Atmospheric Distribution of PAHs and Quinones in the Gas and PM1 Phases in the Guadalajara Metropolitan Area, Mexico: Sources and Health Risk
Abstract
:1. Introduction
2. Experiments
2.1. The Guadalajara Metropolitan Area (GMA)
2.2. Sampling Sites
2.3. Samples Collection
2.4. Chemical Analyses
2.5. Samples’ Quality Control
2.6. Meteorology at the GMA
2.7. Statistical Analysis
2.8. Source Apportionment
2.9. Health-Risk Assessment
2.9.1. Benzo[a]pyrene (BaP) Equivalency
2.9.2. Exposure Concentrations for Assessing Cancer Risks
2.9.3. Cancer Risk Characterized by an Inhalation Unit Risk
3. Results and Discussion
3.1. Polycyclic Aromatic Hydrocarbons (PAHs)
3.1.1. Ambient Levels
3.1.2. Gas-particulate matter (PM1) Distribution
3.1.3. Source Apportionment
3.1.4. Health-Risk Assessment
3.2. Quinones
3.2.1. Ambient levels
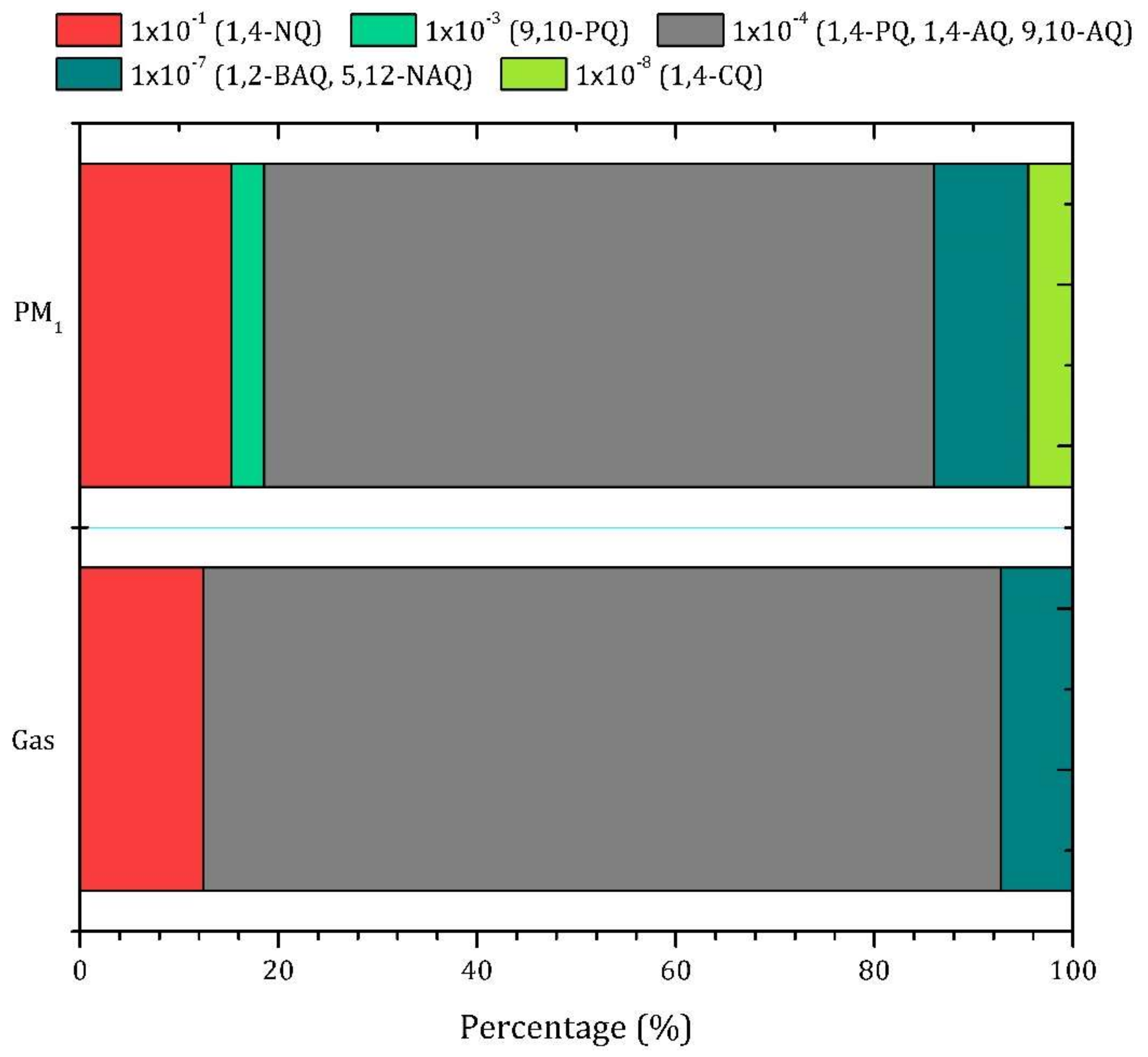
3.2.2. Gas-PM1 Distribution
3.2.3. Source Attribution
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jayakumar, T.; Nishigaki, I.; Nishigaki, Y.; Vetriselvi, J.; Sakthisekaran, D. Immunomodulatory Effect of Mangiferin in Experimental Animals with Benzo(a) Pyrene-induced Lung Carcinogenesis. Int. J. Biomed. Sci. 2013, 9, 61–67. [Google Scholar]
- Wenger, D.; Gerecke, A.C.; Heeb, N.V.; Hueglin, C.; Seiler, C.; Haag, R.; Naegeli, H.; Zenobi, R. Aryl hydrocarbon receptor-mediated activity of atmospheric particulate matter from an urban and a rural site in Switzerland. Atmos. Environ. 2009, 43, 3556–3562. [Google Scholar] [CrossRef]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110, 451–488. [Google Scholar] [CrossRef] [PubMed]
- Cuadras, A.; Rovira, E.; Marcé, R.M.; Marcé, R.M. Lung cancer risk by polycyclic aromatic hydrocarbons in a Mediterranean industrialized area. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Programme on Chemical Safety. Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbon; World Health Organization; International Programme on Chemical Safety: Geneva, Switzerland, 1998. [Google Scholar]
- Karavalakis, G.; Fontaras, G.; Ampatzoglou, D.; Kousoulidou, M.; Stournas, S.; Samaras, Z.; Bakeas, E. Effects of low concentration biodiesel blends application on modern passenger cars. Part 3: Impact on PAH, nitro-PAH, and oxy-PAH emissions. Environ. Pollut. 2010, 158, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Ringuet, J.; Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E. Reactivity of polycyclic aromatic compounds (PAHs, NPAHs and OPAHs) adsorbed on natural aerosol particles exposed to atmospheric oxidants. Atmos. Environ. 2012, 61, 15–22. [Google Scholar] [CrossRef]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone Chemistry and Toxicity. Toxicol. Appl. Pharmacol. 1992, 6, 2–16. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, D.; Bharti, S.K.; Kumar, A.; Pandey, A.K.; Ana, G.R.E.E.; Verma, A.; Khan, A.H.; Patel, D.K.; Mudiam, M.K.R.; Jain, S.K.; et al. Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int. J. Hyg. Environ. Health 2013, 216, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Douglas, A.L.; Yong, P.K. Formation of polyaromatic hydrocarbon (PAH)-quinones during the gas phase reactions of PAHs with the OH radical in the atmosphere. Environ. Chem. 2015, 12, 307–315. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Miguel, A.H.; Lu, R.; Purvis, K.; Grant, B.; Mayo, P.; Di Stefano, E.; Cho, A.K.; Froines, J. Atmospheric formation of 9,10-phenanthraquinone in the Los Angeles air basin. Atmos. Environ. 2008, 42, 2312–2319. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Miguel, A.H.; Di Stefano, E.; Schmitz, D.A.; Cho, A.K.; Thurairatnam, S.; Avol, E.L.; Froines, J.R. Atmospheric Distribution of Gas- and Particle-Phase Quinones in Southern California. Aerosol Sci. Technol. 2008, 42, 854–861. [Google Scholar] [CrossRef]
- Wnorowski, A.; Charland, J.-P. Profiling quinones in ambient air samples collected from the Athabasca region (Canada). Chemosphere 2017. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, M.; Stephanou, E.G. Diurnal Cycle of PAHs, Nitro-PAHs, and Oxy-PAHs in a high Oxidation Capacity Marine Background Atmosphere. Environ. Sci. Technol. 2007, 41, 8011–8017. [Google Scholar] [CrossRef]
- Lee, S.C.; Cheng, Y.; Ho, K.F.; Cao, J.J.; Louie, P.K.-K.; Chow, J.C.; Watson, J.G. PM1.0 and PM2.5 Characteristics in the Roadside Environment of Hong Kong. Aerosol Sci. Technol. 2006, 40, 157–165. [Google Scholar] [CrossRef]
- Perrone, M.R.; Becagli, S.; Garcia Orza, J.A.; Vecchi, R.; Dinoi, A.; Udisti, R.; Cabello, M. The impact of long-range-transport on PM1 and PM2.5 at a Central Mediterranean site. Atmos. Environ. 2013, 71, 176–186. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Alam, M.S.; Godri Pollitt, K.J.; Stark, C.; Harrison, R.M. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 2013, 77, 974–982. [Google Scholar] [CrossRef]
- Tomaz, S.; Jaffrezo, J.L.; Favez, O.; Perraudin, E.; Villenave, E.; Albinet, A. Sources and atmospheric chemistry of oxy- and nitro-PAHs in the ambient air of Grenoble (France). Atmos. Environ. 2017, 161, 144–154. [Google Scholar] [CrossRef]
- Barradas-Gimate, A.; Murillo-Tovar, M.; Díaz-Torres, J.; Hernández-Mena, L.; Saldarriaga-Noreña, H.; Delgado-Saborit, J.; López-López, A. Occurrence and Potential Sources of Quinones Associated with PM2.5 in Guadalajara, Mexico. Atmosphere 2017, 8, 140. [Google Scholar] [CrossRef]
- Sánchez, H.U.R.; García, M.D.A.; Bejaran, R.; Guadalupe, M.E.G.; Vázquez, A.W.; Toledano, A.C.P.; Villasenor, O.D.L.T. The spatial-temporal distribution of the atmospheric polluting agents during the period 2000–2005 in the Urban Area of Guadalajara, Jalisco, Mexico. J. Hazard. Mater. 2009, 165, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Escarela, G. Extreme value modeling for the analysis and prediction of time series of extreme tropospheric ozone levels: A case study. J. Air Waste Manag. Assoc. 2012, 62, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Nájera-Cedillo, M.; Márquez-Azúa, B.; Sánchez-Gómez, R.; Corona, J.P. Los sistemas de información geográfica como herramienta para observar el comportamiento del ozono en la Zona Metropolitana de Guadalajara. GEOS 2005, 25, 368–376. [Google Scholar]
- Tereshchenko, I.E.; Filonov, A.E. Acerca de las causas de las elevadas concentraciones de ozono en la atmósfera de la Zona Metropolitana de Guadalajara, en octubre de 1996. GEOS 1997, 2, 54–59. [Google Scholar]
- Davydova-Belitskaya, V.; Skiba, Y.N. Climate of Guadalajara City (Mexico), Its Variation and Change within Latest 120 Years. World Resour. Rev. 1999, 11, 258–270. [Google Scholar]
- US EPA. Evaluation and Estimation of Potential Carcinogenic Risks of Polynuclear Aromatic Hydrocarbons; US EPA: Washington, DC, USA, 1985. [Google Scholar]
- Jauregui, E.; Godinez, L.; Cruz, F. Aspects of heat-island development in Guadalajara, Mexico. Atmos. Environ. Part B Urban Atmos. 1992, 26, 391–396. [Google Scholar] [CrossRef]
- Tereshchenko, I.E.; Filonov, A.E. Air temperature fluctuations in Guadalajara, Mexico, from 1926 to 1994 in relation to urban growth. Int. J. Climatol. 2001, 21, 483–494. [Google Scholar] [CrossRef]
- Ulloa, H.; García, M.; Pérez, A.; Meulenert, A.; Ávila, D. Clima y radiación solar en las grandes ciudades: zona metropolitana de guadalajara (estado de Jalisco, Mexico). Investig. Geogr. 2011, 56, 165–175. [Google Scholar]
- Kim, M.; Kennicutt, M.C.; Qian, Y. Source characterization using compound composition and stable carbon isotope ratio of PAHs in sediments from lakes, harbor, and shipping waterway. Sci. Total Environ. 2008, 389, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Stout, S.A.; Wang, Z. Chemical Fingerprinting Methods and Factors Affecting Petroleum Fingerprints in the Environment. In Standard Handbook Oil Spill Environmental Forensics: Fingerprinting and Source Identification, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 61–129. ISBN 9780128096598. [Google Scholar]
- Stogiannidis, E.; Laane, R. Source Characterization of Polycyclic Aromatic Hydrocarbons by Using Their Molecular Indices: An Overview of Possibilities. In Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2013; Volume 234, pp. 49–133. ISBN 978-3-642-45397-7. [Google Scholar]
- Li, P.; Kong, S.; Geng, C.; Han, B.; Lu, B.; Sun, R.; Zhao, R.; Bai, Z. Health risk assessment for vehicle inspection workers exposed to airborne polycyclic aromatic hydrocarbons (PAHs) in their work place. Environ. Sci. Process. Impacts 2013, 15, 623. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Development of a relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures (External Review Draft); US EPA: Washington, DC, USA, 2010. [Google Scholar]
- Yang, T.T.; Lin, S.T.; Hung, H.F.; Shie, R.H.; Wu, J.J. Effect of Relative Humidity on Polycyclic Aromatic Hydrocarbon Emissions from Smoldering Incense. Aerosol Air Qual. Res. 2013, 13, 662–671. [Google Scholar] [CrossRef]
- US EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); Official Superfund Remediation Technology Innovation Environmental Protection Agency: Washington, DC, USA, 2009; Volume I, pp. 1–68.
- Agency for Toxic Substances and Disease Registry. Public Health Assessment Guidance Manual (Update); Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- World Health Organization. Polycyclic Aromatic Hydrocarbons; World Health Organization: Copenhagen, Denmark, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- Possanzini, M.; Di Palo, V.; Gigliucci, P.; Tomasi Scianò, M.C.; Cecinato, A. Determination of phase-distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos. Environ. 2004, 38, 1727–1734. [Google Scholar] [CrossRef]
- Melymuk, L.; Bohlin, P.; Pozo, K.; Sáňka, O.; Klánová, J. Current Challenges in Air Sampling of Semivolatile Organic Contaminants: Sampling Artifacts and Their Influence on Data Comparability. Environ. Sci. Technol. 2014, 48, 14077–14091. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Li, X.D.; Qi, S.H.; Liu, G.Q.; Peng, X.Z. Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou, South China. Sci. Total Environ. 2006, 355, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Vasilakos, C.; Levi, N.; Maggos, T.; Hatzianestis, J.; Michopoulos, J.; Helmis, C. Gas-particle concentration and characterization of sources of PAHs in the atmosphere of a suburban area in Athens, Greece. J. Hazard. Mater. 2007, 140, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Morville, S.; Delhomme, O.; Millet, M. Seasonal and diurnal variations of atmospheric PAH concentrations between rural, suburban and urban areas. Atmos. Pollut. Res. 2011, 2, 366–373. [Google Scholar] [CrossRef]
- Hytonen, K.; Yli-Pirila, P.; Tissari, J.; Grohn, A.; Riipinen, I.; Lehtinen, K.E.J.; Jokiniemi, J. Gas-Particle Distribution of PAHs in Wood Combustion Emission Determined with Annular Denuders, Filter, and Polyurethane Foam Adsorbent. Aerosol Sci. Technol. 2009, 43, 442–454. [Google Scholar] [CrossRef]
- Singh, D.K.; Gupta, T. Effect through inhalation on human health of PM1 bound polycyclic aromatic hydrocarbons collected from foggy days in northern part of India. J. Hazard. Mater. 2016, 306, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Wang, Y.-F.; Lin, T.-C.; Chen, Y.-Y.; Lin, W.-C.; Ku, C.-C.; Cheng, J.-T. PAH characteristics in the ambient air of traffic-source. Sci. Total Environ. 1995, 159, 185–200. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Slezakova, K.; Pires, J.C.M.; Castro, D.; Alvim-Ferraz, M.C.M.; Delerue-Matos, C.; Morais, S.; Pereira, M.C. PAH air pollution at a Portuguese urban area: Carcinogenic risks and sources identification. Environ. Sci. Pollut. Res. 2013, 20, 3932–3945. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A. Source Apportionment of Particle Bound Polycyclic Aromatic Hydrocarbons at an Industrial Location in Agra, India. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- De Abrantes, R.; De Assunção, J.V.; Pesquero, C.R. Emission of polycyclic aromatic hydrocarbons from light-duty diesel vehicles exhaust. Atmos. Environ. 2004, 38, 1631–1640. [Google Scholar] [CrossRef]
- Okona-Mensah, K.B.; Battershill, J.; Boobis, A.; Fielder, R. An approach to investigating the importance of high potency polycyclic aromatic hydrocarbons (PAHs) in the induction of lung cancer by air pollution. Food Chem. Toxicol. 2005, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira Mdo, C.; Morais, S. Exposure to polycyclic aromatic hydrocarbons and assessment of potential risks in preschool children. Environ. Sci. Pollut. Res. Int. 2015, 22, 13892–13902. [Google Scholar] [CrossRef] [PubMed]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene—An environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.G.; Toscano, W.A. Risk Assessment for Environmental Health; John Wiley & Sons, Inc.: San Francisco, CA, USA, 2007; ISBN 9780787983192. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Guidance Manual for the Assessment of Joint Toxic Action of Chemical Mixtures; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004.
- Bruce, E.D.; Abusalih, A.A.; McDonald, T.J.; Autenrieth, R.L. Comparing deterministic and probabilistic risk assessments for sites contaminated by polycyclic aromatic hydrocarbons (PAHs). J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2007, 42, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Serrano, J.; Carranco Ortiz, B.; Salmón Muñóz, J.; Amador González, S. Registro Estatal de Cáncer; Guadalajara, Mexico, 2010. Available online: https://ssj.jalisco.gob.mx/registros/51 (accessed on 20 January 2018).
- Chen, H.; Goldberg, M.S. The effects of outdoor air pollution on chronic illnesses. McGill J. Med. MJM 2009, 12, 58–64. [Google Scholar] [PubMed]
- Alam, M.S.; Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Using atmospheric measurements of PAH and quinone compounds at roadside and urban background sites to assess sources and reactivity. Atmos. Environ. 2013, 77, 24–35. [Google Scholar] [CrossRef]
- Sousa, E.T.; Lopes, W.A.; de Andrade, J.B. Fontes, Formação, Reatividade E Determinação De Quinonas Na Atmosfera. Quim. Nova 2016, 39, 486–495. [Google Scholar] [CrossRef]
- Wnorowski, A. Characterization of the ambient air content of parent polycyclic aromatic hydrocarbons in the Fort McKay region (Canada). Chemosphere 2017, 174, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Di Stefano, E.; You, Y.; Rodriguez, C.E.; Schmitz, D.A.; Kumagai, Y.; Miguel, A.H.; Eiguren-Fernandez, A.; Kobayashi, T.; Avol, E.; et al. Determination of Four Quinones in Diesel Exhaust Particles, SRM 1649a, and Atmospheric PM2.5 Special Issue of Aerosol Science and Technology on Findings from the Fine Particulate Matter Supersites Program. Aerosol Sci. Technol. 2004, 38, 68–81. [Google Scholar] [CrossRef]
- Jakober, C.A.; Riddle, S.G.; Robert, M.A.; Destaillats, H.; Charles, M.J.; Green, P.G.; Kleeman, M.J. Quinone emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol. 2007, 41, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Ringuet, J.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E.; Albinet, A. Particle size distribution of nitrated and oxygenated polycyclic aromatic hydrocarbons (NPAHs and OPAHs) on traffic and suburban sites of a European megacity: Paris (France). Atmos. Chem. Phys. 2012, 12, 8877–8887. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.O.; Durant, J.L.; Dookeran, N.M.; Taghizadeh, K.; Plummer, E.F.; Lafleur, A.L.; Sarofim, A.F.; Smith, K.A. Measurement of Oxygenated Polycyclic Aromatic Hydrocarbons associated with a size-segregated urban aerosol. Environ. Sci. Technol. 1997, 31, 2064–2070. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J.; Hering, S.V. Investigation of organic aerosol sampling artifacts in the Los Angeles basin. Atmos. Environ. 1994, 28, 3061–3071. [Google Scholar] [CrossRef]
- Wingfors, H.; Hägglund, L.; Magnusson, R. Characterization of the size-distribution of aerosols and particle-bound content of oxygenated PAHs, PAHs, and n-alkanes in urban environments in Afghanistan. Atmos. Environ. 2011, 45, 4360–4369. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lane, D.A. Unique products from the reaction of naphthalene with the hydroxyl radical. Atmos. Environ. 2009, 43, 4886–4893. [Google Scholar] [CrossRef]
- Jian, L.; Zhu, Y.P.; Zhao, Y. Monitoring fine and ultrafine particles in the atmosphere of a Southeast Chinese city. J. Environ. Monit. 2011, 13, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Patai, S. The Chemistry of the Quinonoid Compounds—Part 1; John Wiley & Sons, Inc.: New York, NY, USA, 1974; ISBN 0471669296. [Google Scholar]
- Moriconi, E.J.; O’Connor, W.F.; Taranko, L.B. Ozonolysis of Polycyclic Aromatics. V. Naphthacene and 5,12-Naphthacenequinone. Arch. Biochem. Biophys. 1959, 83, 283–290. [Google Scholar] [CrossRef]
- Agudelo-Castañeda, D.M.; Teixeira, E.C. Seasonal changes, identification and source apportionment of PAH in PM1.0. Atmos. Environ. 2014, 96, 186–200. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Arey, J.; Zielinska, B. Gas-phase atmospheric chemistry of 1-and 2-nitronaphthalene and 1,4-naphthoquinone. Atmos. Environ. 1989, 23, 2679–2690. [Google Scholar] [CrossRef]
- Guillard, C.; Delprat, H.; Hoang-Van, C.; Pichat, P. Laboratory Study of the Rates and Products of the Phototransformations of Naphthalene Adsorbed on Samples of Titanium Dioxide, Ferric Oxide, Muscovite, and Fly Ash. J. Atmos. Chem. 1993, 16, 47–59. [Google Scholar] [CrossRef]
- Wang, L.; Arey, J.; Atkinson, R. Kinetics and Products of Photolysis and Reaction with OH Radicals of a Series of Aromatic Carbonyl Compounds. Environ. Sci. Technol. 2006, 40, 5465–5471. [Google Scholar] [CrossRef] [PubMed]
- Bayona, J.M.; Maekides, K.E.; Lee, M.L. Characterization of Polar Polycyclic Aromatic Compounds in a Heavy-Duty Diesel Exhaust Particulate by Capillary Column Gas Chromatography and High-Resolution Mass Spectrometry. Environ. Sci. Technol. 1988, 22, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.F.; Carvalho, L.R.F.; Allen, A.G.; Cardoso, A.A. Diurnal and nocturnal measurements of PAH, nitro-PAH, and oxy-PAH compounds in atmospheric particulate matter of a sugar cane burning region. Atmos. Environ. 2014, 83, 193–201. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, T. Formation of nitroanthracene and anthraquinone from the heterogeneous reaction between NO2 and anthracene adsorbed on NaCl particles. Environ. Sci. Technol. 2014, 48, 8671–8678. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T. Atmospheric Chemistry of Polycyclic Aromatic Hydrocarbons and Related Compounds. J. Health Sci. 2011, 57, 504–511. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jariyasopit, N.; Schrlau, J.; Jia, Y.; Tao, S.; Yu, T.W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Simonich, S.L.M. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the beijing olympic games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef] [PubMed]
- Janhäll, S.; Jonsson, Å.M.; Molnár, P.; Svensson, E.A.; Hallquist, M. Size resolved traffic emission factors of submicrometer particles. Atmos. Environ. 2004, 38, 4331–4340. [Google Scholar] [CrossRef]





| Parameter | Minimum | Average | Maximum |
|---|---|---|---|
| Temperature (°C) | 16.1 | 24.3 | 32.4 |
| Relative humidity (%) | 14.0 | 46.0 | 91.0 |
| Wind speed (m s−1) | 0.0 | 1.8 | 6.7 |
| Solar radiation (W m−2) ** | 1.0 | 255.0 | 1111.0 |
| Compound | RPF |
|---|---|
| Flu | 0.08 |
| BaA | 0.2 |
| Chr | 0.1 |
| BbF | 0.8 |
| BkF | 0.03 |
| BaP | 1 |
| Ind | 0.07 |
| Dib | 10 |
| BghiP | 0.009 |
| GAS + PM1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH | CEN (ng m−3) | TLA (ng m−3) | Average of CEN and TLA (ng m−3) | ||||||||||||
| Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | |
| Nap | 61.5 | 18.3 | 36.6 | 103.4 | 59.9 | 50.3 | 22.4 | 9.12 | 83.2 | 49.6 | 52.1 | 18.5 | 30.1 | 93.3 | 52.6 |
| Ace | 34.2 | 15.4 | 12.2 | 67.1 | 30.6 | 33.8 | 15.7 | 4.05 | 72.9 | 32.8 | 31.9 | 12.5 | 15.2 | 56.9 | 28.4 |
| Acy | 16.0 | 4.6 | 10.3 | 24.2 | 15.1 | 11.6 | 4.83 | 2.05 | 22.7 | 13.1 | 12.8 | 4.09 | 6.85 | 18.7 | 13.2 |
| Fl | 7.72 | 5.33 | 0.711 | 14.84 | 8.49 | 5.48 | 3.84 | 0.506 | 11.6 | 3.51 | 5.22 | 3.05 | 1.26 | 12.9 | 5.26 |
| Phe | 17.1 | 4.18 | 10.9 | 24.4 | 17.4 | 12.9 | 4.25 | 2.48 | 19.5 | 13.3 | 13.9 | 4.16 | 6.31 | 20.1 | 15.4 |
| Ant | 3.16 | 2.21 | 0.475 | 6.68 | 1.96 | 3.06 | 2.61 | 0.303 | 10.3 | 1.85 | 2.91 | 1.68 | 0.576 | 7.56 | 2.93 |
| Flu | 2.73 | 2.07 | 0.254 | 6.78 | 2.03 | 2.67 | 2.14 | 0.0227 | 8.97 | 2.24 | 2.45 | 1.34 | 0.274 | 5.57 | 2.36 |
| Pyr | 2.25 | 2.16 | 0.0137 | 6.69 | 1.71 | 2.48 | 2.25 | 0.00163 | 8.34 | 1.99 | 2.22 | 1.26 | 0.0633 | 5.13 | 2.12 |
| BaA | 4.67 | 2.22 | 1.15 | 7.17 | 5.00 | 2.49 | 2.61 | 0.0145 | 6.55 | 1.78 | 2.31 | 1.23 | 0.0142 | 3.86 | 2.49 |
| Chr | 3.52 | 2.47 | 0.0261 | 8.30 | 3.88 | 3.73 | 3.76 | 0.0971 | 10.0 | 3.79 | 2.49 | 2.04 | 0.0812 | 6.94 | 2.29 |
| BbF | 3.76 | 3.53 | 0.0309 | 9.85 | 3.41 | 2.12 | 3.16 | 0.0378 | 12.0 | 0.493 | 2.71 | 2.72 | 0.0381 | 10.9 | 2.19 |
| BkF | 2.08 | 2.54 | 0.0281 | 7.86 | 0.714 | 1.88 | 3.23 | 0.0688 | 12.8 | 0.522 | 1.85 | 1.75 | 0.0484 | 6.74 | 1.62 |
| BaP | 2.25 | 2.44 | 0.00739 | 6.35 | 1.49 | 1.61 | 2.18 | 0.00658 | 6.28 | 0.207 | 1.59 | 1.17 | 0.107 | 3.19 | 1.71 |
| Ind | 2.33 | 2.52 | 0.0298 | 7.59 | 1.20 | 1.39 | 2.24 | 0.0211 | 7.28 | 0.435 | 1.71 | 1.28 | 0.0105 | 3.91 | 1.61 |
| Dib | 4.50 | 3.39 | 0.0383 | 8.99 | 4.71 | 2.52 | 3.27 | 0.0636 | 8.76 | 0.228 | 2.44 | 1.55 | 0.0379 | 4.52 | 2.71 |
| BghiP | 3.01 | 3.30 | 0.112 | 9.99 | 1.90 | 2.02 | 2.54 | 0.0284 | 8.10 | 0.582 | 2.07 | 1.47 | 0.0183 | 4.99 | 1.96 |
| Σ16PAH | 170.0 | 47.5 | - | - | - | 140.0 | 53.4 | - | - | - | 140.0 | 39.1 | - | - | - |
| PAHs | Gas (ng m−3) | PM1 (ng m−3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | |
| Nap | 54.2 | 17.9 | 30.1 | 93.3 | 53.2 | 0.207 | 0.184 | 0.00592 | 0.479 | 0.231 |
| Ace | 31.9 | 12.4 | 15.2 | 56.7 | 27.9 | 0.211 | 0.217 | 0.0187 | 0.726 | 0.113 |
| Acy | 12.8 | 4.01 | 6.85 | 18.7 | 12.8 | 0.229 | 0.237 | 0.00821 | 0.665 | 0.202 |
| Fl | 5.19 | 2.96 | 1.26 | 12.9 | 5.08 | 0.943 | 0.520 | 0.355 | 1.75 | 1.01 |
| Phe | 12.9 | 4.02 | 6.31 | 19.8 | 13.5 | 1.41 | 1.71 | 0.0394 | 4.34 | 0.259 |
| Ant | 2.50 | 1.18 | 0.576 | 4.29 | 2.72 | 0.572 | 1.39 | 0.00533 | 4.91 | 0.0471 |
| Flu | 2.47 | 1.34 | 0.250 | 5.56 | 2.51 | 0.0114 | 0.00839 | 0.00565 | 0.0239 | 0.00813 |
| Pyr | 2.22 | 1.26 | 0.00499 | 5.12 | 2.11 | 0.0171 | 0.0207 | 0.000539 | 0.0583 | 0.00827 |
| BaA | 1.85 | 1.16 | 0.0142 | 3.58 | 2.27 | 1.47 | 1.51 | 0.00726 | 3.59 | 1.03 |
| Chr | 1.89 | 0.999 | 0.0461 | 2.82 | 2.33 | 1.53 | 1.87 | 0.0283 | 4.31 | 0.668 |
| BbF | 1.39 | 1.23 | 0.00648 | 3.68 | 1.57 | 1.62 | 2.90 | 0.134 | 9.49 | 0.288 |
| BkF | 1.41 | 1.46 | 0.0115 | 4.45 | 1.48 | 0.541 | 0.599 | 0.160 | 2.29 | 0.271 |
| BaP | 1.55 | 1.19 | 0.00300 | 3.17 | 1.72 | 0.0947 | 0.0671 | 0.00406 | 0.214 | 0.0968 |
| Ind | 1.40 | 1.32 | 0.0100 | 3.73 | 1.34 | 0.498 | 0.26 | 0.174 | 0.978 | 0.409 |
| Dib | 2.63 | 1.35 | 0.0415 | 4.49 | 2.89 | 0.354 | 0.544 | 0.0318 | 1.29 | 0.0436 |
| BghiP | 1.95 | 1.13 | 0.0123 | 4.05 | 1.98 | 0.584 | 0.863 | 0.0968 | 3.01 | 0.337 |
| Σ16PAH | 137.0 | 37.7 | - | - | - | 7.25 | 8.34 | - | - | - |
| PAH Ratio | Value | Reported Range | Source |
|---|---|---|---|
| Phe/Phe + Ant | 0.83 | >0.70 | Vehicular |
| Flu/Flu + Pyr | 0.53 | >0.50 | Diesel |
| Ind/Ind + BghiP | 0.42 | 0.35–0.70 | Diesel |
| BbF/BkF | 1.5 | >0.50 | Diesel |
| BaP/BaP + Chr | 0.37 | 0.50 | Diesel |
| Compound | BaPeq | LCR |
|---|---|---|
| Flu | 0.195 | 2.15 × 10−4 |
| BaA | 0.433 | 1.89 × 10−4 |
| Chr | 0.249 | 2.17 × 10−4 |
| BbF | 1.35 | 2.36 × 10−4 |
| BkF | 0.0555 | 1.61 × 10−4 |
| BaP | 1.59 | 1.39 × 10−4 |
| Ind | 0.119 | 1.49 × 10−4 |
| Dib | 13.7 | 2.13 × 10−4 |
| BghiP | 0.0175 | 1.81 × 10−4 |
| BaPTeqΣ9PAH | 17.7 | 1.7 × 10−3 |
| GAS + PM1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quinone | CEN (ng m−3) | TLA (ng m−3) | Average of CEN and TLA (ng m−3) * | ||||||||||||
| Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | |
| 1,4-NQ | 1.31 | 1.25 | 0.0589 | 5.25 | 1.07 | 0.782 | 1.07 | 0.0646 | 3.59 | 0.367 | 0.978 | 1.04 | 0.0849 | 3.99 | 0.780 |
| 1,4-PQ | 6.22 | 3.74 | 1.58 | 15.6 | 5.75 | 4.60 | 3.52 | 1.02 | 15.1 | 3.88 | 5.21 | 2.87 | 0.891 | 10.5 | 5.07 |
| 9,10-PQ | 0.224 | 0.153 | 0.0371 | 0.457 | 0.215 | 0.209 | 0.181 | 0.0555 | 0.486 | 0.119 | 0.127 | 0.109 | 0.0185 | 0.375 | 0.0828 |
| 1,4-AQ | 0.776 | 0.140 | 0.645 | 0.925 | 0.759 | 1.12 | 0.518 | 0.491 | 1.75 | 1.12 | 0.567 | 0.185 | 0.322 | 0.872 | 0.561 |
| 9,10-AQ | 0.312 | 0.124 | 0.173 | 0.553 | 0.323 | 0.752 | 0.445 | 0.357 | 1.45 | 0.625 | 0.371 | 0.238 | 0.104 | 0.812 | 0.398 |
| 1,2-BAQ | 0.322 | 0.324 | 0.0320 | 0.950 | 0.180 | 0.328 | 0.385 | 0.0353 | 1.16 | 0.128 | 0.305 | 0.246 | 0.0466 | 0.892 | 0.226 |
| 5,12-NAQ | 0.390 | 0.319 | 0.0424 | 1.06 | 0.439 | 0.478 | 0.467 | 0.0450 | 1.48 | 0.216 | 0.421 | 0.308 | 0.0838 | 0.986 | 0.364 |
| 1,4-CQ | 0.482 | 0.474 | 0.0655 | 0.998 | 0.384 | 0.0687 | 0.0670 | 0.0382 | 0.156 | 0.0592 | 0.172 | 0.193 | 0.0191 | 0.499 | 0.0782 |
| Σ8Quinones | 8.17 | 4.10 | - | - | - | 6.74 | 3.86 | - | - | - | 7.46 | 2.88 | - | - | - |
| Quinones | Gas (ng m−3) | PM1 (ng m−3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. * | SD | Min | Max | Med | Ave. * | SD | Min | Max | Med | |||
| 1,4-NQ | 0.643 | 0.409 | 0.0306 | 1.32 | 0.761 | 0.596 | 1.14 | 0.00586 | 3.16 | 0.0465 | ||
| 1,4-PQ | 4.37 | 3.06 | 0.892 | 10.5 | 3.37 | 1.70 | 0.320 | 1.07 | 2.02 | 1.79 | ||
| 9,10-PQ | - | - | <LOD ** | - | - | 0.127 | 0.109 | 0.0186 | 0.375 | 0.0829 | ||
| 1,4-AQ | - | - | <LOD | - | - | 0.567 | 0.185 | 0.323 | 0.872 | 0.561 | ||
| 9,10-AQ | - | - | <LOD | - | - | 0.371 | 0.238 | 0.104 | 0.812 | 0.398 | ||
| 1,2-BAQ | 0.179 | 0.194 | 0.00770 | 0.564 | 0.068 | 0.155 | 0.198 | 0.00349 | 0.682 | 0.0751 | ||
| 5,12-NAQ | 0.247 | 0.222 | 0.0121 | 0.696 | 0.219 | 0.214 | 0.240 | 0.0447 | 0.795 | 0.0887 | ||
| 1,4-CQ | - | - | <LOD | - | - | 0.172 | 0.193 | 0.0191 | 0.499 | 0.0782 | ||
| Σ8Quinones | 5.44 | 3.44 | 2.32 | 1.16 | ||||||||
| PAHs-Quinones | r |
|---|---|
| Nap(p) vs. 1,4-NQ(p) | 0.83 * |
| Phe(p) vs. 1,4-PQ(p) | 0.77 * |
| Phe(p) vs. 9,10-PQ(p) | 0.57 |
| Ant(p) vs. 1,4-AQ(p) | 0.40 |
| 1,4-NQ(p) vs. 5,12-NAQ(p) | 0.75 * |
| Nap(p) vs. 5,12-NAQ(p) | 0.75 |
| BaA(g) vs. 1,2-BAQ(g) | 0.46 |
| BaA(g) vs. 1,2-BAQ(p) | −0.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Castillo, V.; López-López, A.; Hernández-Mena, L.; Murillo-Tovar, M.A.; Díaz-Torres, J.D.J.; Hernández-Paniagua, I.Y.; Del Real-Olvera, J.; León-Becerril, E. Atmospheric Distribution of PAHs and Quinones in the Gas and PM1 Phases in the Guadalajara Metropolitan Area, Mexico: Sources and Health Risk. Atmosphere 2018, 9, 137. https://doi.org/10.3390/atmos9040137
Ojeda-Castillo V, López-López A, Hernández-Mena L, Murillo-Tovar MA, Díaz-Torres JDJ, Hernández-Paniagua IY, Del Real-Olvera J, León-Becerril E. Atmospheric Distribution of PAHs and Quinones in the Gas and PM1 Phases in the Guadalajara Metropolitan Area, Mexico: Sources and Health Risk. Atmosphere. 2018; 9(4):137. https://doi.org/10.3390/atmos9040137
Chicago/Turabian StyleOjeda-Castillo, Valeria, Alberto López-López, Leonel Hernández-Mena, Mario Alfonso Murillo-Tovar, José De Jesús Díaz-Torres, Iván Y. Hernández-Paniagua, Jorge Del Real-Olvera, and Elizabeth León-Becerril. 2018. "Atmospheric Distribution of PAHs and Quinones in the Gas and PM1 Phases in the Guadalajara Metropolitan Area, Mexico: Sources and Health Risk" Atmosphere 9, no. 4: 137. https://doi.org/10.3390/atmos9040137
APA StyleOjeda-Castillo, V., López-López, A., Hernández-Mena, L., Murillo-Tovar, M. A., Díaz-Torres, J. D. J., Hernández-Paniagua, I. Y., Del Real-Olvera, J., & León-Becerril, E. (2018). Atmospheric Distribution of PAHs and Quinones in the Gas and PM1 Phases in the Guadalajara Metropolitan Area, Mexico: Sources and Health Risk. Atmosphere, 9(4), 137. https://doi.org/10.3390/atmos9040137







