Identification of Phytoplankton Blooms under the Index of Inherent Optical Properties (IOP Index) in Optically Complex Waters
Abstract
:1. Introduction
2. Materials and Methods
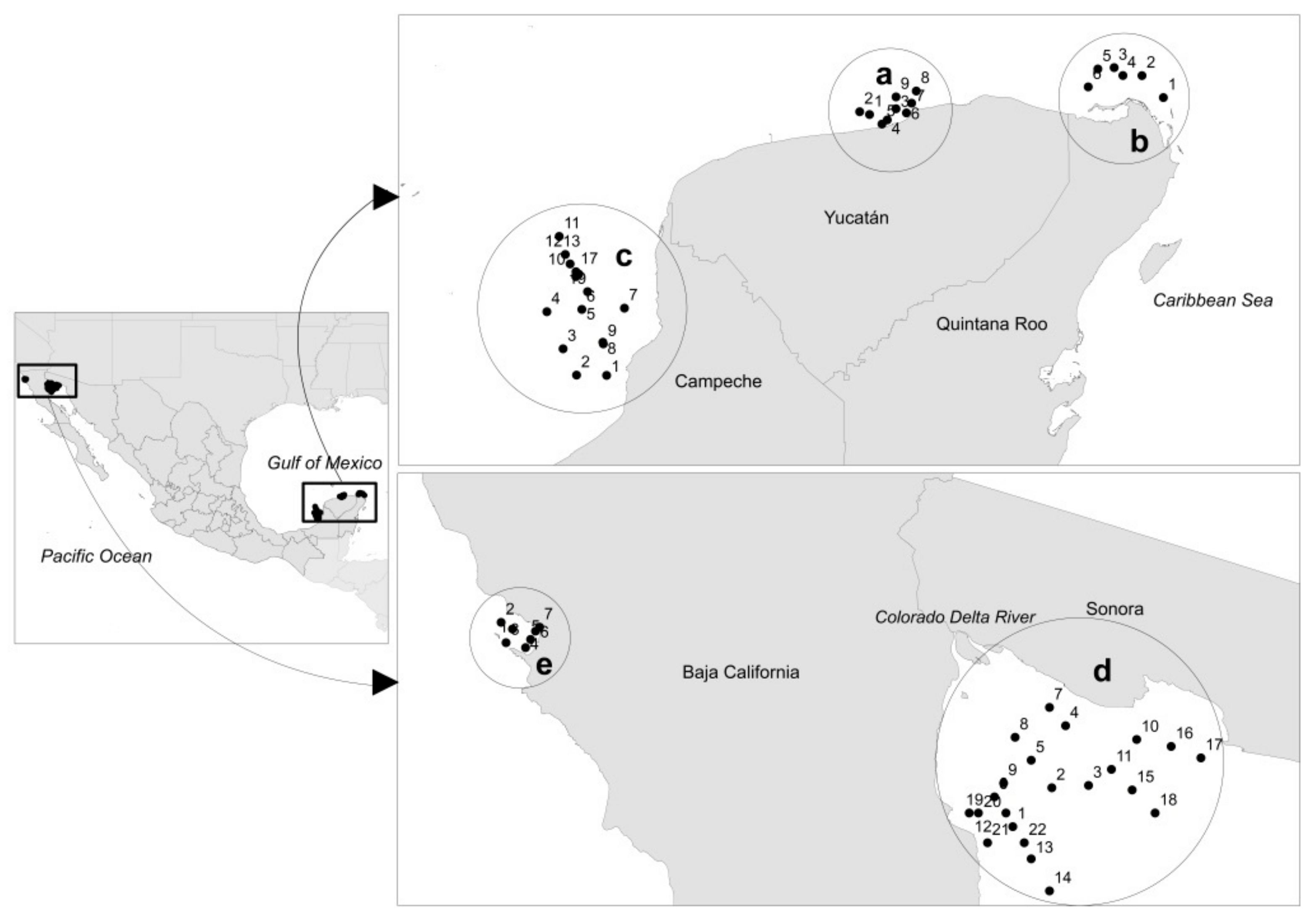
2.1. Study Area
2.2. Collection of Samples
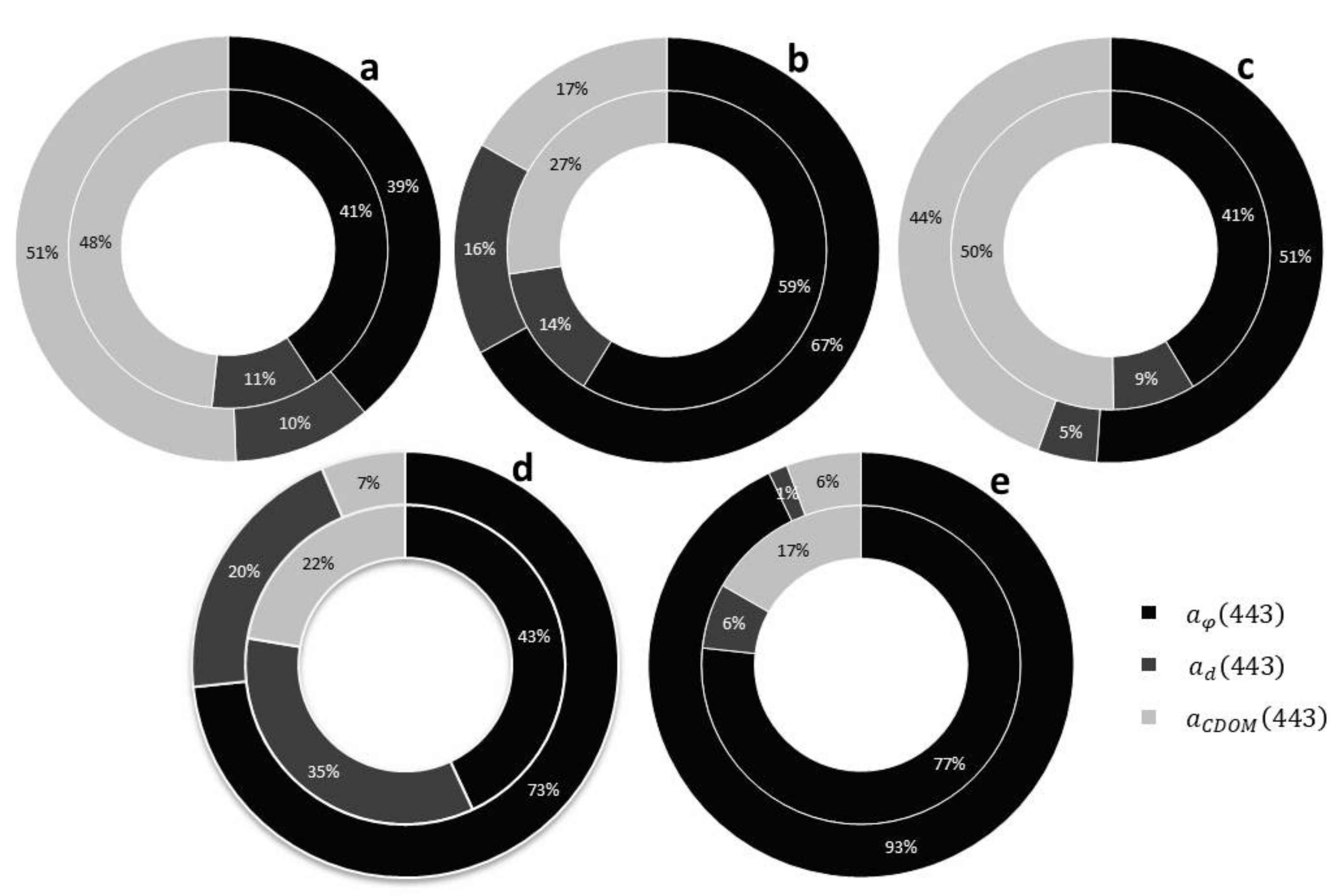
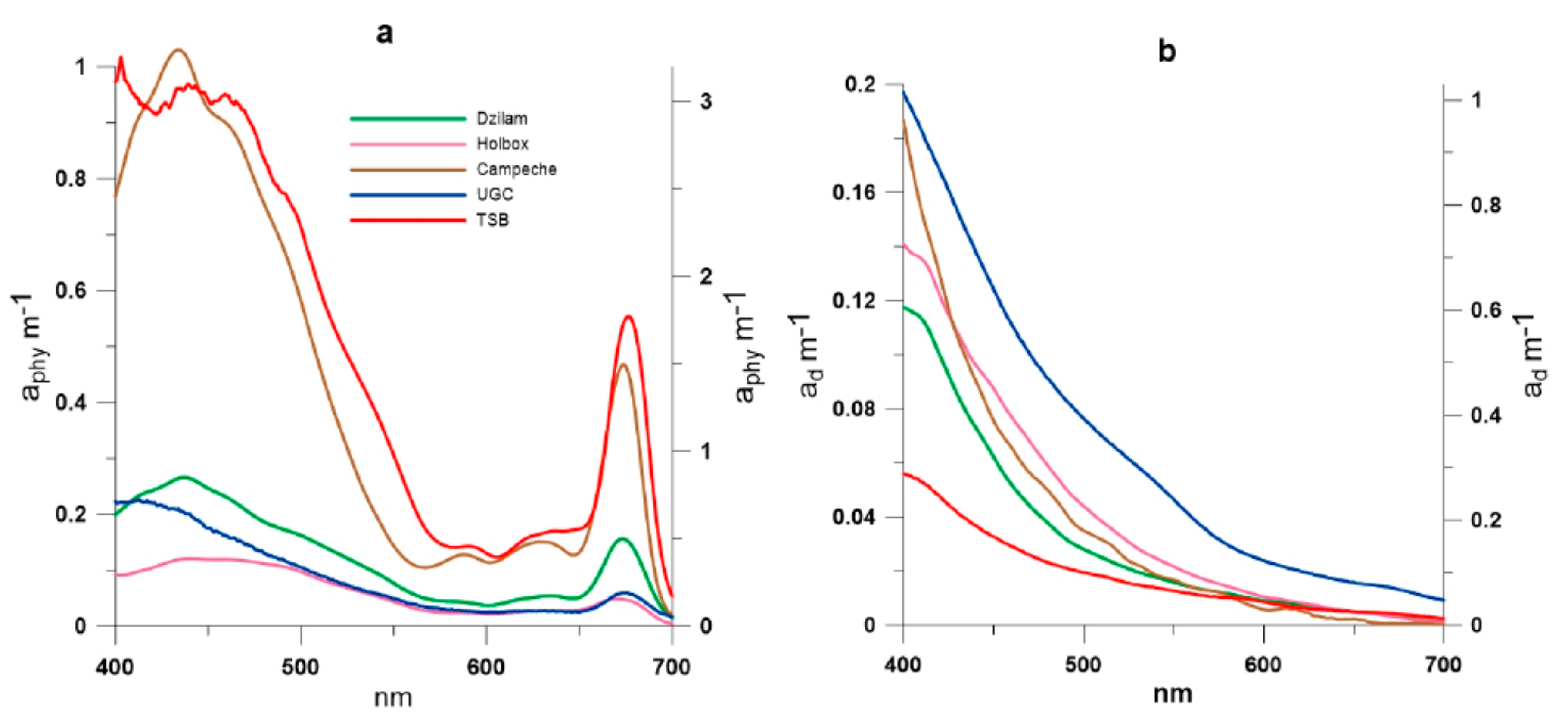
2.3. Absorption Coefficients Determination
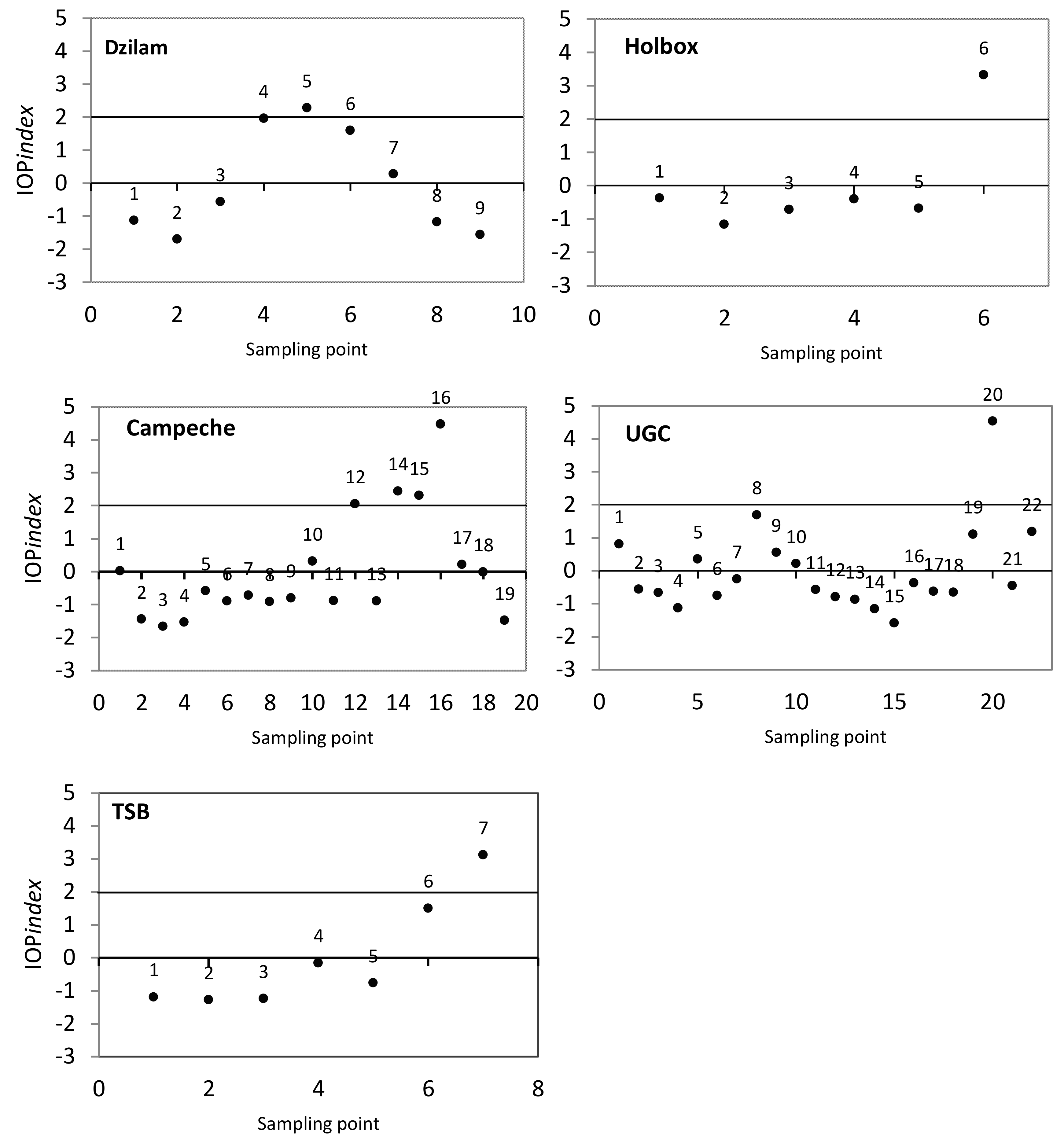
2.4. IOP Index Determination
2.5. Phytoplankton Community Characterization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gower, J.; King, S.; Borstad, G.; Brown, L. Detection of intense plankton blooms using the 709 nm band of the MERIS imaging spectrometer. Int. J. Remote Sens. 2005, 26, 2005–2012. [Google Scholar] [CrossRef]
- Carstensen, J.; Conley, D. Frequency, composition, and causes of summer phytoplankton blooms in a shallow coastal ecosystem, the Kattegat. Limnol. Oceanogr. 2004, 49, 191–201. [Google Scholar] [CrossRef]
- Legendre, L. The significance of microalgal blooms for fisheries and for the export of particulate organic carbón in oceans. J. Plankton Res. 1990, 12, 681–699. [Google Scholar] [CrossRef]
- Ji, R.; Edwards, M.; Mackas, D.; Runge, J.; Thomas, A. Marine plankton phenology and life history in a changing climate: Current research and future directions. J. Plankton Res. 2010, 32, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K. Harmful or exceptional phytoplankton blooms in the marine ecosystem. Adv. Mar. Biol. 1997, 31, 301–385. [Google Scholar] [CrossRef]
- Smayda, T.J. What is a bloom? A commentary. Limnol. Oceanogr. 1997, 42, 1132–1136. [Google Scholar] [CrossRef]
- Brody, S.R.; Lozier, M.S.; Dunne, J.P. A comparison of methods to determine phytoplankton Bloom initiation. J. Geophys. Res. Oceans 2013, 118, 2345–2357. [Google Scholar] [CrossRef]
- Platt, T.; Fuentes-Yaco, C.; Frank, K.T. Spring algal Bloom and larval fish survival. Nature 2007, 423, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Kaitala, S.; Maunula, P. Identification and quantification of plankton bloom events in the Baltic Sea by continuous pCO2 and chlorophyll a measurements on a cargo ship. J. Mar. Syst. 2006, 59, 238–248. [Google Scholar] [CrossRef]
- Gittings, J.A.; Raitsos, D.E.; Racault, M.F.; Brewin, R.J.; Pradhan, Y.; Sathyendranath, S.; Platt, T. Seasonal phytoplankton blooms in the Gulf of Aden revealed by remote sensing. Remote Sens. Environ. 2017, 189, 56–66. [Google Scholar] [CrossRef]
- Huppert, A.; Blasius, B.; Stone, L. A Model of Phytoplankton Blooms. Am. Nat. 2002, 159, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Fleming, V.; Seppo Kaitala, S. Phytoplankton spring bloom intensity index for the Baltic Sea estimated for the years 1992 to 2004. Hydrobiologia 2006, 554, 57–65. [Google Scholar] [CrossRef]
- Carstensen, J.; Henriksen, P.; Heiskanen, A.-S. Summer algal blooms in shallow estuaries: Definition, mechanisms, and link to eutrophication. Limnol. Oceanogr. 2007, 52, 370–384. [Google Scholar] [CrossRef]
- Cetinic, I.; Perry, M.J.; D’Asaro, E.; Briggs, N.; Poulton, N.; Sieracki, M.E.; Lee, C.M. A simple optical index shows spatial and temporal heterogeneity in phytoplankton community composition during the 2008 North Atlantic Bloom Experiment. Biogeosciences 2015, 12, 2179–2194. [Google Scholar] [CrossRef]
- Alikas, K.; Kangro, K.; Reinart, A. Detecting cyanobacterial blooms in large North European lakes using the Maximum Chlorophyll Index. Oceanologia 2010, 52, 237–257. [Google Scholar] [CrossRef]
- Platt, T.; Sathyendranath, S.; White, G.; Fuentes-Yaco, C.; Zhai, L.; Devred, E.; Tang, C. Diagnostic properties of phytoplankton time series from remote sensing. Estuar. Coasts 2009, 33, 428–439. [Google Scholar] [CrossRef]
- Preisendorfer, R.W. Application of Radiative Transfer Theory to Light Measurements in the Sea; IUGG: Potsdam, Germany, 1961; Volume 10, pp. 11–30. [Google Scholar]
- Cui, T.; Cao, W.; Zhang, J.; Hao, Y.; Yu, Y.; Zu, T.; Wang, D. Diurnal variability of ocean optical properties during a coastal algal bloom: Implications for ocean colour remote sensing. Int. J. Remote Sens. 2013, 34, 8301–8318. [Google Scholar] [CrossRef]
- Loisel, H.; Vantrepotte, V.; Norkvist, K.; Mériaux, X.; Kheireddine, M.; Ras, J.; Pujo-Pay, M.; Combet, Y.; Leblanc, K.; Dall’Olmo, G.; et al. Characterization of the Bio-Optical Anomaly and Diurnal Variability of Particulate Matter, as Seen from Scattering and Backscattering Coefficients, in Ultra-Oligotrophic Eddies of the Mediterranean Sea. Biogeosciences 2011, 8, 3295–3317. [Google Scholar] [CrossRef]
- Mercado, J.M.; Ramírez, T.; Cortés, D.; Sebastián, M.; Reul, A.; Bautista, B. Diurnal Changes in the Bio-Optical Properties of the Phytoplankton in the Alborán Sea (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2006, 69, 459–470. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780521151757. [Google Scholar]
- Morel, A. Meeting the Challenge of Monitoring Chlorophyll in the Ocean from Outer Space. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 521–534. ISBN 978-1-4020-4516-5. [Google Scholar]
- Santamaría-del-Angel, E.; Soto, I.; Millán-Nuñez, R.; González-Silvera, A.; Wolny, J.; Cerdeira-Estrada, S.; Cajal-Medrano, R.; Muller-Karger, F.; Cannizzaro, J.; Padilla-Rosas, Y.; et al. Experiences and Recommendations for Environmental Monitoring Programs. In Environmental Science, Engineering and Technology; Sebastia-Frasquet, M.-T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; p. 32. ISBN 978-1-63482-189-6. [Google Scholar]
- Santamaría-del-Angel, E.; González-Silvera, A.; Millán-Nuñez, R.; Callejas-Jiménez, M.E.; Cajal-Medrano, R. Determining Dynamic Biogeographic Regions using Remote Sensing Data. In Handbook of Satellite Remote Sensing Image Interpretation: Applications for Marine Living Resources Conservation and Management; Morales, J., Stuart, V., Platt, T., Sathyendranath, S., Eds.; EU PRESPO and IOCCG: Dartmouth, NS, Canada, 2011; Chapter 19; pp. 273–293. [Google Scholar]
- Hernández-Terrones, L.; Rebolledo-Vieyra, M.; Merrino-Ibarra, M.; Soto, M.; LeCossee, A.; Monroy-Rios, E. Groundwater pollution in karstic region (NE Yucatán): Baseline nutrient content and flux to coastal ecosystems. Water Air Soil Pollut. 2011, 218, 517–528. [Google Scholar] [CrossRef]
- Moore, Y.H.; Stoessell, R.K.; Easley, D.H. Fresh-Water/Sea-Water Relationship within a Ground-Water Flow System, Northeastern Coast of the Yucatan Peninsula. Groundwater 1992, 30, 343–350. [Google Scholar] [CrossRef]
- Beddows, P.A.; Smart, P.L.; Whitaker, F.F.; Smith, S.L. Decoupled fresh-saline groundwater circulation of a coastal carbonate aquifer: Spatial patterns of temperature and specific electrical conductivity. J. Hydrol. 2007, 346, 18–32. [Google Scholar] [CrossRef]
- Hernández-Terrones, L.M.; Null, K.A.; Ortega-Camacho, D.; Paytan, A. Water quality assessment in the Mexican Caribbean: Impacts on the coastal ecosystem. Cont. Shelf Res. 2015, 102, 62–72. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Morales-Ojeda, S.M. Subtropical Karstic Coastal Lagoon Assessment, Southeast Mexico. The Yucatan Peninsula Case. In Coastal Lagoons: Critical Habitats of Environmental Change; Kennish, M.J., Paerl, H.W., Eds.; CRC Press: Boca Raton, FL, USA, 2010; p. 26. ISBN 9781420088304 1420088300. [Google Scholar]
- Sánchez, F.J.; Gámez, D.; Guevara, G.; Shirasago, G.; Obeso, M. Análisis de la circulación superficial de mesoescala en la bahía de Campeche mediante sensores activos y pasivos. Geos 2010, 30, 204. [Google Scholar]
- Monreal-Gómez, M.A.; Salas de León, D.A. Simulación de la circulación en la Bahía de Campeche. Geofís. Int. 1990, 29, 101–111. [Google Scholar]
- Merrell, W., Jr.; Morrison, J. On the circulation of the western Gulf of Mexico with observations from April 1978. J. Geophys. Res. 1981, 86, 4181–4185. [Google Scholar] [CrossRef]
- Cochrane, J.D. Investigations of the Yucatan current; the region of cold surface water. In Oceanography and Meteorology of the Gulf of Mexico; McLellan, H.J., Ed.; Annual Report Rep 61-15F; Department of Oceanography, Texas A&M University: College Station, TX, USA, 1961; pp. 5–6. [Google Scholar]
- Carriquiry, J.D.; Sanchez, A. Sedimentation in the Colorado River delta and Upper Gulf of California after nearly a century of discharge loss. Mar. Geol. 1999, 158, 125–145. [Google Scholar] [CrossRef]
- Brusca, R.C.; Álvarez-Borrego, S.; Hastings, P.A.; Findley, L.T. Colorado River flow and biological productivity in the Northern Gulf of California, Mexico. Earth Sci. Rev. 2017, 164, 1–30. [Google Scholar] [CrossRef]
- Santamaría-del Ángel, E.; Millán-Núñez, R.; De la Peña, G. Efecto de la turbidez en la productividad primaria en dos estaciones en el Área del Delta del Río Colorado. Cienc. Mar. 1996, 22, 483–493. [Google Scholar]
- Daessle, L.W.; Orozco, A.; Struck, U.; Camacho-Ibar, V.F.; van Geldern, R.; Santamaría-del-Ángel, E.; Barth, J.A.C. Sources and sinks of nutrients and organic carbon during the 2014 pulse flow of the Colorado River into Mexico. Ecol. Eng. 2017, 106, 799–808. [Google Scholar] [CrossRef]
- Orozco-Durán, A.; Daesslé, L.W.; Camacho-Ibar, V.F.; Ortiz-Campos, E.; Barth, J.A.C. Turnover and release of P-, N-, Si-nutrients in the Mexicali Valley (Mexico): Interactions between the lower Colorado River and adjacent ground-and surface water systems. Sci. Total Environ. 2015, 512–513, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Maldonado, J.A.; Santamaría-del-Ángel, E.; Sebastiá-Frasquet, M.T. Reflectances of SPOT multispectral images associated with the turbidity of the Upper Gulf of California. Rev. Teledetec. 2017, 49, 1–16. [Google Scholar] [CrossRef]
- Cepeda-Morales, J.; Durazo, R.; Millán-Nuñez, E.; De la Cruz-Orozco, M.; Sosa-Ávalos, R.; Espinosa-Carreón, T.L.; Soto-Mardones, L.; Gaxiola-Castro, G. Response of primary producers to the hydrographic variability in the southern region of the California Current System. Cienc. Mar. 2017, 43, 123–135. [Google Scholar] [CrossRef]
- Delgadillo-Hinojosa, F.; Camacho-Ibar, V.; Huerta-Díaz, M.A.; Torres-Delgado, V.; Pérez-Brunius, P.; Lares, L.; Castro, R. Seasonal behavior of dissolved cadmium and Cd/PO 4 ratio in Todos Santos Bay: A retention site of upwelled waters in the Baja California peninsula, Mexico. Mar. Chem. 2015, 168, 37–48. [Google Scholar] [CrossRef]
- Durazo, R.; Gaxiola-Castro, G.; Lavaniegos, B.; Castro-Valdez, R.; Gómez-Valdés, J.; Da, S.; Mascarenhas, A., Jr. Oceanographic conditions west of the Baja California coast, 2002-2003: A weak El Niño and subarctic water enhancement. Cienc. Mar. 2005, 31, 537–552. [Google Scholar] [CrossRef]
- Linacre, L.; Durazo, R.; Hernández-Ayón, J.M.; Delgadillo-Hinojosa, F.; Cervantes-Díaz, G.; Lara-Lara, J.R.; Camacho-Ibar, V.; Siqueiros-Valencia, A.; Bazán-Guzmán, C. Temporal variability of the physical and chemical water characteristics at a coastal monitoring observatory: Station Ensenada. Cont. Shelf Res. 2010, 30, 1730–1742. [Google Scholar] [CrossRef]
- Espinosa-Carreón, T.L.; Gaxiola-Castro, G.; Durazo, R.; De la Cruz-Orozco, M.E.; Norzagaray-Campos, M.; Solana-Arellano, E. Influence of anomalous subarctic water intrusion on phytoplankton production off Baja California. Cont. Shelf Res. 2015, 92, 108–121. [Google Scholar] [CrossRef]
- Millán-Núñez, E.; Macias-Carballo, M. Phytogeography associated at spectral absorption shapes in the southern region of the California current. Calif. Ocean. Fish. Investig. Rep. 2014, 55, 183–196. [Google Scholar]
- Gutierrez-Mejia, E.; Lares, M.L.; Huerta-Diaz, M.A.; Delgadillo-Hinojosa, F. Cadmium and phosphate variability during algal blooms of the dinoflagellate Lingulodinium polyedrum in Todos Santos Bay, Baja California, Mexico. Sci. Total Environ. 2016, 541, 865–876. [Google Scholar] [CrossRef] [PubMed]
- COFEPRIS. State Sanitary Emergencies by Red Tide (Mexico). Available online: Http://www.cofepris.gob.mx/AZ/Paginas/Marea%20Roja/EmergenciasSanitariasEstatales.aspx (accessed on 24 January 2018).
- Mitchell, B.G.; Kahru, M.; Wieland, J.; Stramska, M. Determination of spectral absorption coefficients of particles, dissolved material and phytoplankton for discrete water samples. In Ocean Optics Protocols for Satellite Ocean Color Sensor Validation; NASA, Mueller, J.L., Fargion, G.S., Eds.; Flight Space Center: Greenbelt, MD, USA, 2002; Volume 3, pp. 231–257. [Google Scholar]
- Santamaría-del-Angel, E.; Millán-Núñez, R.; González-Silvera, A.; Callejas-Jiménez, M.; Cajal-Medrano, R.; Galindo-Bect, M. The response of shrimp fisheries to climate variability off Baja California, México. ICES J. Mar. Sci. 2011, 68, 766–772. [Google Scholar] [CrossRef]
- Hirata, T.; Aiken, J.; Smyth, T.J.; Barlow, R.G. An absorption model to derive phytoplankton size classes from satellite ocean colour. Remote Sens. Environ. 2008, 112, 3153–3159. [Google Scholar] [CrossRef]
- Aiken, J.; Hardman-Mountford, N.; Barlow, R.; Fishwick, J.; Hirata, T.; Smyth, T. Functional links between bioenergetics and bio-optical traits of phytoplankton taxonomic groups: An overarching hypothesis with applications for ocean colour remote sensing. J. Plankton Res. 2008, 30, 165–181. [Google Scholar] [CrossRef]
- Stuart, V.; Sathyendranath, S.; Platt, T.; Maass, H.; Irwin, B.D. Pigments and species composition of natural phytoplankton populations: Effect on the absorption spectra. J. Plankton Res. 1998, 20, 187–217. [Google Scholar] [CrossRef]
- Lohrenz, S.E.; Weidemann, A.D.; Tuel, M. Phytoplankton spectral absorption as influenced by community size structure and pigment composition. J. Plankton Res. 2003, 25, 35–61. [Google Scholar] [CrossRef]
- Wu, J.; Hong, H.; Shang, S.; Dai, M.; Lee, Z. Variation of phytoplankton absorption coefficients in the northern South China Sea during spring and autumn. Biogeosci. Discuss. 2007, 4, 1555–1584. [Google Scholar] [CrossRef]
- Millán-Nuñez, E.; Millán-Nuñez, R. Specific Absorption Coefficient and Phytoplankton Community Structure in the Southern Region of the California Current during January 2002. J. Oceanogr. 2010, 66, 719–730. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur velvollkommung der quantitative phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Haywood, A.J.; Steidinger, K.A.; Truby, E.W.; Bergquist, P.R.; Bergquist, P.L.; Adamson, J.; MacKenzie, L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004, 40, 165–179. [Google Scholar] [CrossRef]
- Steidinger, K.A.; Wolny, J.L.; Haywood, A.J. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. Nova Hedwig. 2008, 133, 269–284. [Google Scholar]
- Gárate-Lizárraga, I.; Okolodkov, Y.; Cortés-Altamirano, R. Microalgas formadoras de florecimientos algales en el Golfo de California. In Florecimientos Algales Nocivos en México; García-Mendoza, E., Quijano-Scheggia, S.I., Olivos-Ortiz, A., Núñez-Vázquez, E.J., Eds.; CICESE: Ensenada, México, 2016; pp. 130–145. [Google Scholar]
- Quijano, S.I.; Barajas, M.; Chang, H.; Bates, S. The inhibitory effect of a non-yessotoxin-producing dinoflagellate, Lingulodinium polyedrum (Stein) Dodge, towards Vibrio vulnificus and Staphylococcus aureus. Rev. Biol. Trop. 2016, 64, 805–816. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a Determination: Improvements in Methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Mendoza, M.; Ortiz-Pérez, M.A. Caracterización geomorfológica del talud y la plataforma continentales de Campeche-Yucatán, México. Investig. Geogr. 2000, 43, 7–31. [Google Scholar]
- Herrera-Silveira, J.A. Ecología de los Productores Primarios en la Laguna de Celestún, México. Patrones de Variación Espacial y Temporal. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 1993. [Google Scholar]
- Aguilar-Trujillo, A.C.; Okolodkov, Y.B.; Herrera-Silveira, J.A.; Merino-Virgilio, F.D.C.; Galicia-García, C. Taxocoenosis of epibenthic dinoflagellates in the coastal waters of the northern Yucatan Peninsula before and after the harmful algal bloom event in 2011–2012. Mar. Pollut. Bull. 2017, 119, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, M.J.; Álvarez-Torres, P.; Horak-Romo, K.P.; Ortega-Izaguirre, R. Harmful algal blooms and eutrophication along the mexican coast of the Gulf of Mexico large marine ecosystem. Environ. Dev. 2017, 22, 120–128. [Google Scholar] [CrossRef]
- Ochoa, J.L.; Hernández-Becerril, D.U.; Lluch-Cota, S.; Arredondo-Vega, B.O.; Nuñez-Vázquez, E.; Heredia-Tapia, A.; Alonso-Rodríguez, R. Marine biotoxins and harmful algal blooms in Mexico’s Pacific littoral. Harmful algal blooms in the PICES region of the North Pacific. PICES Sci. Rep. 2002, 23, 119–128. [Google Scholar]
- Hernández-Becerril, D.U. Morfología y taxonomía de algunas especies de diatomeas del género Coscinodiscus de las costas del Pacífico mexicano. Rev. Biol. Trop. 2000, 48, 7–18. [Google Scholar]
- Liefer, J.D.; Robertson, A.; MacIntyre, H.L.; Smith, W.L.; Dorsey, C.P. Characterization of a toxic Pseudo-nitzschia spp. bloom in the Northern Gulf of Mexico associated with domoic acid accumulation in fish. Harmful Algae 2013, 26, 20–32. [Google Scholar] [CrossRef]
- Schnetzer, A.; Miller, P.E.; Schaffner, R.A.; Stauffer, B.A.; Jones, B.H.; Weisberg, S.B.; Caron, D.A. Blooms of Pseudo-nitzschia and domoic acid in the San Pedro Channel and Los Angeles harbor areas of the Southern California Bight, 2003–2004. Harmful Algae 2007, 6, 372–387. [Google Scholar] [CrossRef]
- Peña Manjarrez, J.; Gaxiola-Castro, G.; Helenes-Escamilla, J. Environmental factors influencing the variability of Lingulodinium polyedrum and Scrippsiella trochoidea (Dinophyceae) cyst production. Cienc. Mar. 2009, 35, 1–14. [Google Scholar] [CrossRef]
- Ruiz-de la Torre, M.C.; Maske, H.; Ochoa, J.; Almeda-Jauregui, C.O. Maintenance of Coastal Surface Blooms by Surface Temperature Stratification and Wind Drift. PLoS ONE 2013, 8, e58958. [Google Scholar] [CrossRef]
- Kudela, R.M.; Bickel, A.; Carter, M.L.; Howard, M.D.; Rosenfeld, L. The monitoring of harmful algal blooms through ocean observing: The development of the California Harmful Algal Bloom Monitoring and Alert Program. Coast. Ocean Obs. Syst. 2015, 58–75. [Google Scholar] [CrossRef]
- Reinart, A.; Paavel, B.; Pierson, D.; Strombeck, N. Inherent and apparent optical properties of Lake Peipsi, Estonia. Boreal Environ. Res. 2004, 9, 429–445. [Google Scholar]
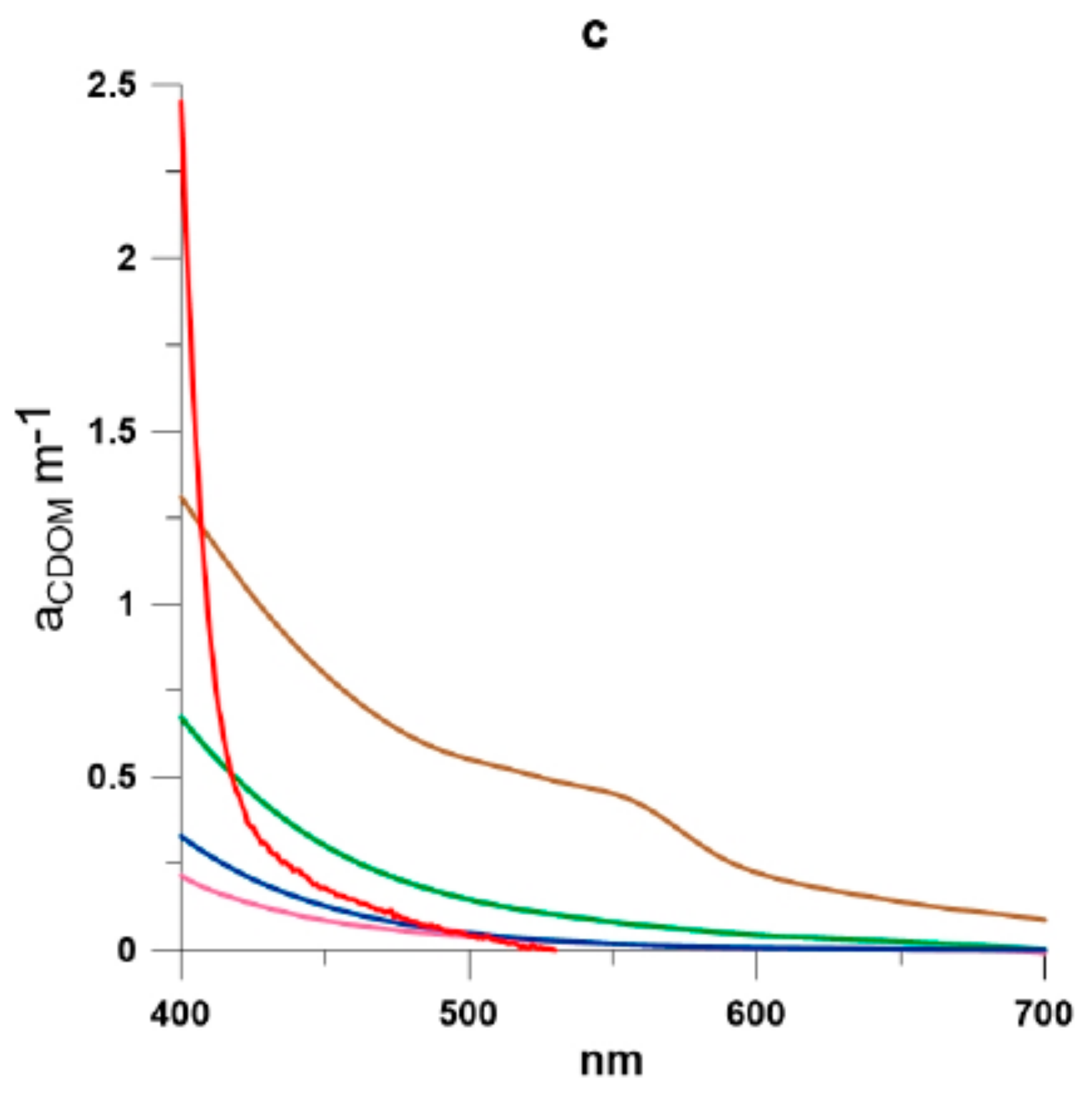
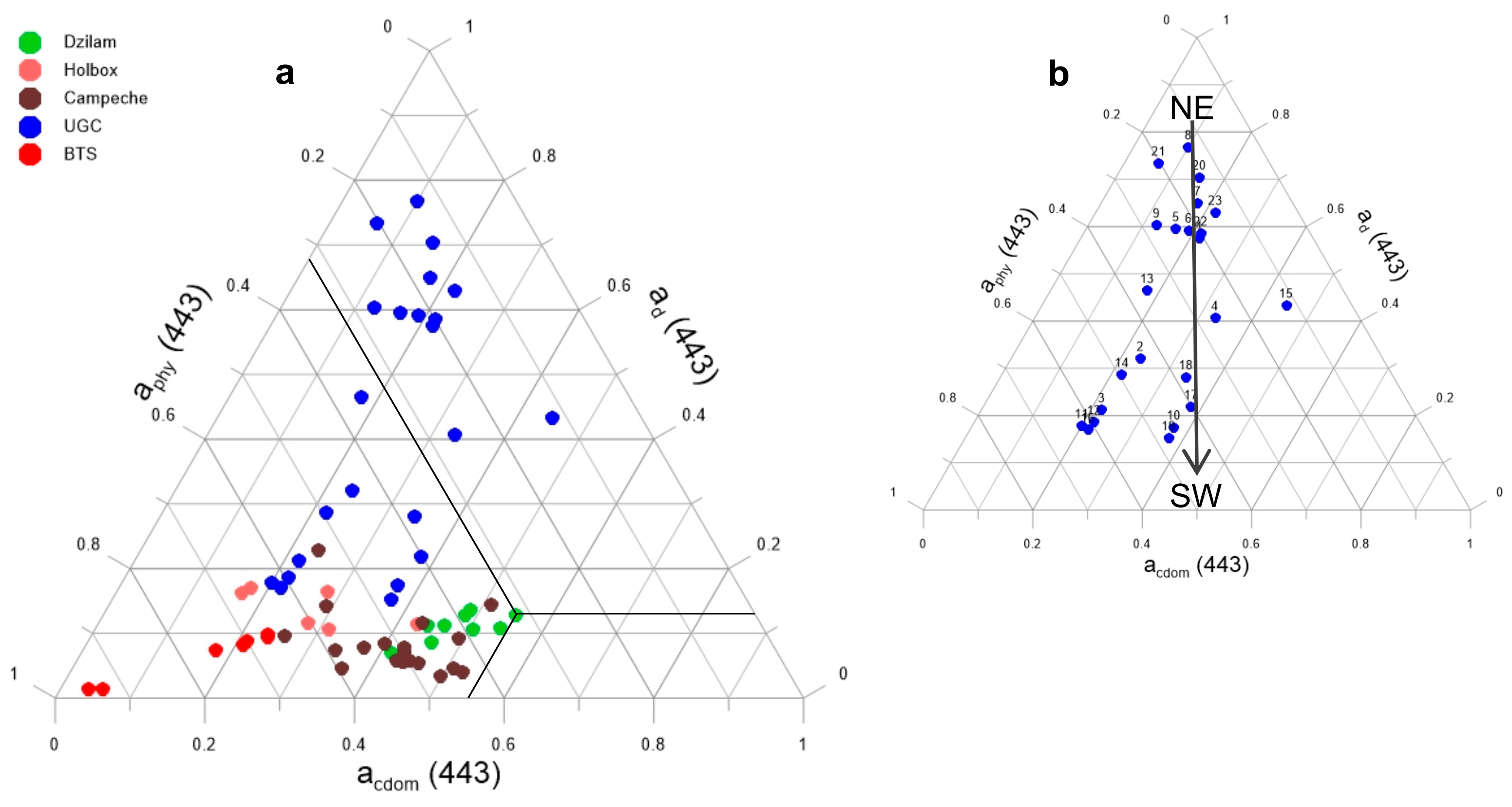
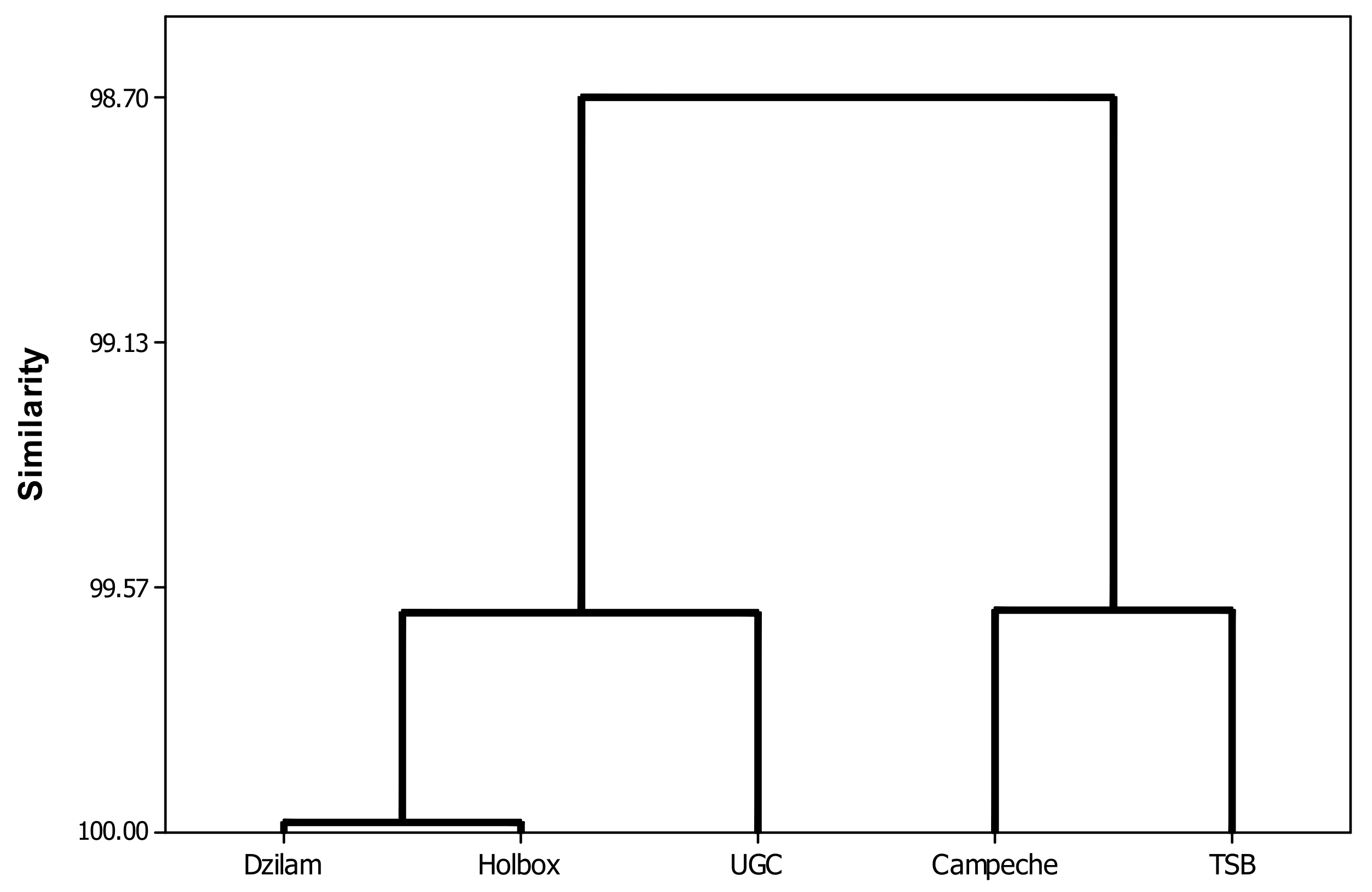
| Campaign | Sta. | aphy (440 nm) | aphy (675 nm) | Ratio B/R | Campaign | Sta. | aphy (440 nm) | aphy (675 nm) | Ratio B/R |
|---|---|---|---|---|---|---|---|---|---|
| Dzilam | 1 | 0.110 | 0.038 | 2.86 | UGC | 1 | 0.039 | 0.078 | 1.99 |
| 2 | 0.091 | 0.032 | 2.83 | 2 | 0.035 | 0.084 | 2.40 | ||
| 3 | 0.167 | 0.061 | 2.75 | 3 | 0.039 | 0.095 | 2.43 | ||
| 4 | 0.371 | 0.229 | 1.62 | 4 | 0.017 | 0.042 | 2.47 | ||
| 5 | 0.264 | 0.155 | 1.71 | 5 | 0.041 | 0.085 | 2.07 | ||
| 6 | 0.179 | 0.064 | 2.80 | 6 | 0.024 | 0.054 | 2.27 | ||
| 7 | 0.170 | 0.072 | 2.36 | 7 | 0.026 | 0.055 | 2.09 | ||
| 8 | 0.131 | 0.035 | 3.79 | 8 | 0.036 | 0.084 | 2.35 | ||
| 9 | 0.131 | 0.028 | 4.76 | 9 | 0.048 | 0.105 | 2.16 | ||
| Holbox | 1 | 0.101 | 0.030 | 3.36 | 10 | 0.038 | 0.093 | 2.43 | |
| 2 | 0.076 | 0.023 | 3.37 | 11 | 0.045 | 0.105 | 2.31 | ||
| 3 | 0.118 | 0.038 | 3.08 | 12 | 0.043 | 0.093 | 2.18 | ||
| 4 | 0.072 | 0.024 | 3.06 | 13 | 0.031 | 0.073 | 2.33 | ||
| 5 | 0.094 | 0.028 | 3.34 | 14 | 0.032 | 0.073 | 2.28 | ||
| 6 | 0.387 | 0.151 | 2.57 | 15 | 0.003 | 0.007 | 2.59 | ||
| Campeche | 1 | 0.067 | 0.016 | 4.28 | 16 | 0.029 | 0.066 | 2.24 | |
| 2 | 0.034 | 0.006 | 5.33 | 17 | 0.032 | 0.071 | 2.21 | ||
| 3 | 0.036 | 0.006 | 6.47 | 18 | 0.028 | 0.066 | 2.35 | ||
| 4 | 0.032 | 0.006 | 5.24 | 19 | 0.032 | 0.073 | 2.27 | ||
| 5 | 0.136 | 0.054 | 2.53 | 20 | 0.020 | 0.072 | 3.54 | ||
| 6 | 0.111 | 0.044 | 2.51 | 21 | 0.059 | 0.201 | 3.39 | ||
| 7 | 0.129 | 0.027 | 4.80 | 22 | 0.021 | 0.053 | 2.57 | ||
| 8 | 0.132 | 0.028 | 4.77 | 23 | 0.026 | 0.067 | 2.55 | ||
| 9 | 0.114 | 0.030 | 3.83 | TSB | 1 | 0.144 | 0.053 | 2.70 | |
| 10 | 0.295 | 0.154 | 1.91 | 2 | 0.139 | 0.047 | 2.96 | ||
| 11 | 0.135 | 0.067 | 2.02 | 3 | 0.172 | 0.061 | 2.82 | ||
| 12 | 0.685 | 0.338 | 2.03 | 4 | 0.365 | 0.150 | 2.43 | ||
| 13 | 0.127 | 0.052 | 2.43 | 5 | 0.219 | 0.085 | 2.58 | ||
| 14 | 0.590 | 0.287 | 2.06 | 6 | 3.077 | 1.773 | 1.74 | ||
| 15 | 0.543 | 0.251 | 2.17 | 7 | 3.617 | 1.815 | 1.99 | ||
| 16 | 1.006 | 0.464 | 2.17 | ||||||
| 17 | 0.370 | 0.172 | 2.16 | ||||||
| 18 | 0.243 | 0.114 | 2.13 | ||||||
| 19 | 0.065 | 0.021 | 3.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Maldonado, J.A.; Santamaría-del-Ángel, E.; González-Silvera, A.; Cervantes-Rosas, O.D.; López, L.M.; Gutiérrez-Magness, A.; Cerdeira-Estrada, S.; Sebastiá-Frasquet, M.-T. Identification of Phytoplankton Blooms under the Index of Inherent Optical Properties (IOP Index) in Optically Complex Waters. Water 2018, 10, 129. https://doi.org/10.3390/w10020129
Aguilar-Maldonado JA, Santamaría-del-Ángel E, González-Silvera A, Cervantes-Rosas OD, López LM, Gutiérrez-Magness A, Cerdeira-Estrada S, Sebastiá-Frasquet M-T. Identification of Phytoplankton Blooms under the Index of Inherent Optical Properties (IOP Index) in Optically Complex Waters. Water. 2018; 10(2):129. https://doi.org/10.3390/w10020129
Chicago/Turabian StyleAguilar-Maldonado, Jesús A., Eduardo Santamaría-del-Ángel, Adriana González-Silvera, Omar D. Cervantes-Rosas, Lus M. López, Angélica Gutiérrez-Magness, Sergio Cerdeira-Estrada, and María-Teresa Sebastiá-Frasquet. 2018. "Identification of Phytoplankton Blooms under the Index of Inherent Optical Properties (IOP Index) in Optically Complex Waters" Water 10, no. 2: 129. https://doi.org/10.3390/w10020129








