Gas Exchanges and Stem Water Potential Define Stress Thresholds for Efficient Irrigation Management in Olive (Olea europea L.)
Abstract
:1. Introduction
2. Materials and Methods
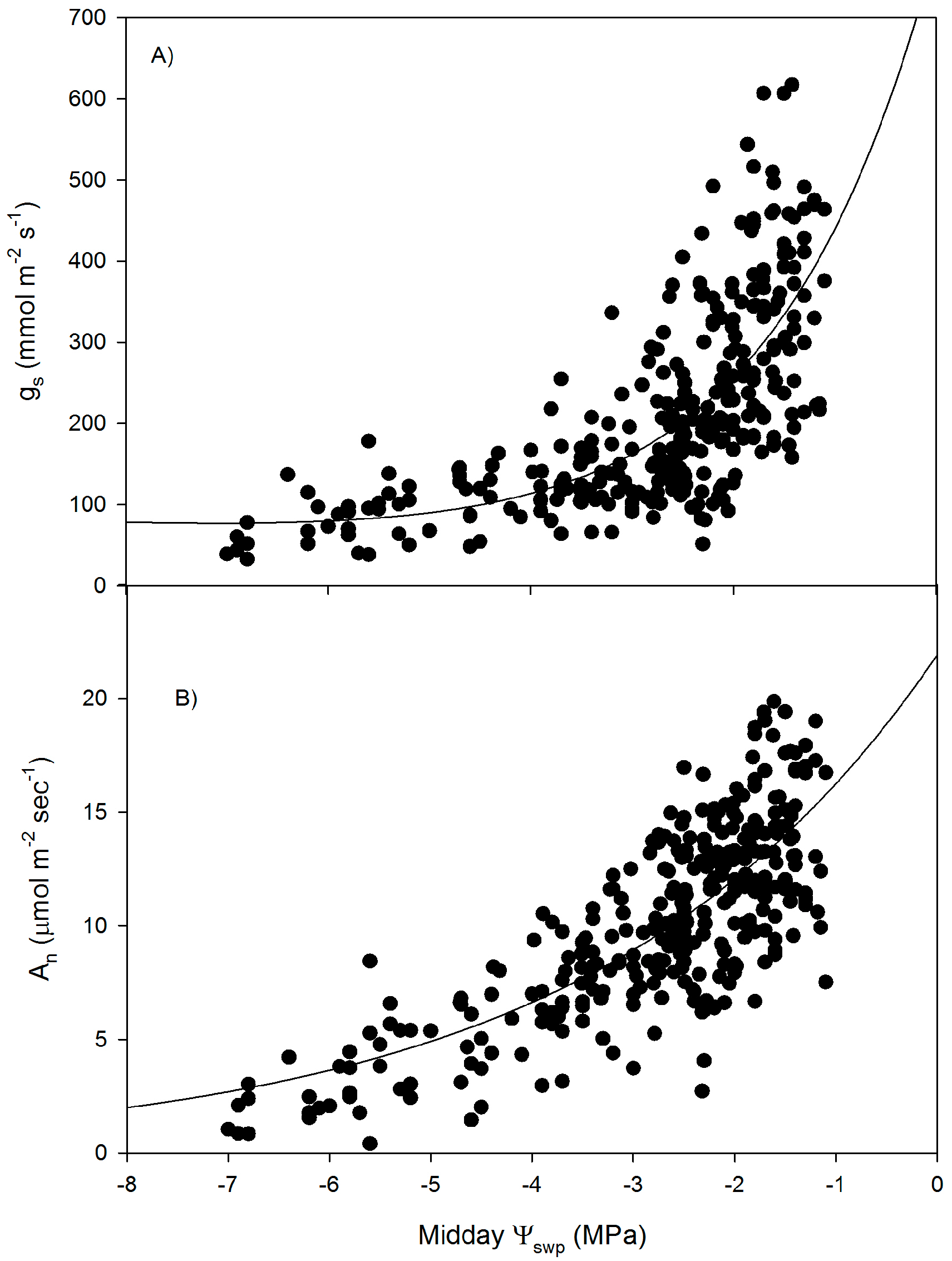
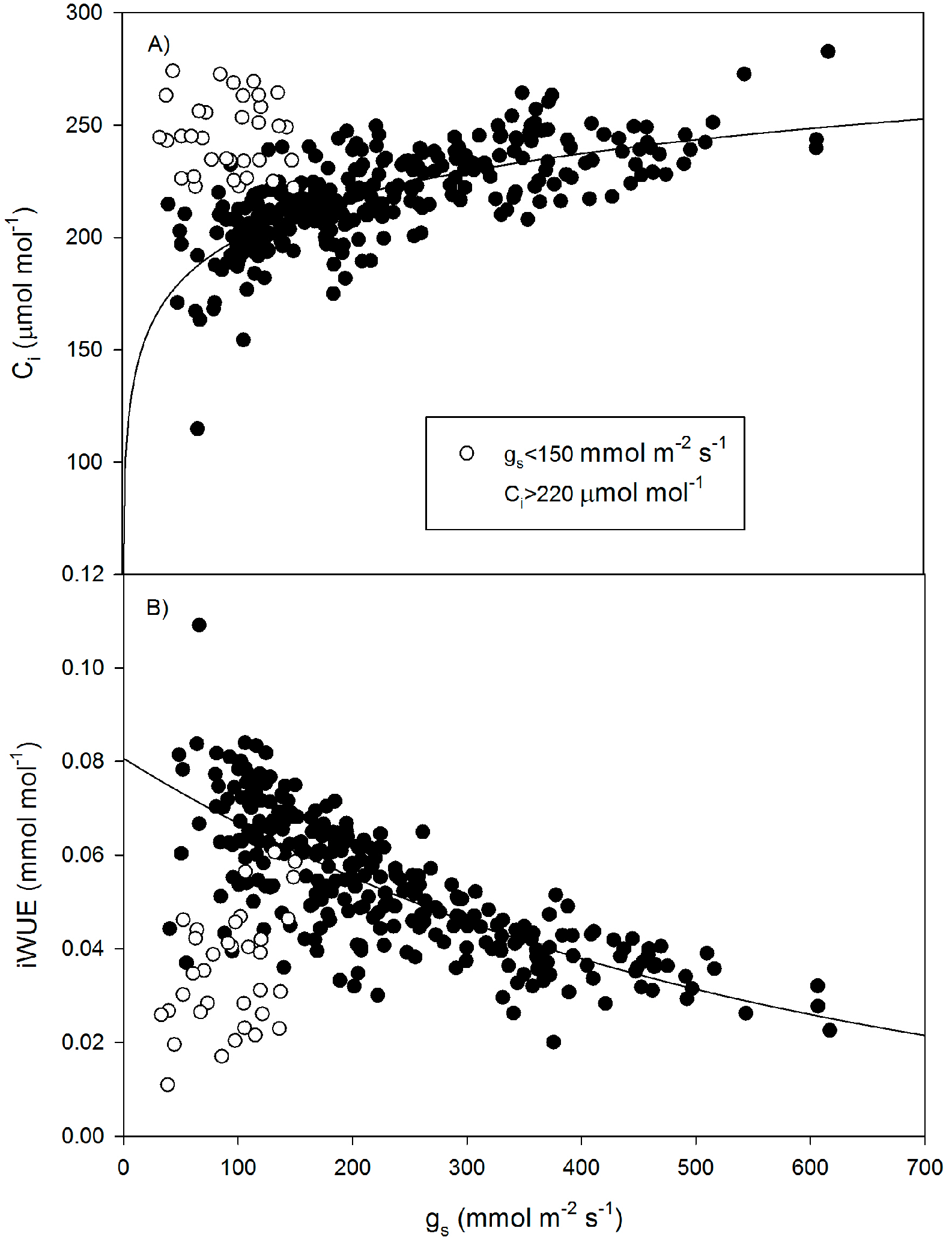
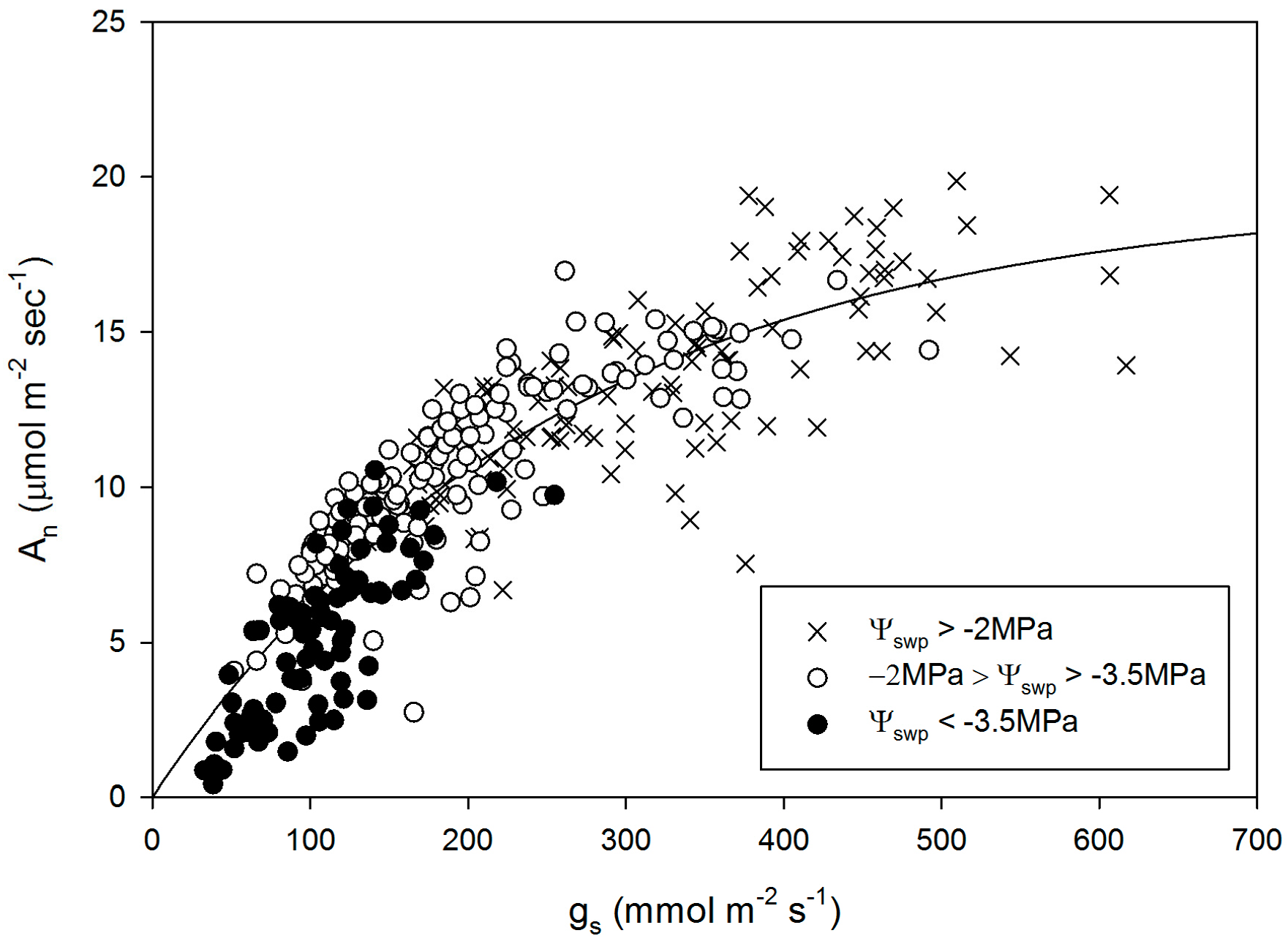
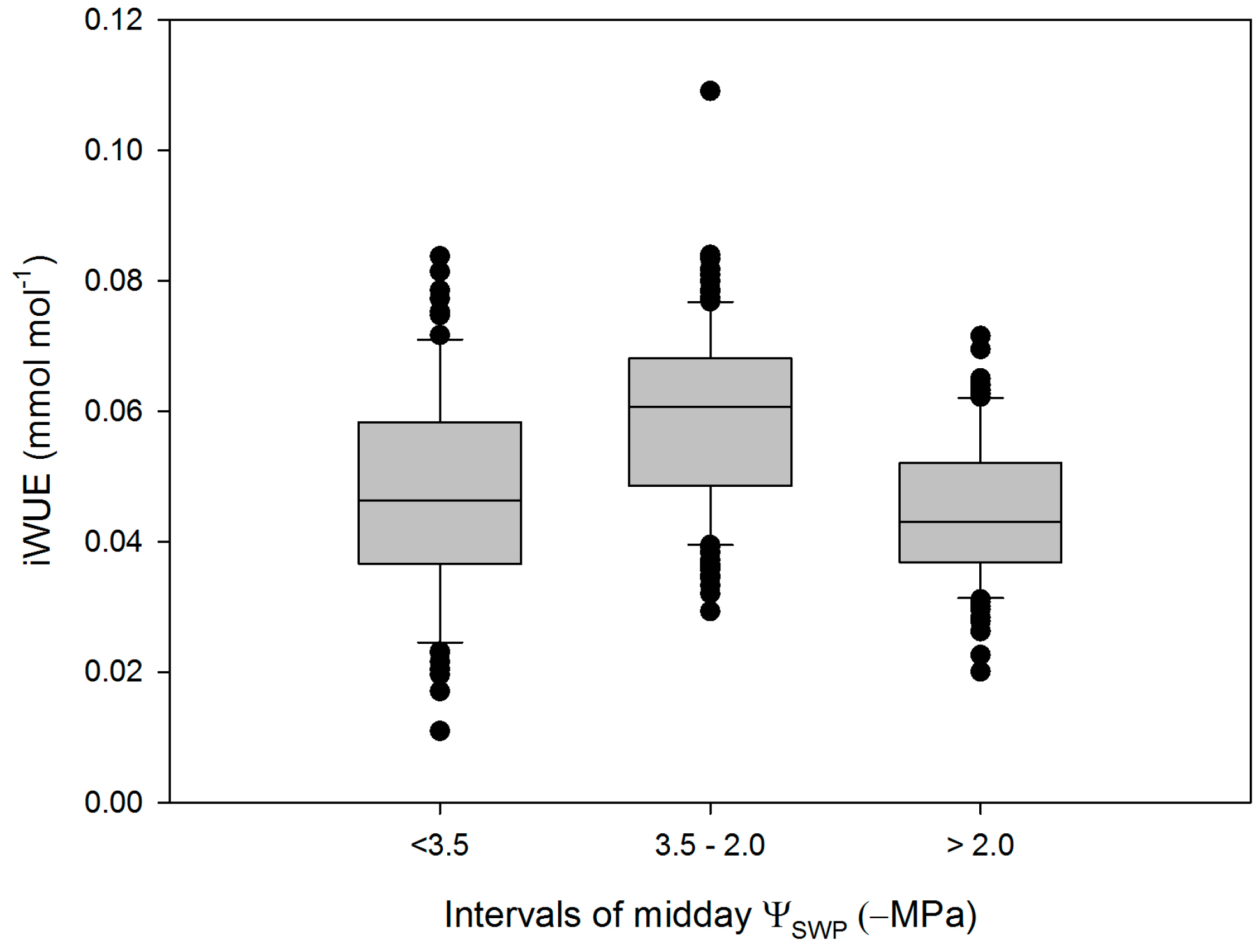
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Camposeo, S.; Godini, A. Preliminary observations about the performance of 13 varieties according to the super high density oliveculture training system in Apulia (southern Italy). Adv. Hortic. Sci. 2010, 24, 16–20. [Google Scholar]
- Tous, J.; Romero, A.; Plana, J.; Hermoso, J.F. Olive oil cultivars suitable for very high density planting conditions. Acta Hortic. 2008, 791, 403–408. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Hermoso, J.F. New trends in olive orchard design for continuous mechanical harvesting. Adv. Hortic. Sci. 2010, 24, 43–52. [Google Scholar]
- Pastor, M.; García-Vila, M.; Soriano, M.A.; Vega, V.; Fereres, E. Productivity of olive orchards in response to tree density. J. Hortic. Sci. Biotechnol. 2007, 82, 555–562. [Google Scholar] [CrossRef]
- Policarpo, M.; Talluto, G.; Lo Bianco, R. Vegetative and productive responses of ‘Conference’ and ‘Williams’ pear trees planted at different in-row spacing. Sci. Hortic. 2006, 109, 322–331. [Google Scholar] [CrossRef]
- Marino, G.; Pernice, F.; Marra, F.P.; Caruso, T. Validation of an online system for the continuous monitoring of tree water status for sustainable irrigation managements in olive (Olea europaea L.). Agric. Water Manag. 2016, 177, 298–307. [Google Scholar] [CrossRef]
- Marra, P.F.; Marino, G.; Marchese, A.; Caruso, T. Effects of different irrigation regimes on a super-high density olive grove cv. ‘Arbequina’, vegetative growth, productivity and polyphenol content of the oil. Irrig. Sci. 2016, 34, 1–13. [Google Scholar] [CrossRef]
- Grattan, S.R.; Berenguer, M.J.; Connell, J.H.; Polito, V.S.; Vossen, P.M. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M. Summer deficit-irrigation strategies in a hedgerow olive orchard cv. ‘Arbequina’: Effect on fruit characteristics and yield. Irrig. Sci. 2013, 31, 259–269. [Google Scholar] [CrossRef]
- Turner, N.C. Plant water relations and irrigation management. Agric. Water Manag. 1990, 17, 59–73. [Google Scholar] [CrossRef]
- Fereres, E.; Goldhamer, D. Deciduous fruit and nut trees. In Irrigation of Agricultural Crops. Agronomy Monograph No. 30; Stewart, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 987–1017. [Google Scholar]
- Moriana, A.; Pérez-López, D.; Prieto, M.H.; Ramírez-Santa-Pau, M.; Pérez-Rodriguez, J.M. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M.; Leal, A.; Pezuela, C. Relationship of stem water potential and leaf conductance to vegetative growth of young olive trees in a hedgerow orchard. Aust. J. Agric. Res. 2008, 59, 270–279. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M. Physiological and growth responses to irrigation of a newly established hedgerow Olive orchard. HortScience 2010, 45, 809–814. [Google Scholar]
- Gómez-del-Campo, M. Summer deficit irrigation in a hedgerow olive orchard cv. Arbequina: Relationship between soil and tree water status, and growth and yield components. Span. J. Agric. Res. 2013, 11, 547–557. [Google Scholar] [CrossRef]
- Corell, M.; Pérez-López, D.; Martín-Palomo, M.J.; Centeno, A.; Girón, I.; Galindo, A.; Moreno, F. Comparison of the water potential baseline in different locations. Usefulness for irrigation scheduling of olive orchards. Agric. Water Manag. 2016, 177, 308–316. [Google Scholar] [CrossRef]
- Di Vaio, C.; Marallo, N.; Marino, G.; Caruso, T. Effect of water stress on dry matter accumulation and partitioning in pot-grown olive trees (cv. Leccino and Racioppella). Sci. Hortic. 2013, 164, 155–159. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano, E.J.; Galmés, J.; Gulías, J.; Lefi, E.-K.; Martínez-Cañellas, S.F.; Moreno, M.T.; Ribas-Carbó, M.; et al. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: The interest of stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Flexas, J.; Escalona, J.M.; Evain, S.; GulÌas, J.; Moya, I.; Osmond, C.B.; Medrano, H. Steady-state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C3 plants. Physiol. Plant. 2002, 114, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E.; Moreno, F.; Girón, I.F.; Blázquez, O.M. Stomatal control of water use in olive tree leaves. Plant Soil 1997, 190, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Giorio, P.; Sorrentino, G.; d’Andria, R. Stomatal behaviour, leaf water status and photosynthetic response in fieldgrown olive trees under water deficit. Environ. Exp. Bot. 1999, 42, 95–104. [Google Scholar] [CrossRef]
- Bongi, G.; Palliotti, A. Olive. In Handbook of Environmental Physiology of Fruit Crops; Temperate Crops Volume I; Shaffer, B., Anderson, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 165–187. [Google Scholar]
- Angelopoulos, K.; Dichio, B.; Xiloyannis, C. Inhibition of photosynthesis in olive trees (Olea europaea L.) during water stress and rewatering. J. Exp. Bot. 1996, 47, 1093–1100. [Google Scholar] [CrossRef]
- Di Vaio, C.; Marra, F.P.; Scaglione, G.; La Mantia, M.; Caruso, T. The effect of different vigour olive clones on growth, dry matter partitioning and gas exchange under water deficit. Sci. Hortic. 2012, 134, 72–78. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitation revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 1985, 8, 95–104. [Google Scholar] [CrossRef]
- Düring, H. CO2 assimilation and photorespiration of grapevine leaves: Responses to light and drought. Vitis 1988, 27, 199–208. [Google Scholar]
- Quick, W.P.; Chaves, M.M.; Wendler, R.; David, M.; Rodrigues, M.L.; Passaharinho, J.A.; Pereira, J.S.; Adcock, M.D.; Leegood, R.C.; Stitt, M. The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant Cell Environ. 1992, 15, 25–35. [Google Scholar] [CrossRef]
- Fernández, J.E.; Diaz-Espejo, A.; D’Andria, R.; Sebastiani, L.; Tognetti, R. Potential and limitations of improving olive orchard design and management through modelling. Plant Biosyst. 2008, 142, 130–137. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration. In Guidelines for Computing Crop Water Requirements; Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Fernández, J.E.; Díaz-Espejo, A.; Infante, J.M.; Durán, P.; Palomo, M.J.; Chamorro, V.; Girón, I.F.; Villagarcía, L. Water relations and gas exchange in olive trees under regulated deficit irrigation and partial rootzone drying. Plant Soil 2006, 284, 273–291. [Google Scholar] [CrossRef]
- Orgaz, F.; Villalobos, F.; Testi, L.; Pastor, M.; Hidalgo, J.C.; Fereres, E. Programación de riegos en plantaciones de olivar. Metodología para el cálculo de las necesidades de agua de riego en el olivar regado por goteo. In Cultivo Del Olivo Con Riego Localizado; Pastor, M., Ed.; Mundi-Prensa: Madrid, Spain, 2005; pp. 83–138. [Google Scholar]
- Orgaz, F.; Fereres, E.; Riego, D.B. El Cultivo Del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 1997; pp. 251–272. [Google Scholar]
- Testi, L.; Villalobos, F.J.; Orgaz, F. Evapotranspiration of a young irrigated olive orchard in southern Spain. Agric. Meteorol. 2004, 121, 1–18. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Env. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Marino, G.; Macaluso, L.; Marra, F.P.; Ferguson, L.; Marchese, A.; Campisi, G.; Volo, P.; Laudicina, V.A.; Caruso, T. Horticultural performance of 23 Sicilian olive genotypes in hedgerow systems: Vegetative growth, productive potential and oil quality. Sci. Hortic. 2017, 217, 217–225. [Google Scholar] [CrossRef]
- Begg, J.E.; Turner, N.C. Water potential gradients in field tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, K.J. Porometry in SEB. In Symposium of Instrumentation for Environmental Physiology; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Parkinson, K.J.; Day, W.; Leach, J.E. A portable system for measuring the photosynthesis and transpiration of graminaceous leaves. J. Exp. Bot. 1980, 31, 1441–1453. [Google Scholar] [CrossRef]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Martín-Aranda, J. Water status of olive trees under dry-farming and drip-irrigation. Acta Hortic. 1993, 335, 157–164. [Google Scholar] [CrossRef]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.D.M.; Cuevas, M.V.; Hernandez-Santana, V.; Rodriguez-Dominguez, C.M.; Padilla-Díaz, C.M.; Fernández, J.E. Classification models for automatic identification of daily states from leaf turgor related measurements in olive. Comput. Electron. Agric. 2017, 142, 181–189. [Google Scholar] [CrossRef]
- Naor, A.; Schneider, D.; Ben-Gal, A.; Zipori, I.; Dag, A.; Kerem, Z.; Birger, R.; Peres, M.; Gal, Y. The effects of crop load and irrigation rate in the oil accumulation stage on oil yield and water relations of ‘Koroneiki’ olives. Irrig. Sci. 2013, 31, 781–791. [Google Scholar] [CrossRef]
- López-Bernal, A.; García-Tejera, O.; Testi, L.; Orgaz, F.; Villalobos, F.J. Stomatal oscillations in olive trees: Analysis and methodological implications. Tree Physiol. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Zimmermann, U.; Rüger, S.; Martín-Palomo, M.J.; Torres-Ruiz, J.M.; Cuevas, M.V.; Sann, C.; Ehren-berger, W.; et al. Online-monitoring of tree water stress in a hedgerow olive orchard using the leaf patch clamp pressure probe. Agric. Water Manag. 2011, 100, 25–35. [Google Scholar] [CrossRef]
- Tognetti, R.; d’Andria, R.; Lavini, A.; Morelli, G. The effect of deficit irrigation on crop yield and vegetative development of Olea europaea L. (cvs. Frantoio and Leccino). Eur. J. Agron. 2006, 25, 356–364. [Google Scholar] [CrossRef]
- Moriana, A.; Villalobos, F.J.; Fereres, E. Stomatal and photosynthetic responses of olive (Olea europaea L.) leaves to water deficits. Plant Cell Environ. 2002, 25, 395–405. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Walcroft, A.S.; Fernandez, J.E.; Hafidi, B.; Palomo, M.J.; Giron, I.F. Modeling photosynthesis in olive leaves under drought conditions. Tree Physiol. 2006, 26, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, M.V.; Torres-Ruiz, J.M.; Álvarez, R.; Jiménez, M.D.; Cuerva, J.; Fernández, J.E. Assessment of trunk diameter variation derived indices as waterstress indicators in mature olive trees. Agron. Water Manag. 2010, 97, 1293–1302. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Michelakis, N.; Tzombanakis, I. Effect of water amount and application date on yield and water utilisation efficiency of ‘Koroneiki’ olives under drip irrigation. Adv. Hortic. Sci. 1992, 2, 82–84. [Google Scholar]
- Wahbi, S.; Wakrim, R.; Aganchich, B.; Serraj, R. Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate. I. Physiological and agronomic responses. Agric. Ecosyst. Environ. 2005, 106, 289–301. [Google Scholar] [CrossRef]
- Lawlor, D.W. The effects of water deficit on photosynthesis. In Environment and Plant Metabolism: Flexibility and Acclimation; Smirnoff, N., Ed.; BIOS Scientific Publishers: Oxford, UK, 1995; pp. 129–160. [Google Scholar]
- Cornic, G.; Massacci, A. Leaf photosynthesis under drought stress. In Advances in Photosynthesis: Photosynthesis and the Environment Volume 5; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 347–366. [Google Scholar]
- Ort, D.R.; Oxborough, K.; Wise, R.R. Depressions of photosynthesis in crops with water deficits, In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; BIOS Scientific Publishers: Oxford, UK, 1994; pp. 315–329. [Google Scholar]
- Brodribb, T. Dynamics of changing intercellular CO2 concentration (ci) during drought and determination of minimum functional ci. Plant Physiol. 1996, 111, 179–185. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, G.; Caruso, T.; Ferguson, L.; Marra, F.P. Gas Exchanges and Stem Water Potential Define Stress Thresholds for Efficient Irrigation Management in Olive (Olea europea L.). Water 2018, 10, 342. https://doi.org/10.3390/w10030342
Marino G, Caruso T, Ferguson L, Marra FP. Gas Exchanges and Stem Water Potential Define Stress Thresholds for Efficient Irrigation Management in Olive (Olea europea L.). Water. 2018; 10(3):342. https://doi.org/10.3390/w10030342
Chicago/Turabian StyleMarino, Giulia, Tiziano Caruso, Louise Ferguson, and Francesco Paolo Marra. 2018. "Gas Exchanges and Stem Water Potential Define Stress Thresholds for Efficient Irrigation Management in Olive (Olea europea L.)" Water 10, no. 3: 342. https://doi.org/10.3390/w10030342




