Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Characteristics
2.2. Reagents and Materials
2.3. Experimental Procedure
2.4. Analytical Procedure
3. Results and Discussion
3.1. Antimony Speciation
3.2. Sb(III) Removal by Fe(III) or Fe(II) Coagulation
3.2.1. Fe(III) Addition
- GFH (Q5 = 1.4 μg Sb(III)/mg GFH, or 2.5 μg Sb(III)/mg Fe), which was supplied by SIEMENS and mainly consists of akaganeite with an iron content 55 ± 1% w/w at dry basis, and
3.2.2. Fe(II) Addition
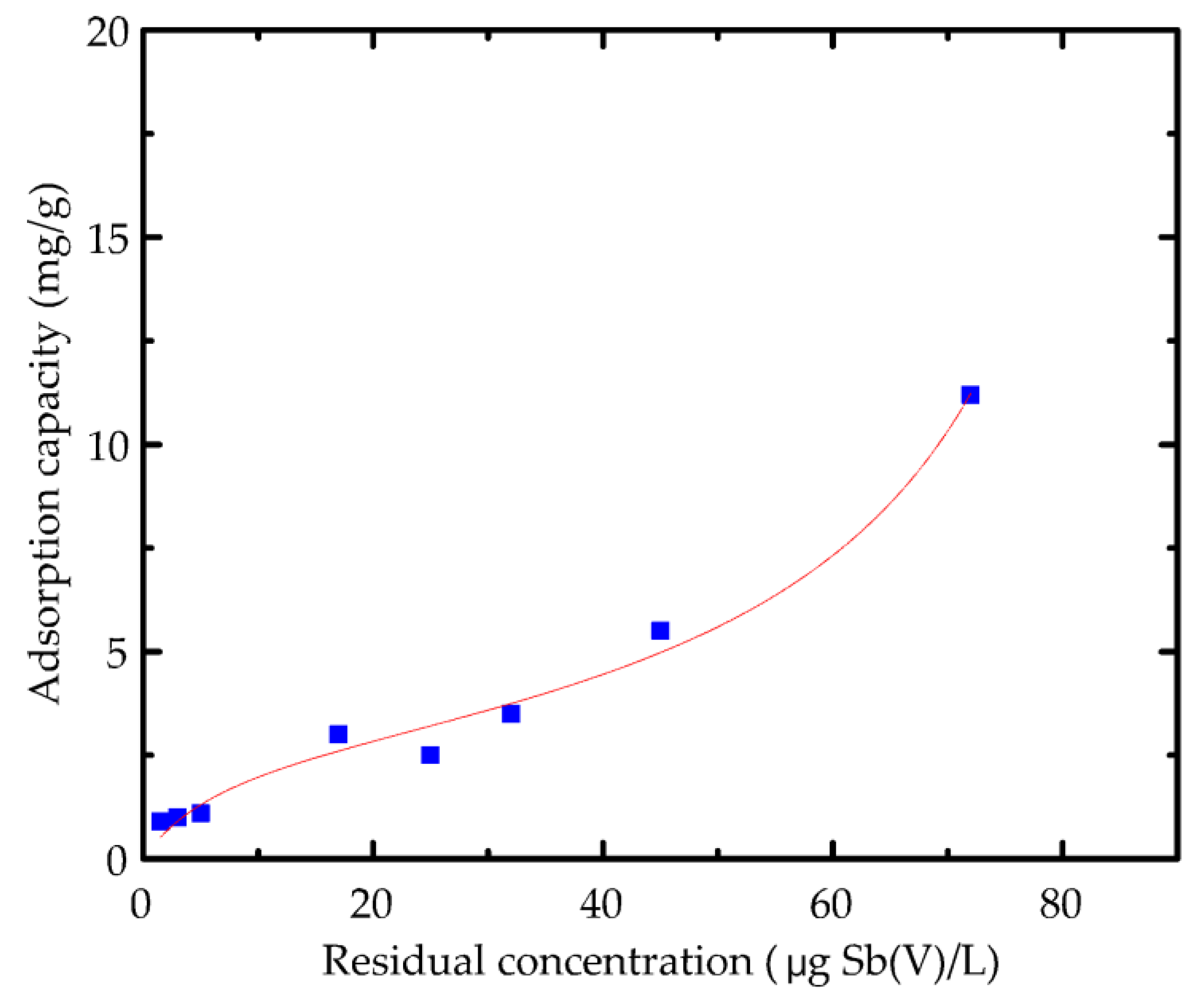
3.3. Sb(V) Removal by Fe(III) or Fe(II) Coagulation
3.3.1. Fe(III)
3.3.2. Fe(II) or Equimolar Fe(II)/Fe(III) Additions
4. Conclusions
- ✔
- Coagulation is generally an effective treatment technique for antimony removal from polluted aqueous sources, with much more efficient Sb(III) removal induced by Fe(III) coagulant, that is, Q5 = 4.7 μg Sb(III)/mg Fe(III), than by Fe(II), that is, Q5 = 0.45 μg Sb(III)/mg Fe(II) at pH 7. Furthermore, Fe(III)-based coagulant addition proved also more efficient than the Fe(II) or Fe(III)/Fe(II) coagulants for Sb(V) removal. However, the Fe(III) uptake capacity for Sb(V), that is, Q5 = 1.82 μg Sb(V)/mg Fe(III), was found almost equal to 39% of the corresponding value for the case of Sb(III) and 2.5% of the corresponding value for the tetrahedral geometry As(V) oxy-anions (i.e., Q5 = 44 μg As(V)/mg Fe(III)).
- ✔
- Fe(II) coagulant seems to contribute to Sb(V) reduction to Sb(III), since its adsorption capacity for Sb(V), that is, Q5 = 1.3 μg Sb(V)/mg Fe(II), was found to be almost three times higher than the corresponding for Sb(III), that is, Q5 = 0.45 μg Sb(III)/mg Fe(II).
- ✔
- The water pH value does not influence Sb(III) removal by the Fe(III) addition at pH range 6–8, commonly encountered in most natural waters with Q5 = 4.8 ± 0.1 μg Sb(III)/mg Fe(III), because Sb(III) is present mainly as a neutral molecule in the form of Sb(OH)3. However, at pH 5 the uptake capacity proved to be significantly higher, that is, Q5 = 10.5 μg Sb(III)/mg Fe(III), due to the increase of positive surface charge density of FeOOH precipitates.
- ✔
- By lowering the water pH below the IEP value of FeOOH precipitates, the uptake of Sb(OH)6− was gradually increased, due to the increase of positively charged Fe(OH)2+/Fe(OH)2+ hydrolysis species of Fe(III), for example, for Co = 60 μg Sb(V)/L and iron dose 2.5 mg Fe(III)/L the residual concentrations at water pH 8, 7, 6, 5 were found to be 59, 56, 40, 25 μg Sb(V)/L, respectively.
- ✔
- The fitting of adsorption isotherms data to sorption models, regarding the equilibrium antimony concentrations in the range of 5–100 μg/L, showed that the Sb(III) data were better fitted to the Freundlich model, while the corresponding data for Sb(V) were better fitted to the BET model.
- ✔
- Finally, the experimental data of this study were focused in antimony concentrations commonly found in polluted natural waters (around or lower than 100 μg/L), hence allowing the accurate determination of respective adsorption capacities by the coagulation produced precipitates-solids at the drinking water regulation limit (Q5), and therefore, supplying the fundamental information for upscaling the results of this study.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Du, Χ.; Qu, F.; Liang, H.; Li, K.; Yu, H.; Bai, L.; Li, G. Removal of antimony(III) from polluted surface water using a hybrid coagulation–flocculation–ultrafiltration (CF–UF) process. Chem. Eng. J. 2014, 254, 293–301. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Aksoy, N.; Simsek, C.; Gunduz, O. Groundwater contamination mechanism in a geothermal field: A case study of Balcova, Turkey. J. Contam. Hydrol. 2009, 103, 13–28. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimony in Drinking-water Background Document for Development of WHO Guidelines for Drinking-Water Quality. WHO/SDE/WSH/03.04/74. 2003. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/antimony.pdf (accessed on 23 March 2018).
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchem. J. 2011, 97, 85–91. [Google Scholar] [CrossRef]
- Kang, M.; Kawasaki, M.; Tamada, S.; Kamei, T.; Magara, Y. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 2000, 131, 293–298. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Sar, A.; Tuzen, M. Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chem. Eng. J. 2010, 163, 382–388. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, F.; Pan, X.; Guo, J.; Wen, D. Removal of antimony from antimony mine flotation wastewater by electrocoagulation with aluminum electrodes. J. Environ. Sci. 2011, 23, 1066–1071. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Dou, X.M.; Mohan, D.; Pittman, C.U.; Ok, Y.S.; Jin, X. Antimonate and antimonite adsorption by a polyvinyl alcohol stabilized granular adsorbent containing nanoscale zero-valent iron. Chem. Eng. J. 2014, 247, 250–257. [Google Scholar] [CrossRef]
- Simeonidis, K.; Papadopoulou, V.; Tresintsi, S.; Kokkinos, E.; Katsoyiannis, I.A.; Zouboulis, A.I.; Mitrakas, M. Efficiency of iron-based oxy-hydroxides in removing antimony from groundwater to levels below the drinking water regulation limit. Sustainability 2017, 9, 238. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation–flocculation–sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kamei, T.; Magara, Y. Comparing poly-aluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Hering, J.G.; Katsoyiannis, I.A.; Theoduloz, G.A.; Berg, M.; Hug, S.J. Arsenic removal from drinking water: Experiences with technologies and constraints in practice. J. Environ. Eng. 2017, 143, 0311700. [Google Scholar] [CrossRef]
- Gregor, J. Arsenic removal during conventional aluminum-based drinking water treatment. Water Res. 2001, 35, 1659–1664. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Ouzounis, K.; Katsoyiannis, I.A.; Zouboulis, A.I.; Mitrakas, M. Is the coagulation-filtration process with Fe(III) efficient for As(III) removal from groundwaters? Sep. Sci. Technol. 2015, 50, 1587–1592. [Google Scholar] [CrossRef]
- Inam, M.A.; Rizwan, K.; Du, R.P.; Yong-Woo, L.; Ich, T.Y. Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water 2018, 10, 418. [Google Scholar] [CrossRef]
- Niedzielski, P.; Siepak, M. The occurrence and speciation of arsenic, antimony and selenium in ground water of Poznań city (Poland). Chem. Geol. 2005, 21, 241–253. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Zouboulis, A.; Mitrakas, M. Comparative study of As(V) removal by ferric coagulation and oxy-hydroxides adsorption: Laboratory and full scale case studies. Desalin. Water Treat. 2013, 51, 2872–2880. [Google Scholar] [CrossRef]
- Ebadi, A.; Soltan Mohammadzadeh, J.S.; Khudiev, A. What is the correct form of BET isotherms for modeling liquid phase adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Mitrakas, Μ.; Panteliadis, P.; Keramidas, V.; Tzimou-Tsitouridou, R.; Sikalidis, C. Predicting Fe3+ dose for As(V) removal at pHs and temperatures commonly encountered in natural waters. Chem. Eng. J. 2009, 155, 716–721. [Google Scholar] [CrossRef]






| Parameter | Average Value |
|---|---|
| pH | 7.30 |
| Conductivity, μS/cm | 590 |
| Na, mg/L | 35 |
| Ca, mg/L | 80 |
| Mg, mg/L | 24 |
| HCO3−, mg/L | 342 |
| Fe, mg/L | <0.02 |
| Mn, mg/L | <0.005 |
| NO3−, mg/L | 9 |
| SO42−, mg/L | 8 |
| Cl−, mg/L | 13 |
| TOC, mg/L | 0.4 |
| Coagulant | pH | ΚF (μg/mg)/(μg/L)n | n | R2 | Q5 μg Sb(III)/mg Fe |
|---|---|---|---|---|---|
| Fe(III) | 5 | 2.964 | 0.7900 | 0.989 | 10.5 |
| Fe(III) | 6 | 0.985 | 1.0019 | 0.997 | 4.9 |
| Fe(III) | 7 | 0.887 | 1.0265 | 0.999 | 4.7 |
| Fe(III) | 8 | 0.995 | 0.9877 | 0.993 | 4.8 |
| Fe(II) | 7 | 0.032 | 1.6354 | 0.994 | 0.45 |
| Coagulant | a | b | c | R2 | Q5 μg Sb(V)/mg Fe |
|---|---|---|---|---|---|
| Fe(III) (Figure 4) | 0.7732 | 0.2357 | −0.0023 | 0.981 | 1.82 |
| Fe(II) (Figure 6) | 0.2239 | 0.0102 | −0.0002 | 0.997 | 1.30 |
| [Fe(III)]/[Fe(II)] = 1 (Figure 7) | 0.4164 | 0.1251 | −0.0014 | 0.985 | 1.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrakas, M.; Mantha, Z.; Tzollas, N.; Stylianou, S.; Katsoyiannis, I.; Zouboulis, A. Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants. Water 2018, 10, 1328. https://doi.org/10.3390/w10101328
Mitrakas M, Mantha Z, Tzollas N, Stylianou S, Katsoyiannis I, Zouboulis A. Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants. Water. 2018; 10(10):1328. https://doi.org/10.3390/w10101328
Chicago/Turabian StyleMitrakas, Manassis, Zoi Mantha, Nikos Tzollas, Stelios Stylianou, Ioannis Katsoyiannis, and Anastasios Zouboulis. 2018. "Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants" Water 10, no. 10: 1328. https://doi.org/10.3390/w10101328
APA StyleMitrakas, M., Mantha, Z., Tzollas, N., Stylianou, S., Katsoyiannis, I., & Zouboulis, A. (2018). Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants. Water, 10(10), 1328. https://doi.org/10.3390/w10101328







