 | 0.2 | Optimum |
| 1961–1990 | 0.2 | 0.5 | ||
| 1971–2000 | −0.2 | 0.6 | ||
| 1981–2010 | −0.4 | 0.6 | ||
| Istocna Boranja | 1951–1980 | 0.1 | 0.3 | |
| 1961–1990 | −0.1 | 0.5 | ||
| 1971–2000 | −0.4 | 0.7 | ||
| 1981–2010 | −0.4 | 0.7 | ||
| Mrkonjic Grad | 1951–1980 | - | - | |
| 1961–1990 | - | - | ||
| 1971–2000 | - | - | ||
| 1981–2010 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stjepanović, S.; Matović, B.; Stojanović, D.; Lalić, B.; Levanič, T.; Orlović, S.; Gutalj, M. The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe. Atmosphere 2018, 9, 451. https://doi.org/10.3390/atmos9110451
Stjepanović S, Matović B, Stojanović D, Lalić B, Levanič T, Orlović S, Gutalj M. The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe. Atmosphere. 2018; 9(11):451. https://doi.org/10.3390/atmos9110451
Chicago/Turabian StyleStjepanović, Stefan, Bratislav Matović, Dejan Stojanović, Branislava Lalić, Tom Levanič, Saša Orlović, and Marko Gutalj. 2018. "The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe" Atmosphere 9, no. 11: 451. https://doi.org/10.3390/atmos9110451
APA StyleStjepanović, S., Matović, B., Stojanović, D., Lalić, B., Levanič, T., Orlović, S., & Gutalj, M. (2018). The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe. Atmosphere, 9(11), 451. https://doi.org/10.3390/atmos9110451





