Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays
Abstract
Highlights
- Pyrotechnics emit PM10-bound heavy metals that degrade ambient air quality
- Pb/Ca, Pb/Al, Pb/Mg, and Pb/Cu ratios can pinpoint emissions from firework displays
- Pb and Ba are possible tracers, with elevated concentrations during firework displays
- PM10 metals in ambient air are mainly crustal emissions regardless of firework events
- Limited combined risk associated with Pb, Cr, Co., Ni, Zn, As, Cd, V, and Mn levels
- As and Cr levels exceed US-EPA guidelines for lifetime cancer risk
1. Introduction
2. Materials & Methods
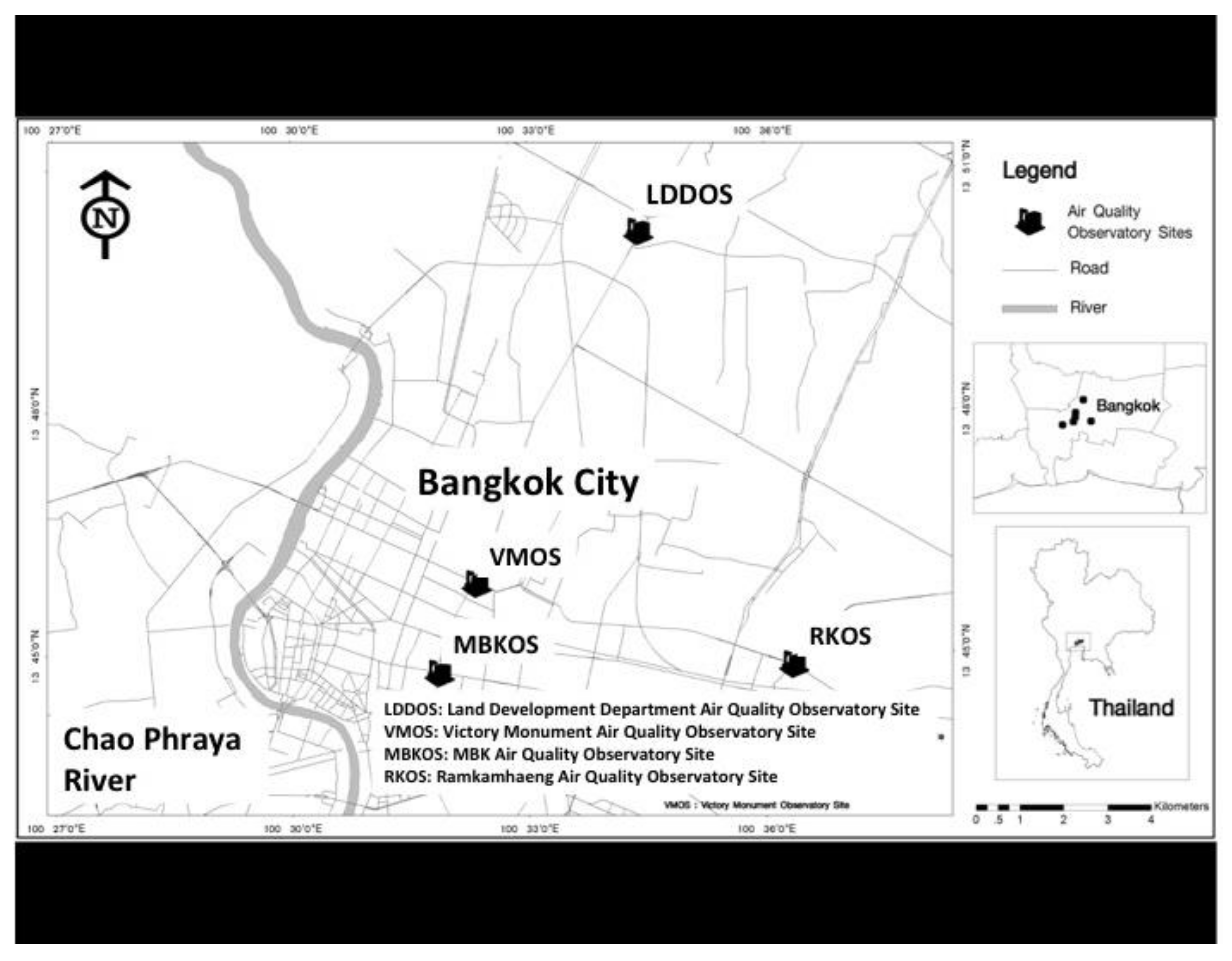
2.1. Air Quality Observation Sites
2.2. Chemical Analysis of Selected Metals
2.3. Health Risk Assessment of Selected Metals
2.4. Enrichment Factors of Selected Metals
2.5. Estimation of Mineral Matter in PM10
2.6. Back Trajectory Analysis
3. Results and Discussion
3.1. Diagnostic Binary Ratios of Selected Metals
3.2. Enrichment Factors of Selected Metals
3.3. Hazard Quotients, Hazard Indexes, and Cancer Risks of Selected Metals
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amodio, M.; Caselli, M.; de Gennaro, G.; Tutino, M. Particulate PAHs in two urban areas of Southern Italy: Impact of the sources, meteorological and background conditions on air quality. Environ. Res. 2009, 109, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Bozlaker, A.; Muezzinoglu, A.; Odabasi, M. Atmospheric concentrations, dry deposition and air–soil exchange of polycyclic aromatic hydrocarbons (PAHs) in an industrial region in Turkey. J. Hazard. Mater. 2008, 153, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Pochmanová, D.; Pastorková, A.; Beneš, I.; Leníček, J.; Topinka, J.; Binková, B. Genotoxicity of urban air pollutants in the Czech Republic: Part I. bacterial mutagenic potencies of organic compounds adsorbed on PM10 particulates. Mutat Res. Gen. Tox. Environ. 2002, 469, 71–82. [Google Scholar] [CrossRef]
- Garban, B.; Blanchoud, H.; Motelay-Massei, A.; Chevreuil, M.; Ollivon, D. Atmospheric bulk deposition of PAHs onto France: Trends from urban to remote sites. Atmos. Environ. 2002, 36, 5395–5403. [Google Scholar] [CrossRef]
- Guo, H.; Lee, S.C.; Ho, K.F.; Wang, X.M.; Zou, S.C. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos. Environ. 2003, 37, 5307–5317. [Google Scholar] [CrossRef]
- Halsall, C.J.; Lee, R.G.; Coleman, P.J.; Burnett, V.; Harding-Jones, P.; Jones, K.C. PCBs in UK urban air. Environ. Sci. Technol. 1995, 29, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, S. Diurnal variation, vertical distribution and source apportionment of carcinogenic polycyclic aromatic hydrocarbons (PAHs) in Chiang-Mai, Thailand. Asian Pac. J. Cancer 2013, 14, 1851–1863. [Google Scholar] [CrossRef]
- Pongpiachan, S. Vertical distribution and potential risk of particulate polycyclic aromatic hydrocarbons in high buildings of Bangkok, Thailand. Asian Pac. J. Cancer 2013, 14, 1865–1877. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Choochuay, C.; Hattayanone, M.; Kositanont, C. Temporal and spatial distribution of particulate carcinogens and mutagens in Bangkok, Thailand. Asian Pac. J. Cancer 2013, 14, 1879–1887. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Kositanont, C.; Palakun, J.; Liu, S.; Ho, K.F.; Cao, J. Effects of day-of-week trends and vehicle types on PM2.5-bounded carbonaceous compositions. Sci. Total Environ. 2015, 532, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, S.; Hattayanone, M.; Choochuay, C.; Mekmok, R.; Wuttijak, N.; Ketratanakul, A. Enhanced PM10 bounded PAHs from shipping emissions. Atmos. Environ. 2015, 108, 13–19. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Hattayanone, M.; Suttinun, O.; Khumsup, C.; Kittikoon, I.; Hiruyatrakul, P.; Cao, J. Assessing human exposure to PM10-bound polycyclic aromatic hydrocarbons during fireworks displays. Atmos. Pollut. Res. 2017, 8, 816–827. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Liu, S.; Huang, R.; Zhao, Z.; Palakun, J.; Kositanont, C.; Cao, J. Variation in day-of-week and seasonal concentrations of atmospheric PM2.5-bound metals and associated health risks in Bangkok, Thailand. Arch. Environ. Contam. Toxicol. 2017, 72, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, S.; Mattanawadee, H.; Junji, C. Effect of agricultural waste burning season on PM 2.5-bound polycyclic aromatic hydrocarbon (PAH) levels in Northern Thailand. Atmos. Pollut. Res. 2017, 8, 1069–1080. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Hattayanone, M.; Tipmanee, D.; Suttinun, O.; Khumsup, C.; Kittikoon, I.; Hirunyatrakul, P. Chemical characterization of polycyclic aromatic hydrocarbons (PAHs) in 2013 Rayong oil spill-affected coastal areas of Thailand. Environ. Pollut. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, H.E.; Swanson, G.M.; Fischer, L.J. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J. Toxicol. Environ. Health 1996, 49, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, P.J.; Shao, L.M. Fate of heavy metals during municipal solid waste incineration in Shanghai. J. Hazard. Mater. 2008, 156, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, S.; Everett, M.; McCarthy, R.; Ordóñez, A.; de Miguel, E. A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry, West Midlands, UK. Environ. Int. 2003, 29, 563–573. [Google Scholar] [CrossRef]
- Madrid, F.; Barrientos, E.D.; Madrid, L. Availability and bio-accessibility of metals in the clay fraction of urban soils of Sevilla. Environ. Pollut. 2008, 156, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Yatkin, S.; Bayram, A. Determination of major natural and anthropogenic source profiles for particulate matter and trace elements in Izmir, Turkey. Chemosphere 2008, 71, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Yatkin, S.; Bayram, A. Source apportionment of PM10 and PM2.5 using positive matrix factorization and chemical mass balance in Izmir, Turkey. Sci. Total Environ. 2008, 390, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, Y.; Xu, Z. PM10 and PM2.5 and health risk assessment for heavy metals in a typical factory for cathode ray tube television recycling. Environ. Sci. Technol. 2013, 47, 12469–12476. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Lu, B.; Ji, Y.; Zhao, X.; Chen, L.; Li, Z.; Han, B.; Bai, Z. Levels, risk assessment and sources of PM 10 fraction heavy metals in four types dust from a coal-based city. Microchem. J. 2011, 98, 280–290. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Li, L.Y.; Chen, H. Assessment of metals pollution and health risk in dust from nursery schools in Xi’an, China. Environ. Res. 2014, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, S.; Zhang, X.; Wu, D.; Luo, W.; Zhang, T.; Zhou, S.; Yang, G.; Wan, H.; Wu, L. Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J. Geochem. Explor. 2015, 148, 71–78. [Google Scholar] [CrossRef]
- Christoforidis, A.; Stamatis, N. Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 2009, 151, 257–263. [Google Scholar] [CrossRef]
- Duong, T.T.; Lee, B.K. Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. J. Environ. Manag. 2011, 92, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Norman, M.; Burman, L. Road traffic emission factors for heavy metals. Atmos. Environ. 2009, 43, 4681–4688. [Google Scholar] [CrossRef]
- Ndiokwere, C.L. A study of heavy metal pollution from motor vehicle emissions and its effect on roadside soil, vegetation and crops in Nigeria. Environ. Pollut. B 1984, 7, 35–42. [Google Scholar] [CrossRef]
- Jianguo, J.; Jun, W.; Xin, X.; Wei, W.; Zhou, D.; Yan, Z. Heavy metal stabilization in municipal solid waste incineration flyash using heavy metal chelating agents. J. Hazard. Mater. 2004, 113, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Meij, R.; te Winkel, H. The emissions of heavy metals and persistent organic pollutants from modern coal-fired power stations. Atmos. Environ. 2007, 41, 9262–9272. [Google Scholar] [CrossRef]
- Reddy, M.S.; Basha, S.; Joshi, H.V.; Jha, B. Evaluation of the emission characteristics of trace metals from coal and fuel oil fired power plants and their fate during combustion. J. Hazard. Mater. 2005, 123, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sushil, S.; Batra, V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in India. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Dahl, O.; Nurmesniemi, H.; Pöykiö, R.; Watkins, G. Comparison of the characteristics of bottom ash and fly ash from a medium-size (32 MW) municipal district heating plant incinerating forest residues and peat in a fluidized-bed boiler. Fuel Process. Technol. 2009, 90, 871–878. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, K.H.; Jang, H.N.; Seo, Y.C.; Seok, K.S.; Hong, J.H.; Jang, M. Emission characteristics of particulate matter and heavy metals from small incinerators and boilers. Atmos. Environ. 2002, 36, 5057–5066. [Google Scholar] [CrossRef]
- Fujimori, T.; Itai, T.; Goto, A.; Asante, K.A.; Otsuka, M.; Takahashi, S.; Tanabe, S. Interplay of metals and bromine with dioxin-related compounds concentrated in e-waste open burning soil from Agbogbloshie in Accra, Ghana. Environ. Pollut. 2016, 209, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, K.; Wu, W.; Tian, H.; Yi, P.; Zhi, G.; Fan, J.; Liu, S. Atmospheric emissions of typical toxic heavy metals from open burning of municipal solid waste in China. Atmos. Environ. 2017, 152, 6–15. [Google Scholar] [CrossRef]
- Betha, R.; Pradani, M.; Lestari, P.; Joshi, U.M.; Reid, J.S.; Balasubramanian, R. Chemical speciation of trace metals emitted from Indonesian peat fires for health risk assessment. Atmos. Res. 2013, 122, 571–578. [Google Scholar] [CrossRef]
- Breulmann, G.; Markert, B.; Weckert, V.; Herpin, U.; Yoneda, R.; Ogino, K. Heavy metals in emergent trees and pioneers from tropical forest with special reference to forest fires and local pollution sources in Sarawak, Malaysia. Sci. Total Environ. 2002, 285, 107–115. [Google Scholar] [CrossRef]
- Camilleri, R.; Vella, A.J. Effect of fireworks on ambient air quality in Malta. Atmos. Environ. 2010, 44, 4521–4527. [Google Scholar] [CrossRef]
- Kulshrestha, U.C.; Rao, T.N.; Azhaguvel, S.; Kulshrestha, M.J. Emissions and accumulation of metals in the atmosphere due to crackers and sparkles during Diwali festival in India. Atmos. Environ. 2004, 38, 4421–4425. [Google Scholar] [CrossRef]
- Sarkar, S.; Khillare, P.S.; Jyethi, D.S.; Hasan, A.; Parween, M. Chemical speciation of respirable suspended particulate matter during a major firework festival in India. J. Hazard. Mater. 2010, 184, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.J.; Birnbaum, A.N. Effects of Independence Day fireworks on atmospheric concentrations of fine particulate matter in the United States. Atmos. Environ. 2015, 115, 192–198. [Google Scholar] [CrossRef]
- Steinhauser, G.; Sterba, J.H.; Foster, M.; Grass, F.; Bichler, M. Heavy metals from pyrotechnics in New Years Eve snow. Atmos. Environ. 2008, 42, 8616–8622. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Wang, J.; Peng, X.; Shi, G.L.; Feng, Y.C. Estimation of the direct and indirect impacts of fireworks on the physicochemical characteristics of atmospheric PM10 and PM2.5. Atmos. Chem. Phys. 2014, 14, 9469–9479. [Google Scholar] [CrossRef]
- Feng, J.; Yu, H.; Su, X.; Liu, S.; Li, Y.; Pan, Y.; Sun, J.H. Chemical composition and source apportionment of PM 2.5 during Chinese spring festival at Xinxiang, a heavily polluted city in north China: Fireworks and health risks. Atmos. Res. 2016, 182, 176–188. [Google Scholar] [CrossRef]
- Tsai, H.H.; Chien, L.H.; Yuan, C.S.; Lin, Y.C.; Jen, Y.H.; Ie, I.R. Influences of fireworks on chemical characteristics of atmospheric fine and coarse particles during Taiwan’s Lantern Festival. Atmos. Environ. 2012, 62, 256–264. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Xu, C.; An, Z. The air pollution caused by the burning of fireworks during the lantern festival in Beijing. Atmos. Environ. 2007, 41, 417–431. [Google Scholar] [CrossRef]
- Winberry, W.T.; Murphy, N.T.; Riggin, R.M. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air; Atmospheric Research and Exposure Assessment Laboratory, Office of Research and Development, US Environmental Protection Agency: Triangle Park, NC, USA, 1988. [Google Scholar]
- Iijima, A.; Sato, K.; Fujitani, Y.; Fujimori, E.; Saitoh, Y.; Tanabe, K.; Ohara, T.; Kozawa, K.; Furuta, N. Clarification of the predominant emission sources of antimony in airborne particulate matter and estimation of their effects on the atmosphere in Japan. Environ. Chem. 2009, 6, 122–135. [Google Scholar] [CrossRef]
- Iijima, A.; Sato, K.; Ikeda, T.; Sato, H.; Kozawa, K.; Furuta, N. Concentration distributions of dissolved Sb(III) and Sb(V) species in size-classified inhalable airborne particulate matter. J. Anal. Atomic Spectrom. 2010, 25, 356–363. [Google Scholar] [CrossRef]
- Granero, S.; Domingo, J. Levels of metals in soils of Alcala de Henares, Spain: Human health risks. Environ. Int. 2012, 28, 159–164. [Google Scholar] [CrossRef]
- Kong, S.F.; Lu, B.; Ji, Y.Q.; Zhao, X.Y.; Bai, Z.P.; Xu, Y.H.; Liu, Y.; Jiang, H. Risk assessment of heavy metals in road and soil dust within PM2.5, PM10 and PM100 fractions in Dongying city, Shandong Province, China. J. Environ. Monit. 2012, 14, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Pan, Y.P.; Wang, Y.S.; Wang, Y.F.; Li, X.R. Chemical characteristics and sources of trace metals in precipitation collected from a typical industrial city in Northern China. Huan Jing Ke Xue 2012, 33, 3712–3717. (In Chinese) [Google Scholar] [PubMed]
- Wang, W.; Cheng, S.; Zhang, D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014, 135, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Zhang, Y.; Ding, M.; Li, L. Identification of traffic-related metals and the effects of different environments on their enrichment in roadside soils along the Qinghai-Tibet highway. Sci. Total Environ. 2015, 521–522, 160–172. [Google Scholar] [CrossRef] [PubMed]
- López, J.M.; Callén, M.S.; Murillo, R.; García, T.; Navarro, M.V.; de la Cruz, M.T.; Mastral, A.M. Levels of selected metals in ambient air PM10 in an urban site of Zaragoza (Spain). Environ. Res. 2005, 99, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, R.L. The Crust. In Treatise on Geochemistry; Elsevier: New York, NY, USA, 2003; Volume 3. [Google Scholar]
- Kong, S.F.; Li, L.; Li, X.X.; Yin, Y.; Chen, K.; Liu, D.T.; Yuan, L.; Zhang, Y.J.; Shan, Y.P.; Ji, Y.Q. The impacts of firework burning at the Chinese Spring Festival on air quality: Insights of tracers, source evolution and aging processes. Atmos. Chem. Phys. 2015, 15, 2167–2184. [Google Scholar] [CrossRef]
- Terzi, E.; Argyropoulos, G.; Bougatioti, A.; Mihalopoulos, N.; Nikolaou, K.; Samara, C. Chemical composition and mass closure of ambient PM10 at urban sites. Atmos. Environ. 2010, 44, 2231–2239. [Google Scholar] [CrossRef]
- Draxler, R.R.; Hess, G.D. An overview of the HYSPLIT_4 modelling system for trajectories, dispersion and deposition. Aust. Meteorol. Mag. 1998, 47, 295–308. [Google Scholar]
- Draxler, R.R. Evaluation of an ensemble dispersion calculation. J. Appl. Meteorol. 2003, 42, 308–317. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.K.; Murari, V.; Singh, A.K.; Singh, R.S.; Banerjee, T. Fireworks induced particle pollution: A spatio-temporal analysis. Atmos. Res. 2016, 180, 78–91. [Google Scholar] [CrossRef]
- Vecchi, R.; Bernardoni, V.; Cricchio, D.; D’Alessandro, A.; Fermo, P.; Lucarelli, F.; Nava, S.; Piazzalunga, A.; Valli, G. The impact of fireworks on airborne particles. Atmos. Environ. 2008, 42, 1121–1132. [Google Scholar] [CrossRef]
- Cançado, J.E.; Saldiva, P.H.; Pereira, L.A.; Lara, L.B.; Artaxo, P.; Martinelli, L.A.; Arbex, M.A.; Zanobetti, A.; Braga, A.L. The impact of sugar cane–burning emissions on the respiratory system of children and the elderly. Environ. Health Perspect. 2006, 114, 725. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Hasan, M.; Begum, B.A.; Begum, M.; Biswas, S.K. Chemical characterization of biomass burning deposits from cooking stoves in Bangladesh. Biomass Bioenergy 2013, 52, 122–130. [Google Scholar] [CrossRef]
- Deka, P.; Hoque, R.R. Diwali fireworks: Early signs of impact on PM10 properties of rural Brahmaputra valley. Aerosol Air Qual. Res. 2014, 14, 1752–1762. [Google Scholar] [CrossRef]
- Moreno, T.; Querol, X.; Alastuey, A.; Amato, F.; Pey, J.; Pandolfi, M.; Kuenzli, N.; Bouso, L.; Rivera, M.; Gibbons, W. Effect of fireworks events on urban background trace metal aerosol concentrations: Is the cocktail worth the show? J. Hazard. Mater. 2010, 183, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Tiwari, S.; Catrambone, M.; Dalla Torre, S.; Rantica, E.; Canepari, S. Chemical characterization of atmospheric PM in Delhi, India, during different periods of the year including Diwali festival. Atmos. Pollut. Res. 2011, 2, 418–427. [Google Scholar] [CrossRef]
- Russell, M.S. The Chemistry of Fireworks; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Grima, M.; Butler, M.; Hanson, R.; Mohameden, A. Firework displays as sources of particles similar to gunshot residue. Sci. Justice 2012, 52, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tian, S.; Li, X.; Ying, S.; Yi, L.; Wentworth, G.R.; Wang, Y. Trace elements in particulate matter from metropolitan regions of northern China: Sources, concentrations and size distributions. Sci. Total Environ. 2015, 537, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.C.; Laux, J.M.; Vogt, R.; Finlayson-Pitts, B.J.; Hemminger, J.C. Water-induced reorganization of ultrathin nitrate films on NaCl: Implications for the tropospheric chemistry of sea salt particles. J. Phys. Chem. 1996, 100, 6371–6375. [Google Scholar] [CrossRef]
- Laskin, A.; Moffet, R.C.; Gilles, M.K.; Fast, J.D.; Zaveri, R.A.; Wang, B.; Nigge, P.; Shutthanandan, J. Tropospheric chemistry of internally mixed sea salt and organic particles: Surprising reactivity of NaCl with weak organic acids. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Zhuang, H.; Chan, C.K.; Fang, M.; Wexler, A.S. Formation of nitrate and non-sea-salt sulfate on coarse particles. Atmos. Environ. 1999, 33, 4223–4233. [Google Scholar] [CrossRef]
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjövall, P. Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Li, J.; Pósfai, M.; Hobbs, P.V.; Buseck, P.R. Individual aerosol particles from biomass burning in southern Africa: 2, Compositions and aging of inorganic particles. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Saarikoski, S.; Sillanpää, M.; Sofiev, M.; Timonen, H.; Saarnio, K.; Teinilä, K.; Karppinen, A.; Kukkonen, J.; Hillamo, R. Chemical composition of aerosols during a major biomass burning episode over northern Europe in spring 2006: Experimental and modelling assessments. Atmos. Environ. 2006, 41, 3577–3589. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Iijima, A. Assessment of selected metals in the ambient air PM10 in urban sites of Bangkok (Thailand). Environ. Sci. Pollut. Res. 2016, 23, 2948–2961. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Font, A.; de Hoogh, K.; Leal-Sanchez, M.; Ashworth, D.C.; Brown, R.J.; Hansell, A.L.; Fuller, G.W. Using metal ratios to detect emissions from municipal waste incinerators in ambient air pollution data. Atmos. Environ. 2015, 113, 177–186. [Google Scholar] [CrossRef]
- Hieu, N.T.; Lee, B.K. Characteristics of particulate matter and metals in the ambient air from a residential area in the largest industrial city in Korea. Atmos. Res. 2010, 98, 526–537. [Google Scholar] [CrossRef]
- Weckwerth, G. Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmos. Environ. 2001, 35, 5525–5536. [Google Scholar] [CrossRef]
- Karageorgis, A.P.; Katsanevakis, S.; Kaberi, H. Use of enrichment factors for the assessment of heavy metal contamination in the sediments of Koumoundourou Lake, Greece. Water Air Soil Pollut. 2009, 204, 243–258. [Google Scholar] [CrossRef]
- Almeida, S.M.; Pio, C.A.; Freitas, M.C.; Reis, M.A.; Trancoso, M.A. Source apportionment of atmospheric urban aerosol based on weekdays/weekend variability: Evaluation of road re-suspended dust contribution. Atmos. Environ. 2006, 40, 2058–2067. [Google Scholar] [CrossRef]
- Crawford, J.; Chambers, S.; Cohen, D.D.; Dyer, L.; Wang, T.; Zahorowski, R. Receptor modelling using positive matrix factorization, back trajectories and Radon-222. Atmos. Environ. 2007, 41, 6823–6837. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.S.; Shafer, M.M.; Deminter, J.T.; Weinstein, J.P. Emissions of metals associated with motor vehicle roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Warr, P.; Menon, J. Cambodia’s Special Economic Zones. JSEAE 2016, 33, 273–290. [Google Scholar] [CrossRef]
- Bhanarkar, A.D.; Rao, P.S.; Gajghate, D.G.; Nema, P. Inventory of SO2, PM and toxic metals emissions from industrial sources in Greater Mumbai, India. Atmos. Environ. 2005, 39, 3851–3864. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Fudala, J.; Strzelecka-Jastrzab, E.; Hlawiczka, S.; Panasiuk, D.; Nitter, S.; Pregger, T.; Pfeiffer, H.; Friedrich, R. Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos. Environ. 2007, 41, 8557–8566. [Google Scholar] [CrossRef]
- Skodras, G.; Grammelis, P.; Samaras, P.; Vourliotis, P.; Kakaras, E.; Sakellaropoulos, G.P. Emissions monitoring during coal waste wood co-combustion in an industrial steam boiler. Fuel 2002, 81, 547–554. [Google Scholar] [CrossRef]
- Marcazzan, G.M.; Vaccaro, S.; Valli, G.; Vecchi, R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy). Atmos. Environ. 2001, 35, 4639–4650. [Google Scholar] [CrossRef]
- Samara, C.; Voutsa, D. Size distribution of airborne particulate matter and associated heavy metals in the roadside environment. Chemosphere 2005, 59, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Chen, Y.; Chen, Z.; Xu, S. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- Yang, L.X.; Gao, X.M.; Wang, X.F.; Nie, W.; Wang, J.; Gao, R.; Xu, P.J.; Shou, Y.P.; Zhang, Q.Z.; Wang, W.X. Impacts of firecracker burning on aerosol chemical characteristics and human health risk levels during the Chinese New Year celebration in Jinan, China. Sci. Total Environ. 2014, 476–477, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, S. Incremental lifetime cancer risk of PM2.5 bound polycyclic aromatic hydrocarbons (PAHs) before and after the wildland fire episode. Aerosol Air Qual. Res. 2016, 16, 2907–2919. [Google Scholar] [CrossRef]
- Feng, J.L.; Sun, P.; Hu, X.L.; Zhao, W.; Wu, M.H.; Fu, J.M. The chemical composition and sources of PM2.5 during the 2009 Chinese New Year’s holiday in Shanghai. Atmos. Res. 2012, 118, 435–444. [Google Scholar] [CrossRef]
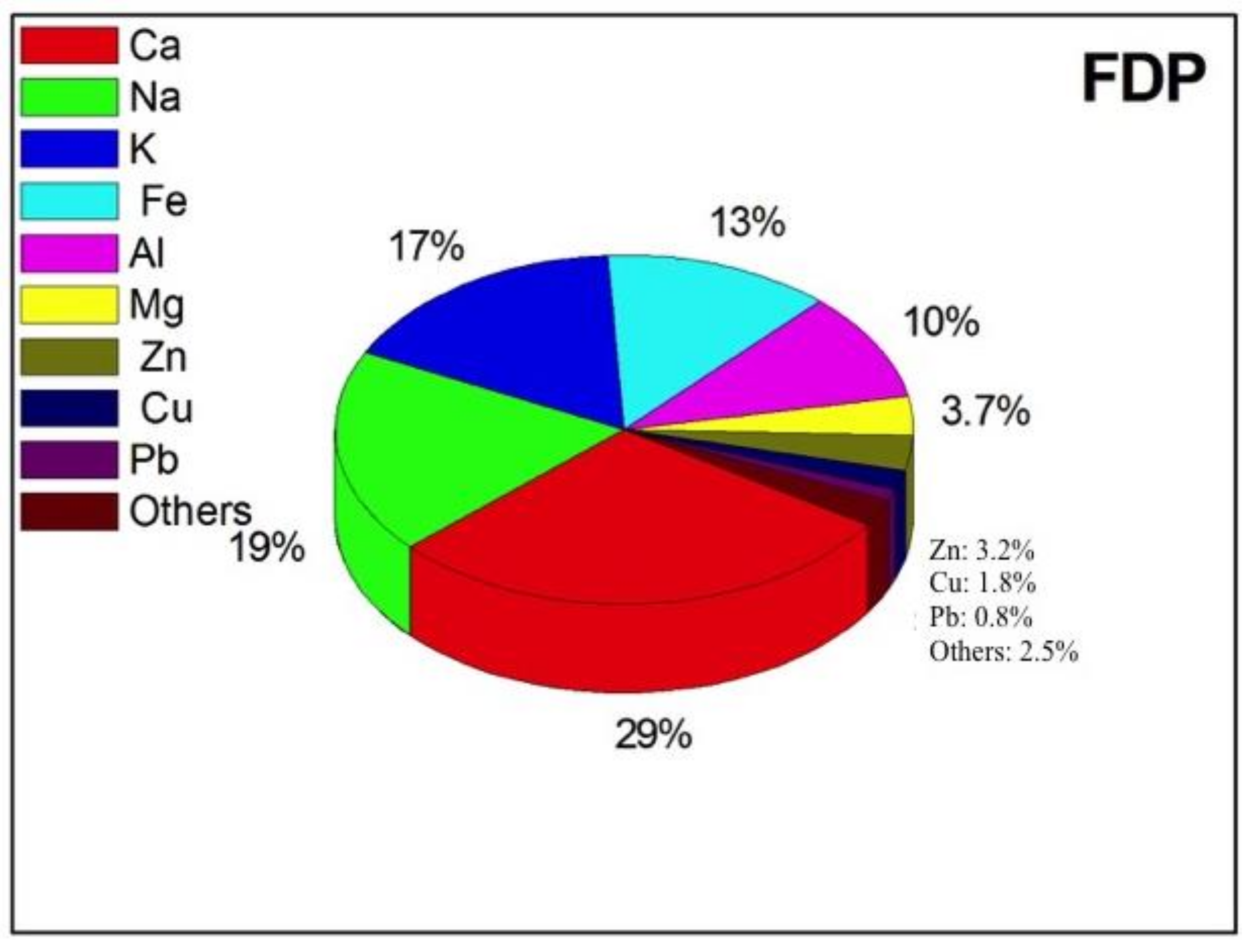
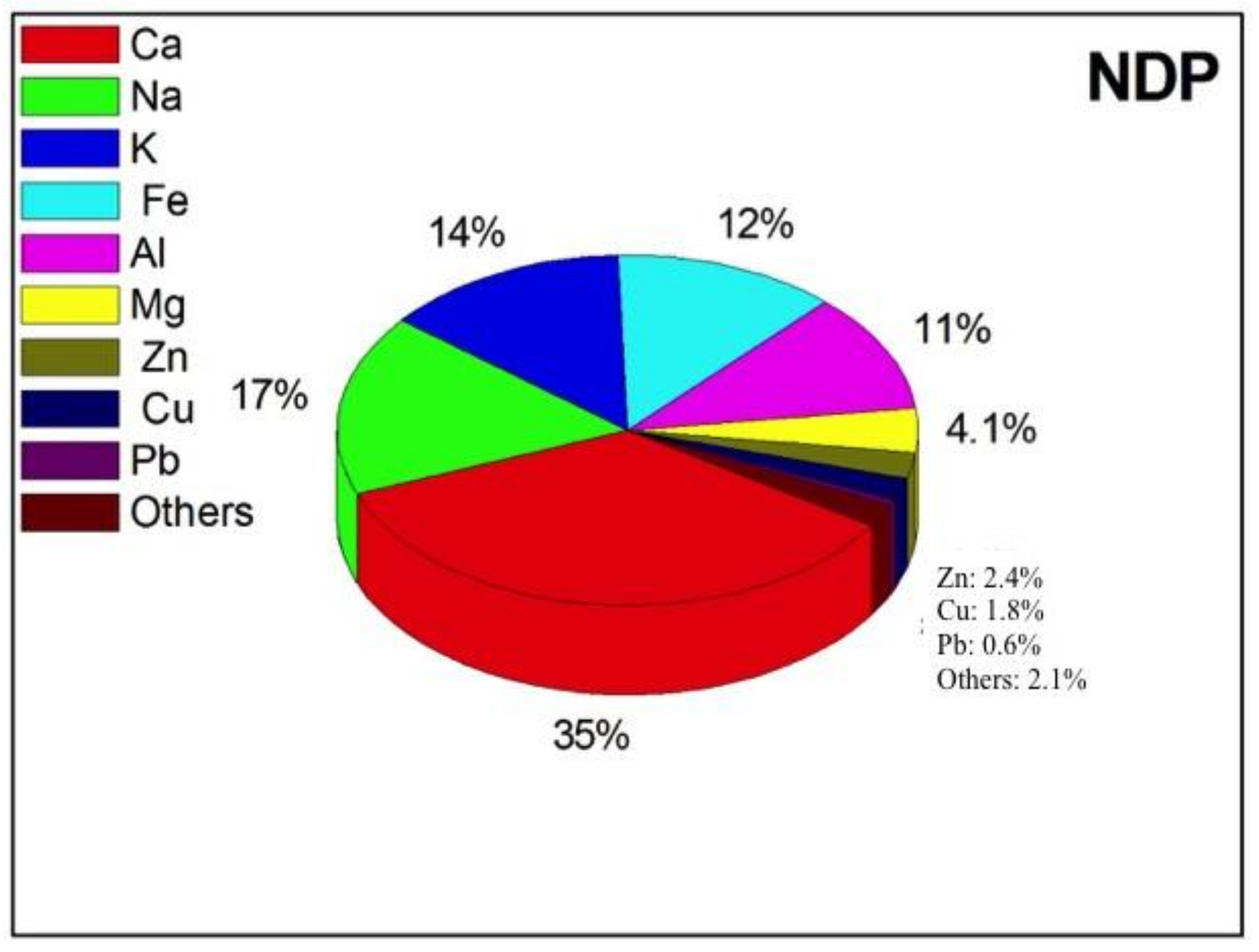
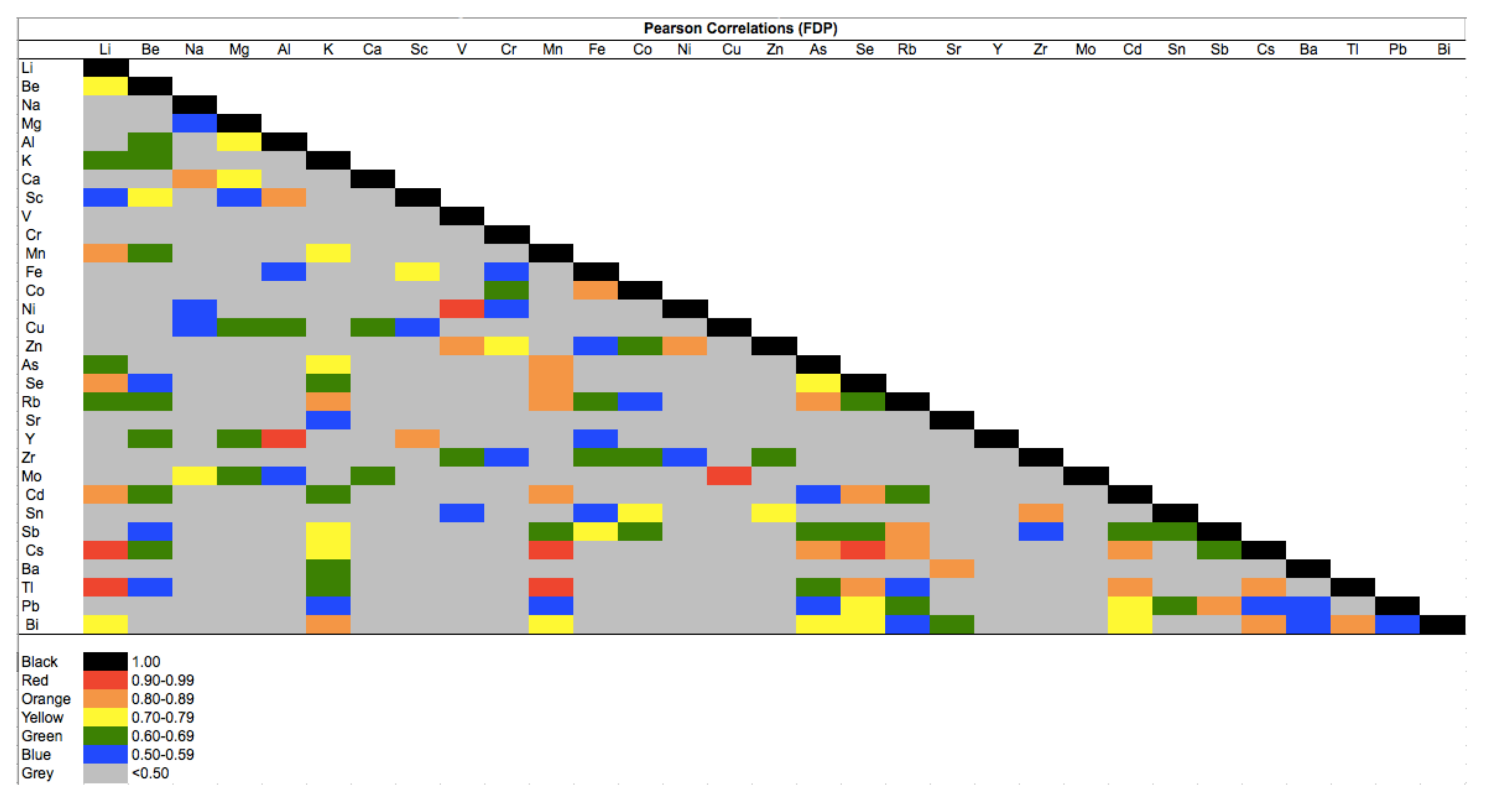
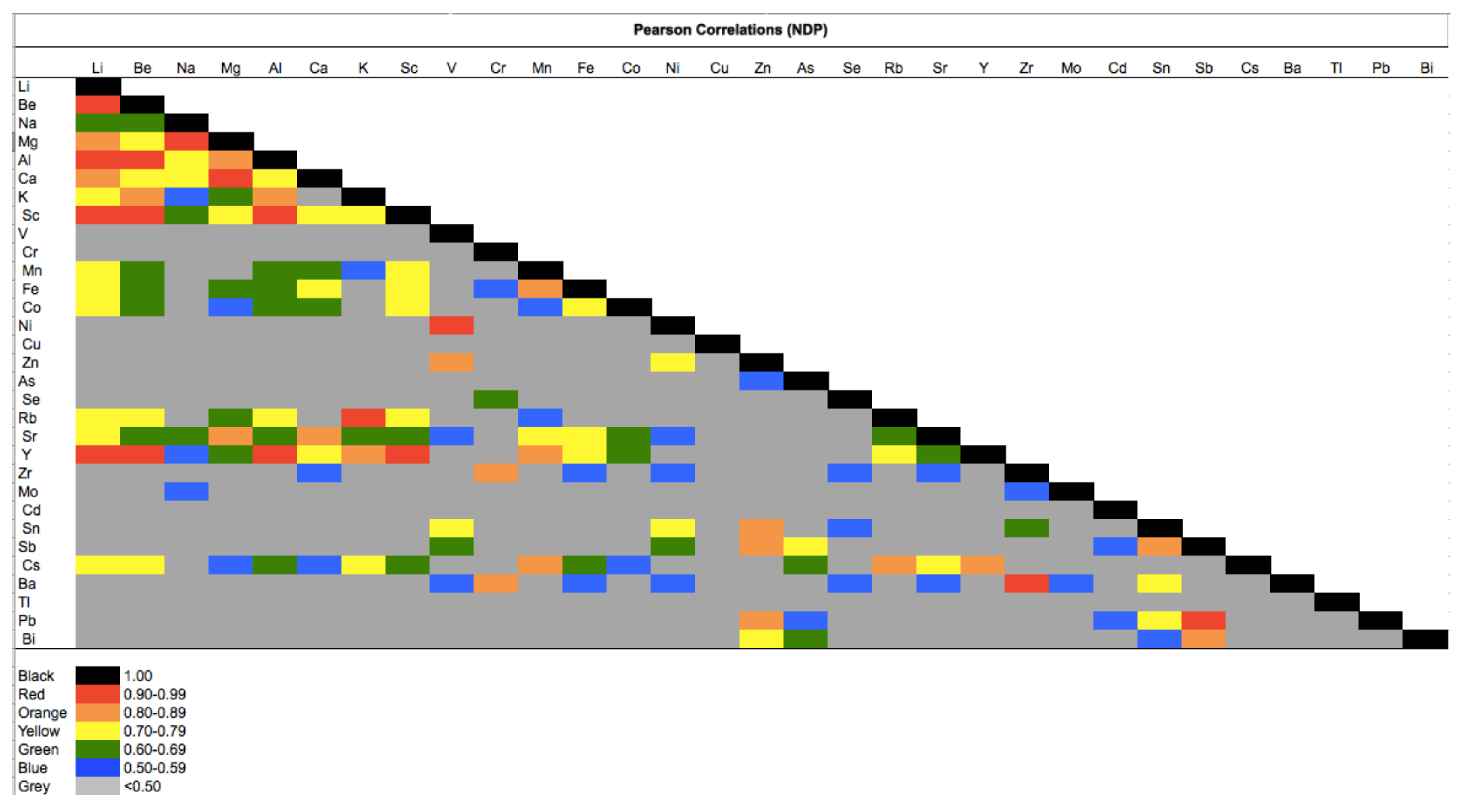
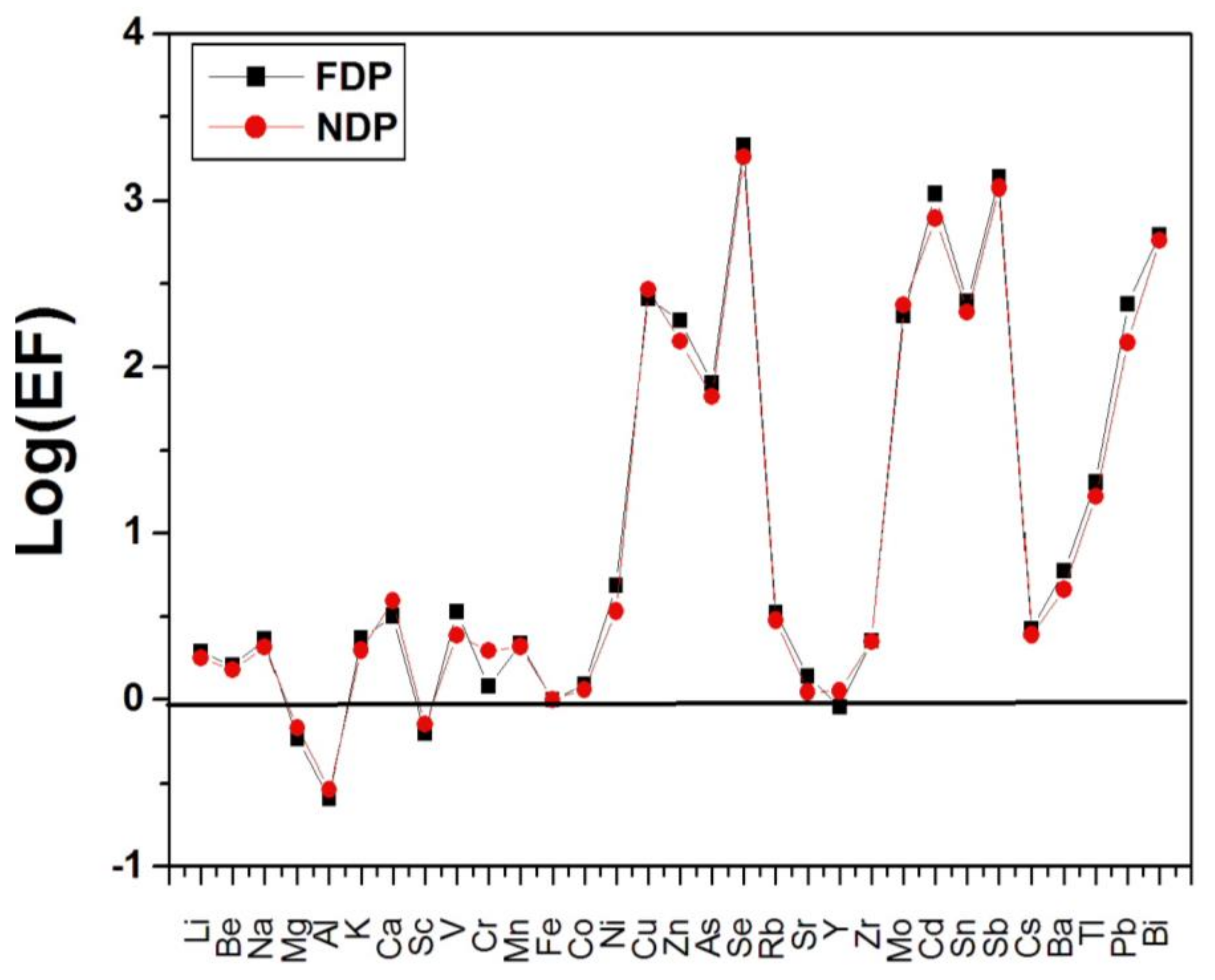
| FDP | NDP | t-Test | |
|---|---|---|---|
| Conc. (ng m−3) | Conc. (ng m−3) | (p < 0.05) | |
| Li | 0.852 (0.425~1.26) | 0.844 (0.280~1.63) | NS * |
| Be | 0.0614 (0.0444~0.0766) | 0.0624 (0.0300~0.100) | NS |
| Na | 1374 (198~2855) | 1339 (77.6~3006) | NS |
| Mg | 263 (26.5~492) | 330 (109~641) | NS |
| Al | 722 (N.D.~1028) | 883 (237~1,768) | S ** |
| K | 1206 (741~1969) | 1094 (199~2542) | NS |
| Ca | 2096 (1219~2977) | 2794 (973~5274) | S |
| Sc | 0.160 (0.0751~0.210) | 0.196 (0.0800~0.360) | S |
| V | 5.97 (3.20~12.1) | 4.67 (1.38~11.15) | NS |
| Cr | 2.03 (0.600~7.05) | 3.58 (0.220~27.10) | S |
| Mn | 39.6 (20.2~64.0) | 41.1 (12.2~93.6) | NS |
| Fe | 922 (569~1167) | 997 (380~1446) | NS |
| Co. | 0.391 (0.212~0.546) | 0.393 (0.140~0.690) | NS |
| Ni | 4.16 (2.18~7.30) | 3.16 (0.670~6.59) | NS |
| Cu | 133 (85.8~264) | 163 (45.9~410) | NS |
| Zn | 233 (123~411) | 189 (35.4~496) | NS |
| As | 7.00 (2.35~13.3) | 6.32 (0.950~22.4) | NS |
| Se | 3.52 (0.916~6.12) | 3.24 (0.590~10.4) | NS |
| Rb | 5.09 (2.57~8.22) | 4.99 (1.27~11.0) | NS |
| Sr | 8.05 (3.44~20.7) | 7.04 (2.63~13.7) | NS |
| Y | 0.347 (N.D.~0.506) | 0.465 (0.220~0.870) | S |
| Zr | 7.95 (5.55~11.8) | 8.54 (3.64~12.5) | NS |
| Mo | 4.11 (0.757~15.1) | 5.15 (0.0300~17.6) | NS |
| Cd | 1.80 (0.289~3.26) | 1.40 (0.260~3.89) | NS |
| Sn | 9.55 (4.62~20.6) | 8.85 (2.26~17.5) | NS |
| Sb | 10.1 (5.33~15.2) | 9.43 (3.01~28.8) | NS |
| Cs | 0.239 (0.0347~0.458) | 0.237 (N.D.~0.710) | NS |
| Ba | 68.1 (38.3~106) | 57.0 (19.5~95.6) | S |
| Tl | 0.334 (0.0357~0.798) | 0.295 (0.0400~1.19) | NS |
| Pb | 74.7 (29.2~145) | 47.2 (7.96~119) | S |
| Bi | 1.82 (0.449~4.63) | 1.83 (0.310~6.89) | NS |
| Diagnostic Binary Ratios | FDP | NDP | FDP/NDP |
|---|---|---|---|
| Pb/Ca | 0.036 | 0.017 | 2.1 |
| Pb/Na | 0.054 | 0.035 | 1.5 |
| Pb/K | 0.062 | 0.043 | 1.4 |
| Pb/Fe | 0.081 | 0.047 | 1.7 |
| Pb/Al | 0.10 | 0.053 | 1.9 |
| Pb/Mg | 0.28 | 0.14 | 2.0 |
| Pb/Zn | 0.32 | 0.25 | 1.3 |
| Pb/Cu | 0.56 | 0.29 | 1.9 |
| Ba/Ca | 0.033 | 0.020 | 1.6 |
| Ba/Na | 0.050 | 0.043 | 1.2 |
| Ba/K | 0.057 | 0.052 | 1.1 |
| Ba/Fe | 0.074 | 0.057 | 1.3 |
| Ba/Al | 0.094 | 0.065 | 1.5 |
| Ba/Mg | 0.26 | 0.17 | 1.5 |
| Ba/Zn | 0.29 | 0.30 | 1.0 |
| Ba/Cu | 0.51 | 0.35 | 1.5 |
| Li/Cs | 3.6 | 3.56 | 1.00 |
| Li/Tl | 2.6 | 2.86 | 0.89 |
| Al/Y | 2077 | 1897 | 1.09 |
| V/Ni | 1.4 | 1.5 | 0.97 |
| Mn/Cs | 165 | 173 | 0.96 |
| Mn/Tl | 119 | 139 | 0.85 |
| Cu/Mo | 32 | 32 | 1.03 |
| Cu/Sb | 13 | 17 | 0.8 |
| Cd/Cu | 0.014 | 0.0086 | 1.6 |
| Cd/Pb | 0.024 | 0.030 | 0.8 |
| Se/Cd | 2.0 | 2.3 | 0.8 |
| Elements | FDP | NDP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dinh-children | Dinh-adults | HQchildren | HQadult | Dinh-children | Dinh-adults | HQchildren | HQadult | Rf | |
| Pb | 1.72 × 107 | 9.67 × 108 | 4.87 × 105 | 2.75 × 105 | 1.08× 107 | 6.12 × 108 | 3.08 × 105 | 1.74 × 105 | 3.52 × 103 |
| Cr | 4.65 × 109 | 2.62 × 109 | 1.63 × 104 | 9.17 × 105 | 8.22 × 109 | 4.63 × 109 | 2.87 × 104 | 1.62 × 104 | 2.86 × 105 |
| Co. | 8.97 × 1010 | 5.06 × 1010 | 1.57 × 104 | 8.86 × 105 | 9.03 × 1010 | 5.09 × 1010 | 1.58 × 104 | 8.92 × 105 | 5.71 × 106 |
| Ni | 9.56 × 109 | 5.39 × 109 | 4.64 × 107 | 2.62 × 107 | 7.26 × 109 | 4.09 × 109 | 3.52 × 107 | 1.99 × 107 | 2.06 × 102 |
| Zn | 5.34 × 107 | 3.01 × 107 | 1.77 × 106 | 1.00 × 106 | 4.34 × 107 | 2.45 × 107 | 1.44 × 106 | 8.14 × 107 | 3.01 × 101 |
| As | 1.61 × 108 | 9.07 × 109 | 5.34 × 105 | 3.01 × 105 | 1.45 × 108 | 8.19 × 109 | 4.82 × 105 | 2.72 × 105 | 3.01 × 104 |
| Cd | 4.13 × 109 | 2.33 × 109 | 4.13 × 106 | 2.33 × 106 | 3.21 × 109 | 1.81 × 109 | 3.21 × 106 | 1.81 × 106 | 1.00 × 103 |
| V | 1.37 × 108 | 7.73 × 109 | 1.96 × 106 | 1.10 × 106 | 1.07 × 108 | 6.04 × 109 | 1.53 × 106 | 8.63 × 107 | 7.00 × 103 |
| Mn | 9.09 × 108 | 5.12 × 108 | 6.49 × 103 | 3.66 × 103 | 9.44 × 108 | 5.33 × 108 | 6.75 × 103 | 3.80 × 103 | 1.40 × 105 |
| Σ | 8.46 × 107 | 4.77 × 107 | 6.92 × 103 | 3.90 × 103 | 6.82 × 107 | 3.85 × 107 | 7.28 × 103 | 4.10 × 103 | - |
| Elements | FDP | NDP | FDP | NDP | |
|---|---|---|---|---|---|
| LADD | LADD | SFa | R | R | |
| Pb | 1.27 × 104 | 8.03 × 105 | - | - | - |
| Cr | 3.44 × 106 | 6.08 × 106 | 42 | 1.45 × 104 | 2.56 × 104 |
| Co. | 6.65 × 107 | 6.69 × 107 | 9.8 | 6.51 × 106 | 6.55 × 106 |
| Ni | 7.08 × 106 | 5.37 × 106 | 0.84 | 5.94 × 106 | 4.51 × 106 |
| Zn | 3.95 × 104 | 3.22 × 104 | - | - | - |
| As | 1.19 × 105 | 1.07 × 105 | 15.1 | 1.80 × 104 | 1.62 × 104 |
| Cd | 3.06 × 106 | 2.38 × 106 | 6.4 | 1.96 × 105 | 1.52 × 105 |
| V | 1.02 × 105 | 7.94 × 106 | - | - | - |
| Mn | 6.73 × 105 | 6.99 × 105 | - | - | - |
| Σ | 6.26 × 104 | 5.05 × 104 | - | 3.57 × 104 | 4.44 × 104 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongpiachan, S.; Iijima, A.; Cao, J. Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays. Atmosphere 2018, 9, 144. https://doi.org/10.3390/atmos9040144
Pongpiachan S, Iijima A, Cao J. Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays. Atmosphere. 2018; 9(4):144. https://doi.org/10.3390/atmos9040144
Chicago/Turabian StylePongpiachan, Siwatt, Akihiro Iijima, and Junji Cao. 2018. "Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays" Atmosphere 9, no. 4: 144. https://doi.org/10.3390/atmos9040144
APA StylePongpiachan, S., Iijima, A., & Cao, J. (2018). Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays. Atmosphere, 9(4), 144. https://doi.org/10.3390/atmos9040144






