Assessment of the Water Treatment Process’s Empirical Model Predictions for the Management of Aesthetic and Health Risks Associated with Cyanobacteria
Abstract
:1. Introduction
- Sudden changes in raw water characteristics (for example, pH), disruption of treatment processes including malfunction of flocculation reactions, increasing the consumption of coagulant and generation of disinfection by-products due to organic materials associated with bloom matrix;
- Production of potent toxins with human health hazards from hepatotoxins to neurotoxins;
- Production of T&O compounds such as the most commonly occurring geosmin and 2-methylisoborneol (MIB). These T&O compounds are very difficult to remove from water, for example, conventional treatment (coagulation/clarification, filtration, and oxidation using chlorine and hydrogen peroxide) is inefficient for their treatment;
- How can utilities have confidence in the application of various treatment processes when they are often site/system specific?
- What is the evidence of the removals of cyanobacteria and metabolites reported in the literature and of the achievability of guidance manuals at the full-scale?
- How can we assess and validate the efficiencies of unit processes at individual treatment plants?
- What is the combined mitigation effect of the entire treatment train?
- In the case of a toxic bloom, how can we determine the point at which we are at risk of distributing water that does not meet the guidelines for toxin concentrations?
2. Materials and Methods
2.1. Development of Empirical Models for the Removal of Cyanobacteria and Their Metabolites
- The removals of the metabolites by PAC are the same in all water quality
- The PAC used is the equivalent of Activated Carbon Technologies’ PS1000 (unless otherwise stated)
- PAC adsorption will achieve the same removal of metabolites as determined in batch experiments undertaken in the laboratory
- PAC application removes only dissolved metabolites and does not affect the intracellular component
- The removal of the cyanobacteria through coagulation and clarification is constant at 95% regardless of the type of cyanobacteria, coagulant, or clarification process
- Coagulation and clarification does not affect the concentration of the dissolved metabolites
- Filtration does not remove dissolved or intracellular metabolites
- Chorine will lyse any cyanobacteria reaching the disinfection point
- Chlorination will achieve the same oxidation of toxins as determined in batch experiments undertaken in the laboratory
- The removals of the cyanotoxins by chlorination are the same in all water quality
2.1.1. PAC and Chlorine Removal Equations
2.1.2. Cyanobacteria and Metabolite Removal Tools
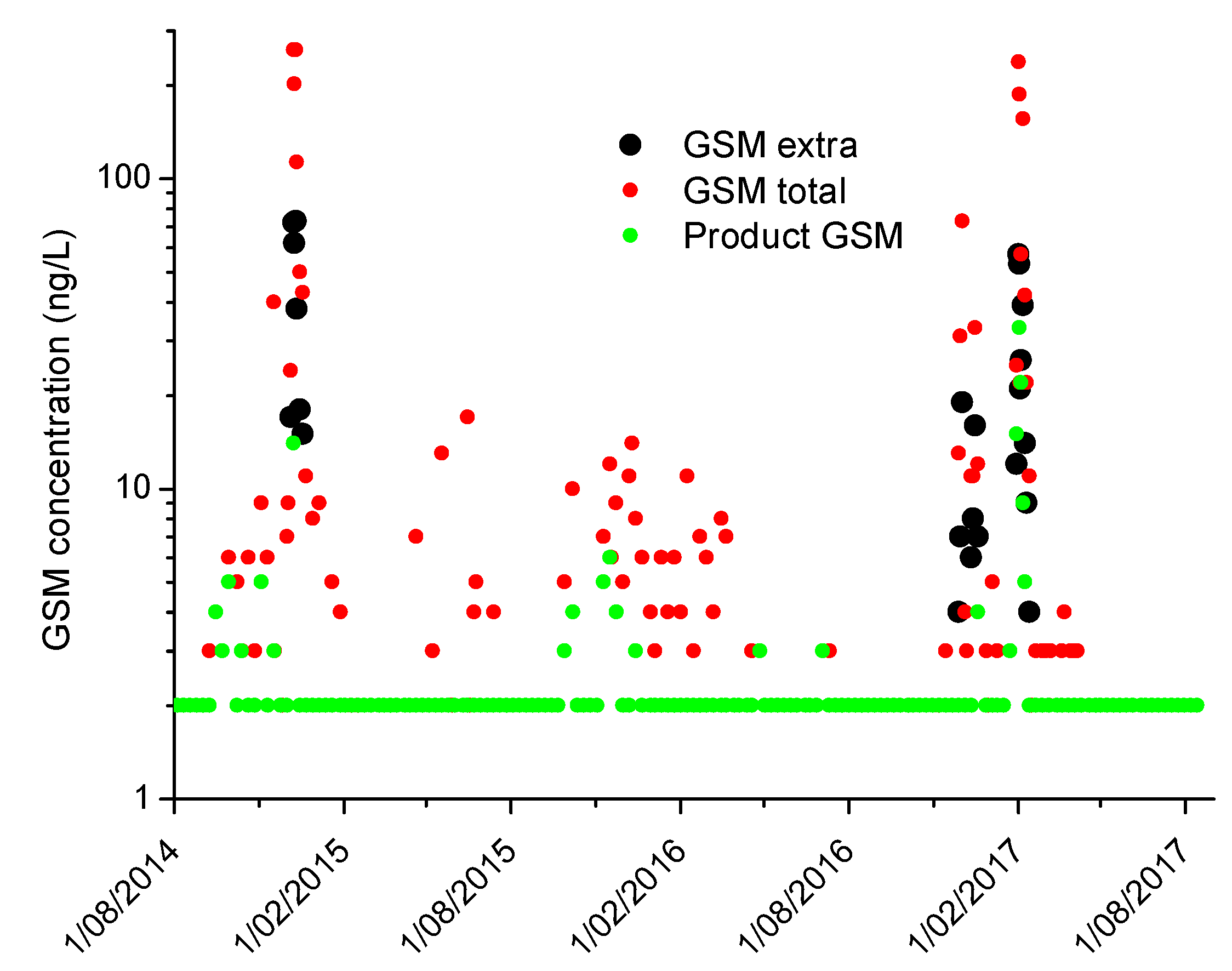
2.2. Monitoring in Full-Scale Plants for Verification of the Estimates Produced by the Tools
3. Results and Discussion
3.1. Guide Development to Perform Plant Audits and Investigative Sampling to Assess Risk Associated with Cyanobacteria
3.2. Model Development and Validation
3.2.1. Model Development
3.2.2. Model Validation
- Conventional treatment with PAC where there is no (or very low) contact time prior to coagulant addition (conv with PAC no contact)
- Conventional treatment with PAC where there is a contact time prior to coagulant addition (conv with PAC)
- Dissolved air flotation with PAC (DAF with PAC)
- Dissolved air flotation without PAC (DAF no PAC)
- Dissolved air flotation followed by GAC (DAF GAC)
- Plants where biological filtration is known to occur through comprehensive laboratory testing (biological filtration)
- For the plants with pre-contact tanks for PAC followed by conventional treatment, the spreadsheet predicted the actual final concentrations within +/−5 ng/L in 8 of the 9 sampling events. The other sampling represented an extreme event with raw water geosmin concentration of 611 ng/L where the PAC may have lysed cells and released further geosmin.
- For the two conventional plants without a pre-coagulation contact time, the predictions were within 2 ng/L and 11 ng/L.
- The plants utilizing DAF, and where biological filtration was effective, deviated from the predictions to a greater degree.
- Where there is known and consistent biological removal, the spreadsheet could easily be modified to take this into account.
- For the DAF plants, the calculator spreadsheet predictions deviated from the actual by between 1 ng/L and 127 ng/L for the sample prior to filtration and between 0 and 36 ng/L for the filtered samples.
4. Conclusions
- The investigative sampling procedures developed are effective and have been successfully applied by three project partners;
- While routine monitoring data is important, investigative sampling within the WTP provides more detailed and insightful information about the effectiveness of unit processes within the plant;
- PAC can be effective for the removal of dissolved T&O, with the optimum application point at least 20 min prior to coagulant addition;
- When there is a pre-contact time there is little or no further removal of dissolved T&O during the subsequent treatment processes;
- Water transfer and reactive spiking techniques may damage cyanobacteria cells and release metabolites into the dissolved state. Particularly at high T&O concentrations, this could lead to lower removals than expected and/or higher PAC dose requirements;
- Coagulation and clarification using sedimentation or UF are very effective for the removal of intracellular metabolites, generally with removals of >95%;
- Dissolved air flotation may lead to lysis of cyanobacteria within the plant. With no further removal processes, this may lead to a breakthrough of unacceptable T&O (or toxins if present) levels into the treated water;
- Biological processes can take place within full-scale treatment plants and can be extremely effective for T&O. However, biological removal does not take place on filters that are backwashed with chlorinated or chloraminated water.
- Further work is required to quantify the damage to cells during PAC mixing at the full-scale plants. Some laboratory testing has suggested that cultured cells are robust and are not damaged by mixing with PAC in a jar test environment. At the full-scale, the bloom may be in decline, resulting in cells that are more prone to damage, or the more rigorous mixing regime, for example, turbulent flow in the pipeline at WTP-A, may result in damage to cells and release of T&O.
- Clearly, DAF can result in damages to the cells, possibly due to the points outlined above. The flotation process may be more disruptive than the settling process, and the float may be subject to further stresses as the bubbles continue to impact the flocculated cells, potentially causing metabolite release. This finding requires further clarification and the identification of operational mitigation strategies.
- While based on a very simple concept, the tools show potential for use at the full-scale to aid in operational decisions. For more accurate predictions, it is recommended that the empirical models used in the calculations are generated for individual WTPs (PAC type and contact time, water quality conditions, and so forth). In addition, it should be noted that there is no full-scale verification for the toxin calculators. The limited full-scale data in the literature suggests that the cyanobacteria are effectively removed by conventional processes, there may be accumulation in sedimentation tanks, and that chlorination of toxins in filtered water is not as effective as suggested by laboratory tests. Therefore it is recommended that the toxin calculators are not relied upon to aid in operational decisions at this stage. When further full-scale verification data is available improvements can be made to these calculators to allow more confident application.
- Further research is required to develop a real-time, in-situ technique for the measurement of metabolites entering WTPs. Currently, the lead time for cell counts and/or metabolite concentration analysis prevents the tools and predictions being applied as they are required for PAC dosing for example.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; World Health Organization (WHO): Geneva, Switzerland, 1999. [Google Scholar]
- Boyer, G.L.; Yang, X.; Patchett, E.A.; Satchwell, M.F. Cyanobacteria toxins in upstate New York waters: A comparison on Onondaga Lake and Oneida Lake (summary of a poster presentation). In Proceedings of the 2nd Annual Onondaga Lake Conference, Syracuse, NY, USA, 20 November 2000. [Google Scholar]
- Carmichael, W.W. Assessment of Blue-Green Algal Toxins in Raw and Finished Drinking Wate; American Water Works Association Research Foundation; American Water Works Association: Denver, CO, USA, 2001. [Google Scholar]
- Tarczyriska, M.; Romanowska-Duda, Z.; Jurczak, T.; Zalewski, M. Toxic cyanobacterial blooms in a drinking water reservoir-causes, consequences and management strategy. Water Sci. Technol. Water Supply 2001, 1, 237–246. [Google Scholar]
- Svrcek, C.; Smith, D.W. Cyanobacteria toxins and the current state of knowledge on water treatment options: A review. J. Environ. Eng. Sci. 2004, 3, 155–185. [Google Scholar] [CrossRef]
- Newcombe, G.; House, J.; Ho, L.; Baker, P.; Burch, M. Management Strategies for Cyanobacteria (Blue-Green Algae): A Guide for Water Utilities; Water Quality Research Australia (WQRA): Adelaide, Australia, 2010. [Google Scholar]
- Zamyadi, A. Emerging toxic cyanobacterial issues in freshwater sources: Influence of climate change. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; Taylor & Francis: New York, NY, USA, 2014; Chapter 5. [Google Scholar]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Pearson, L.A.; Davis, T.W.; Burford, M.A.; Orr, P.T.; Neilan, B.A. Increased incidence of Cylindrospermopsis raciborskii in temperate zones—Is climate change responsible? Water Res. 2012, 46, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, I.; Bouaïcha, N.; Fontan, D.; Levi, Y. Seasonal variation of microcystin concentrations in the Saint-Caprais reservoir (France) and their removal in a small full-scale treatment plant. Water Res. 2002, 36, 2891–2897. [Google Scholar] [CrossRef]
- Rapala, J.; Niemela, M.; Berg, K.; Lepisto, L.; Lahti, K. Removal of cyanobacteria, cyanotoxins, heterotrophic bacteria and endotoxins at an operating surface water treatment plant. Water Sci. Technol. 2006, 54, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, S.; Amante, K.; Karnik, B.; Sommerfeld, M.; Dempster, T. Strategies for Controlling and Mitigating Algal Growth within Water Treatment Plants; Water Research Foundation: Denver, CO, USA, 2009. [Google Scholar]
- Zamyadi, A.; MacLeod, S.L.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Ho, L.; Newcombe, G.; Bustamante, H.; Prevost, M. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Res. 2012, 46, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Dorner, S.; Sauvé, S.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013, 47, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.; Henderson, M.; Henderson, R.K.; Stuetz, R.; Gladman, B.; McDowall, B.; Zamyadi, A. Removal of algal taste and odour compounds by granular and biological activated carbon in full-scale water treatment plants. Water Sci. Technol. Water Supply 2018, 17. [Google Scholar] [CrossRef]
- Agence Française de Sécurité Sanitaire des Aliments (AFSSA); l’Agence Française de Sécurité Sanitaire de l’Environnement et du Travail (AFSSET). Rapport sur L’évaluation des Risques liés à La présence de Cyanobactéries et de Leurs Toxines Dans les Eaux Destinées à L’alimentation, à la Baignade et Autres Activités Récréatives; AFSSA, Maisons-Alfort: Paris, France, 2006. [Google Scholar]
- Fitzgerald, D.J.; Cunliffe, D.A.; Burch, M.D. Development of heath alerts for cyanobacteria related toxins in drinking water in South Australia. Environ. Toxicol. 1999, 14, 203–209. [Google Scholar] [CrossRef]
- Hoeger, S.J.; Shaw, G.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon 2004, 43, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G. Identifying and Quantifying the Outcomes of Cyanobacteria Research: Impacts on the Australian Water Industry; WaterRA Impact Study: Adelaide, Australia, 2013. [Google Scholar]
- Newcombe, G.; Newton, K. Assessment and Management of Aesthetic and Health Risks Associated with Cyanobacteria; WaterRA Project 1078; Water Research Australia (WaterRA): Adelaide, Australia, 2018. [Google Scholar]
- Drikas, M.; Chow, C.W.K.; House, J.; Burch, M.D. Using coagulation, and settling to remove toxic cyanobacteria. J. Am. Water Works Assoc. 2001, 93, 100–111. [Google Scholar] [CrossRef]
- Schmidt, W.; Willmitzer, H.; Bornmann, K.; Pietsch, J. Production of drinking water from raw water containing cyanobacteria—Pilot plant studies for assessing the risk of microcystin breakthrough. Environ. Toxicol. 2002, 17, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, S.J.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharmacol. 2005, 203, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.I.; Ho, L.; Brookes, J.D. Effect of chlorination on Microcystis aeruginosa cell integrity and subsequent microcystin release and degradation. Environ. Sci. Technol. 2007, 41, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shi, H.; Timmons, T.; Adams, C.D. Release and removal of microcystins from Microcystis during oxidative-, physical-, and UV-based disinfection. J. Environ. Eng. 2010, 136, 2–11. [Google Scholar] [CrossRef]
- Zamyadi, A.; Fan, Y.; Daly, R.; Prévost, M. Chlorination of Microcystis aeruginosa: Toxin release and oxidation, cellular chlorine demand and disinfection by-products formation. Water Res. 2013, 47, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Onstad, G.D.; Kull, T.P.J.; Metcalf, J.S.; Acero, J.L.; von Gunten, U. Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Res. 2007, 41, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-F.; Chang, D.; Lien, S.; Tseng, Y.; Chiu, Y.; Wang, Y. Effect of chlorination on the cell integrity of two noxious cyanobacteria and their releases of odorants. J. Water Supply Res. Technol. AQUA 2009, 58, 539–551. [Google Scholar] [CrossRef]
- Zamyadi, A.; Ho, L.; Newcombe, G.; Daly, R.I.; Burch, M.; Baker, P.; Prévost, M. Release and oxidation of cell-bound saxitoxins during chlorination of Anabaena circinalis cells. Environ. Sci. Technol. 2010, 44, 9055–9061. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamyadi, A.; Henderson, R.K.; Newton, K.; Capelo-Neto, J.; Newcombe, G. Assessment of the Water Treatment Process’s Empirical Model Predictions for the Management of Aesthetic and Health Risks Associated with Cyanobacteria. Water 2018, 10, 590. https://doi.org/10.3390/w10050590
Zamyadi A, Henderson RK, Newton K, Capelo-Neto J, Newcombe G. Assessment of the Water Treatment Process’s Empirical Model Predictions for the Management of Aesthetic and Health Risks Associated with Cyanobacteria. Water. 2018; 10(5):590. https://doi.org/10.3390/w10050590
Chicago/Turabian StyleZamyadi, Arash, Rita K. Henderson, Kelly Newton, Jose Capelo-Neto, and Gayle Newcombe. 2018. "Assessment of the Water Treatment Process’s Empirical Model Predictions for the Management of Aesthetic and Health Risks Associated with Cyanobacteria" Water 10, no. 5: 590. https://doi.org/10.3390/w10050590
APA StyleZamyadi, A., Henderson, R. K., Newton, K., Capelo-Neto, J., & Newcombe, G. (2018). Assessment of the Water Treatment Process’s Empirical Model Predictions for the Management of Aesthetic and Health Risks Associated with Cyanobacteria. Water, 10(5), 590. https://doi.org/10.3390/w10050590





