The Importance of Habitat and Lake Morphometry for the Summer Diet Choice of Landlocked Arctic Char in Two West Greenland Lakes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
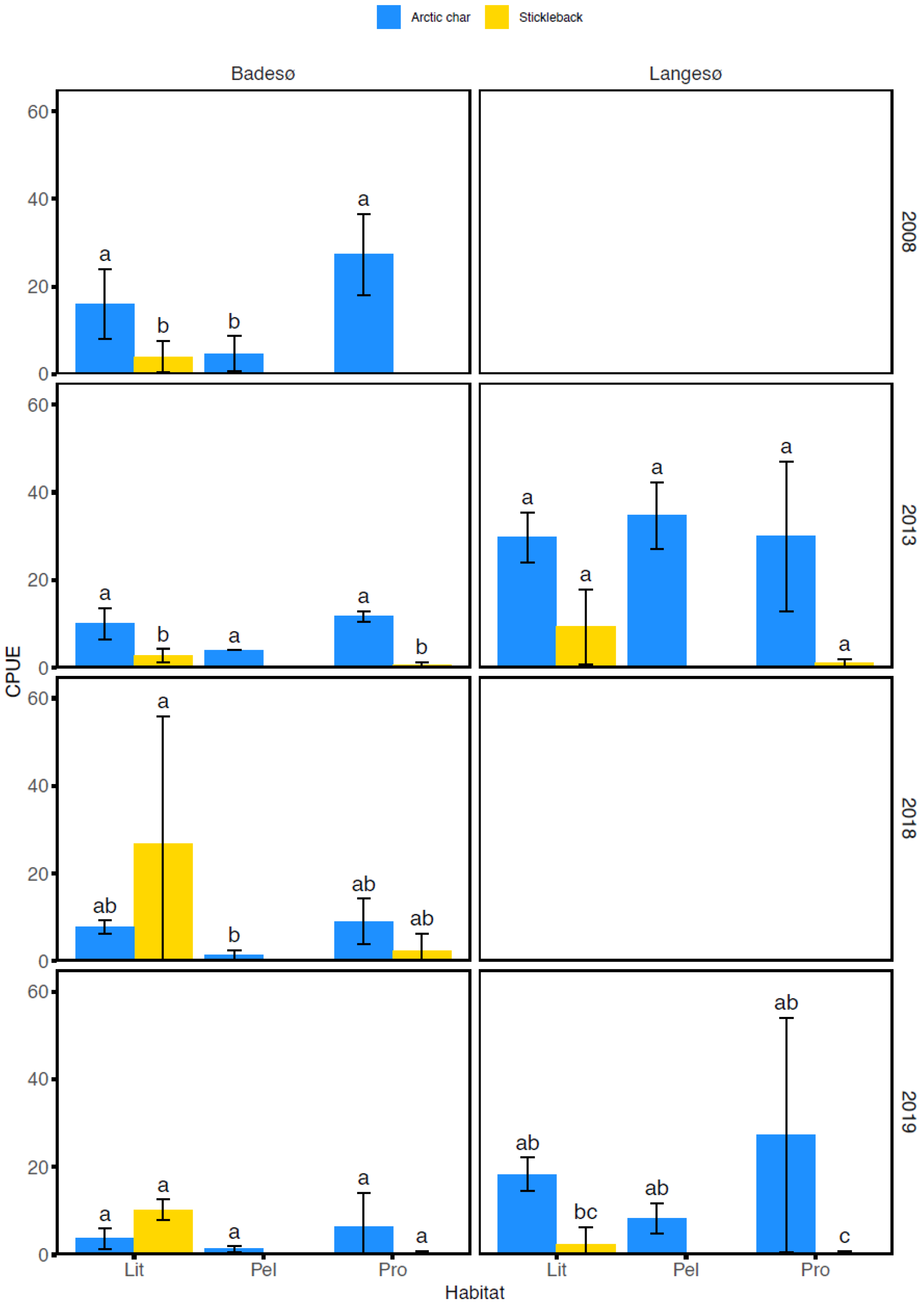
3.1. Stomach Content Analysis
3.2. Isotopes and Simmr Mixing Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.B. Exotic fishes and freshwater fish diversity. Zoos’ Print J. 2000, 15, 363–367. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater gradient. Annu. Rev. Ecol. Syst. 1996, 27, 337–363. Available online: http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.27.1.337 (accessed on 15 March 2023). [CrossRef] [Green Version]
- Mehner, T.; Diekmann, M.; Brämick, U.; Lemcke, R. Composition of fish communities in German lakes as related to lake morphology, trophic state, shore structure and human-use intensity. Freshw. Biol. 2005, 50, 70–85. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Anneville, O.; Chandra, S.; Dix, M.; Kuusisto, E.; Livingstone, D.M.; Rimmer, A.; Schladow, S.G.; Silow, E.; Sitoki, L.M.; et al. Morphometry and average temperature affect lake stratification responses to climate change. Geophys. Res. Lett. 2015, 42, 4981–4988. [Google Scholar] [CrossRef] [Green Version]
- Persson, L.; Diehl, S.; Johansson, L.; Andersson, G.; Hamrin, S.F. Shifts in fish communities along the productivity gradient of temperate lakes-patterns and the importance of size-structured interactions. J. Fish Biol. 1991, 38, 281–293. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Schindler, D.E.; Scheuerell, M.D. Habitat coupling in lake ecosystems. Oikos 2002, 98, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Lauridsen, T.L.; Christoffersen, K.S.; Landkildehus, F.; Geertz-Hansen, P.; Amsinck, S.L.; Søndergaard, M.; Davidson, T.A.; Riget, F. The structuring role of fish in Greenland lakes: An overview based on contemporary and paleoecological studies of 87 lakes from the low and the high Arctic. Hydrobiologia 2017, 800, 99–113. [Google Scholar] [CrossRef]
- Laske, S.M.; Amundsen, P.A.; Christoffersen, K.S.; Erkinaro, J.; Guðbergsson, G.; Hayden, B.; Heino, J.; Holmgren, K.; Kahilainen, K.K.; Lento, J.; et al. Circumpolar patterns of Arctic freshwater fish biodiversity: A baseline for monitoring. Freshw. Biol. 2019, 67, 176–193. [Google Scholar] [CrossRef]
- Knopp, J.A. Investigating the effects of environmental change on Arctic char (Salvelinus alpinus) growth using scientific and inuit traditional knowledge. Arctic 2010, 63, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Fournier, E.B.; Schindler, D.E. Depth-specific benthic specialization of Arctic char in an oligotrophic subarctic lake. Aquat. Sci. 2021, 83, 76. [Google Scholar] [CrossRef]
- Eloranta, A.P.; Mariash, H.L.; Rautio, M.; Power, M. Lipid-rich zooplankton subsidise the winter diet of benthivorous Arctic charr (Salvelinus alpinus) in a subarctic lake. Freshw. Biol. 2013, 58, 2541–2554. [Google Scholar] [CrossRef]
- Kristjánsson, B.K.; Malmquist, H.J.; Ingimarsson, F.; Antonsson, T.; Snorrason, S.U.S.; Skúlason, S. Relationships between lake ecology and morphological characters in Icelandic Arctic charr, Salvelinus alpinus. Biol. J. Linn. Soc. 2011, 103, 761–771. [Google Scholar] [CrossRef]
- Klemetsen, A. The Charr Problem Revisited: Exceptional Phenotypic Plasticity Promotes Ecological Speciation in Postglacial Lakes. Freshw. Rev. 2010, 3, 49–74. [Google Scholar] [CrossRef]
- Riget, F.; Jeppesen, E.; Landkildehus, F.; Lauridsen, T.L.; Geertz-Hansen, P.; Christoffersen, K.; Sparholt, H. Landlocked Arctic charr (Salvelinus alpinus) population structure and lake morphometry in Greenland—Is there a connection? Polar Biol. 2000, 23, 550–558. [Google Scholar] [CrossRef]
- Guiguer, K.R.R.A.; Reist, J.D.; Power, M.; Babaluk, J.A. Using stable isotopes to confirm the trophic ecology of Arctic charr morphotypes from Lake Hazen, Nunavut, Canada. J. Fish Biol. 2002, 60, 348–362. [Google Scholar] [CrossRef]
- Knudsen, R.; Klemetsen, A.; Amundsen, P.A.; Hermansen, B. Incipient speciation through niche expansion: An example from the Arctic charr in a subarctic lake. Proc. R. Soc. B Biol. Sci. 2006, 273, 2291–2298. [Google Scholar] [CrossRef] [Green Version]
- Hawley, K.L.; Rosten, C.M.; Christensen, G.; Lucas, M.C. Fine-scale behavioural differences distinguish resource use by ecomorphs in a closed ecosystem. Sci. Rep. 2016, 6, 24369. [Google Scholar] [CrossRef] [Green Version]
- Eloranta, A.P.; Kahilainen, K.K.; Amundsen, P.A.; Knudsen, R.; Harrod, C.; Jones, R.I. Lake size and fish diversity determine resource use and trophic position of a top predator in high-latitude lakes. Ecol. Evol. 2015, 5, 1664–1675. [Google Scholar] [CrossRef] [Green Version]
- Sandlund, O.T.; Eloranta, A.P.; Borgstrøm, R.; Hesthagen, T.; Johnsen, S.I.; Museth, J.; Rognerud, S. The trophic niche of Arctic charr in large southern Scandinavian lakes is determined by fish community and lake morphometry. Hydrobiologia 2016, 783, 117–130. [Google Scholar] [CrossRef]
- Klobucar, S.L.; Budy, P. Trophic structure of apex fish communities in closed versus leaky lakes of arctic Alaska. Oecologia 2020, 194, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Font, L.; Shuter, B.J.; Blanchfield, P.J.; Minns, C.K.; Rennie, M.D. Life at the top: Lake ecotype influences the foraging pattern, metabolic costs and life history of an apex fish predator. J. Anim. Ecol. 2019, 88, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.B.; Christensen, K.D. The Biodiversity of Greenland—A Country Study; Technical Report No. 55; Pinngortitaleriffik, Grønlands Naturinstitut: Nuuk, Greenland, 2003. [Google Scholar]
- Arranz, I.; Davidson, T.A.; Benejam, L.; Brucet, S.; Sanchez-Hernandez, J.; Landkildehus, F.; Lauridsen, T.L.; Mazzeo, N.; Vidal, N.; Ozkan, K.; et al. Interactive Effects of Lake Morphometry and Sticklebacks on the Trophic Position of Arctic charr, Salvelinus alpinus (L.), across Lakes in Western Greenland. LIMNOFISH-J. Limnol. Freshw. Fish. Res. 2022, 8, 101–115. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Jeppesen, E.; Landkildehus, F.; Søndergaard, M. Horizontal distribution of cladocerans in arctic Greenland lakes—Impact of macrophytes and fish. Hydrobiologia 2001, 442, 107–116. [Google Scholar] [CrossRef]
- Bergersen, R. Sticklebacks from Greenland. J. Fish Biol. 1996, 48, 799–801. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Jeppesen, E.; Vander Zanden, M.J.; Schierup, H.H.; Christoffersen, K.; Lodge, D.M. From Greenland to green lakes: Cultural eutrophication and the loss of benthic pathways in lakes. Limnol. Oceanogr. 2003, 48, 1408–1418. [Google Scholar] [CrossRef] [Green Version]
- Vander Zanden, M.J.; Vadeboncoeur, Y. Putting the lake back together 20 years later: What in the benthos have we learned about habitat linkages in lakes? Inland Waters 2020, 10, 305–321. [Google Scholar] [CrossRef]
- Karlsson, J.; Byström, P. Littoral energy mobilization dominates energy supply for top consumers in subarctic lakes. Limnol. Oceanogr. 2005, 50, 538–543. [Google Scholar] [CrossRef]
- Persson, A.; Hansson, L.A. Diet shift in fish following competitive release. Can. J. Fish. Aquat. Sci. 1999, 56, 70–78. [Google Scholar] [CrossRef]
- Eloranta, A.P.; Kahilainen, K.K.; Jones, R.I. Seasonal and ontogenetic shifts in the diet of Arctic charr Salvelinus alpinus in a subarctic lake. J. Fish Biol. 2010, 77, 80–97. [Google Scholar] [CrossRef] [Green Version]
- Matley, J.K.; Maes, G.E.; Devloo-Delva, F.; Huerlimann, R.; Chua, G.; Tobin, A.J.; Fisk, A.T.; Simpendorfer, C.A.; Heupel, M.R. Integrating complementary methods to improve diet analysis in fishery-targeted species. Ecol. Evol. 2018, 8, 9503–9515. [Google Scholar] [CrossRef]
- Griffiths, D. The size structure of lacustrine Arctic charr (Pisces: Salmonidae) populations. Biol. J. Linn. Soc. 1994, 51, 337–357. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Food of Fresh-Water Sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a Review of Methods Used in Studies of the Food of Fishes. J. Anim. Ecol. 1950, 19, 36. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- De Crespin De Billy, V.; Doledec, S.; Chessel, D. Biplot presentation of diet composition data: An alternative for fish stomach contents analysis. J. Fish Biol. 2000, 56, 961–973. [Google Scholar] [CrossRef]
- Qi, H.; Coplen, T.B.; Geilmann, H.; Brand, W.A.; Böhlke, J.K. Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Commun. Mass Spectrom. 2003, 17, 2483–2487. [Google Scholar] [CrossRef]
- Friedman, I.; Neil, J.O.; Cebula, G. Two New Carbonate Stable Isotope Standards. Geostand. Newsl. 1982, 6, 1–173. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source Partitioning Using Stable Isotopes: Coping with Too Much Variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef]
- Kiljunen, M.; Grey, J.; Sinisalo, T.; Chris, H.; Immonen, H.; Jones, R.I. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J. Appl. Ecol. 2006, 43, 1213–1222. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Schindler, D.E.; Holtgrieve, G.W. Does lipid-correction introduce biases into isotopic mixing models? Implications for diet reconstruction studies. Oecologia 2019, 191, 745–755. [Google Scholar] [CrossRef]
- Newsome, S.D.; Wolf, N.; Peters, J.; Fogel, M.L. Integrative and Comparative Biology Amino Acid d 13 C Analysis Shows Flexibility in the Routing of Dietary Protein and Lipids to the Tissue of an Omnivore. Integr. Comp. Biol. 2014, 54, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Snorrasson, S.S.; Skulason, S.; Jonsson, B.; Malmquist, H.J.; Jonasson, P.M.; Sandlund, O.T.; Lindem, T. Trophic specialization in Arctic charr Salvelinus alpinus morphological divergence and ontogenetic niche shifts. Biol. J. Linn. Soc. 1994, 52, 1–18. [Google Scholar] [CrossRef]
- Malmquist, H.J.; Ingimarsson, F.; Jóhannsdóttir, E.E.; Gíslason, D.; Snorrason, S.S. Biology of Brown Trout (Salmo trutta) and Arctic Charr (Salvelinus alpinus) in Four Faroese Lakes. Ann. Soc. Sci. Færoensis Suppl. 2002, 36, 94–113. [Google Scholar]
- Klemetsen, A.; Amundsen, A.; Muladal, H.; Rubach, S.; Solbakken, J.I. Habitat Shifts in a Dense, Resident Arctic Charr Salvelinus Alpinus Population. Physiol. Ecol. Japan Spec. 1989, 1, 187–200. Available online: https://www.researchgate.net/profile/Per-Arne_Amundsen/publication/267266272_Habitat_shifts_in_a_resident_Arctic_charr_Salvelinus_alpinus_population/links/563388c508aeb786b70134f0.pdf (accessed on 15 March 2023).
- L’Abée-Lund, J.H.; Langeland, A.; Jonsson, B.; Ugedal, O. Spatial Segregation by Age and Size in Arctic Charr: A Trade-Off between Feeding Possibility and Risk of Predation. J. Anim. Ecol. 1993, 62, 160–168. [Google Scholar] [CrossRef]
- Bay, E.C. Chironomid Midges; Washington State University: Pullman, WA, USA, 2003; Available online: https://s3.wp.wsu.edu/uploads/sites/408/2015/02/PLS-45-Chironomid-Midges.pdf (accessed on 15 March 2023).
- Oliver, D.R. Adaptations of Arctic Chironomidae. Finn. Zool. Bot. Publ. Board 1968, 5, 111–118. Available online: https://www.jstor.org/stable/23731452 (accessed on 15 March 2023).
- Lindegaard, C.; Mæhl, P. Abundance, population dynamics and production of Chironomidae (Diptera) in an ultraoligotrophic lake in South Greenland. Neth. J. Aquat. Ecol. 1992, 26, 297–308. [Google Scholar] [CrossRef]
- Svenning, M.A.; Borgstrøm, R. Cannibalism in Arctic charr: Do all individuals have the same propensity to be cannibals? J. Fish Biol. 2005, 66, 957–965. [Google Scholar] [CrossRef]
- Sierszen, M.E.; McDonald, M.E.; Jensen, D.A. Benthos as the basis for arctic lake food webs. Aquat. Ecol. 2003, 37, 437–445. [Google Scholar] [CrossRef]
- Jørgensen, L.; Klemetsen, A. Food resource partitioning of Arctic charr, Salvelinus alpinus (L.) and three-spined stickleback, Gasterosteus aculeatus L., in the littoral zone of lake Takvatn in northern Norway. Ecol. Freshw. Fish 1995, 4, 77–84. [Google Scholar] [CrossRef]
- Primicerio, R.; Klemetsen, A. Zooplankton seasonal dynamics in the neighbouring lakes Takvatn and Lombola (Northern Norway). Hydrobiologia 1999, 411, 19–29. [Google Scholar] [CrossRef]
- Rautio, M.; Sorvari, S.; Korhola, A. Diatom and crustacean Zooplankton communities, their seasonal variability and representation in the sediments of subarctic Lake Saanajärvi. J. Limnol. 2000, 59 (Suppl. S1), 81–96. [Google Scholar] [CrossRef] [Green Version]
- Bøhn, T.; Amundsen, P.A. The competitive edge of an invading specialist. Ecology 2001, 82, 2150–2163. [Google Scholar] [CrossRef]
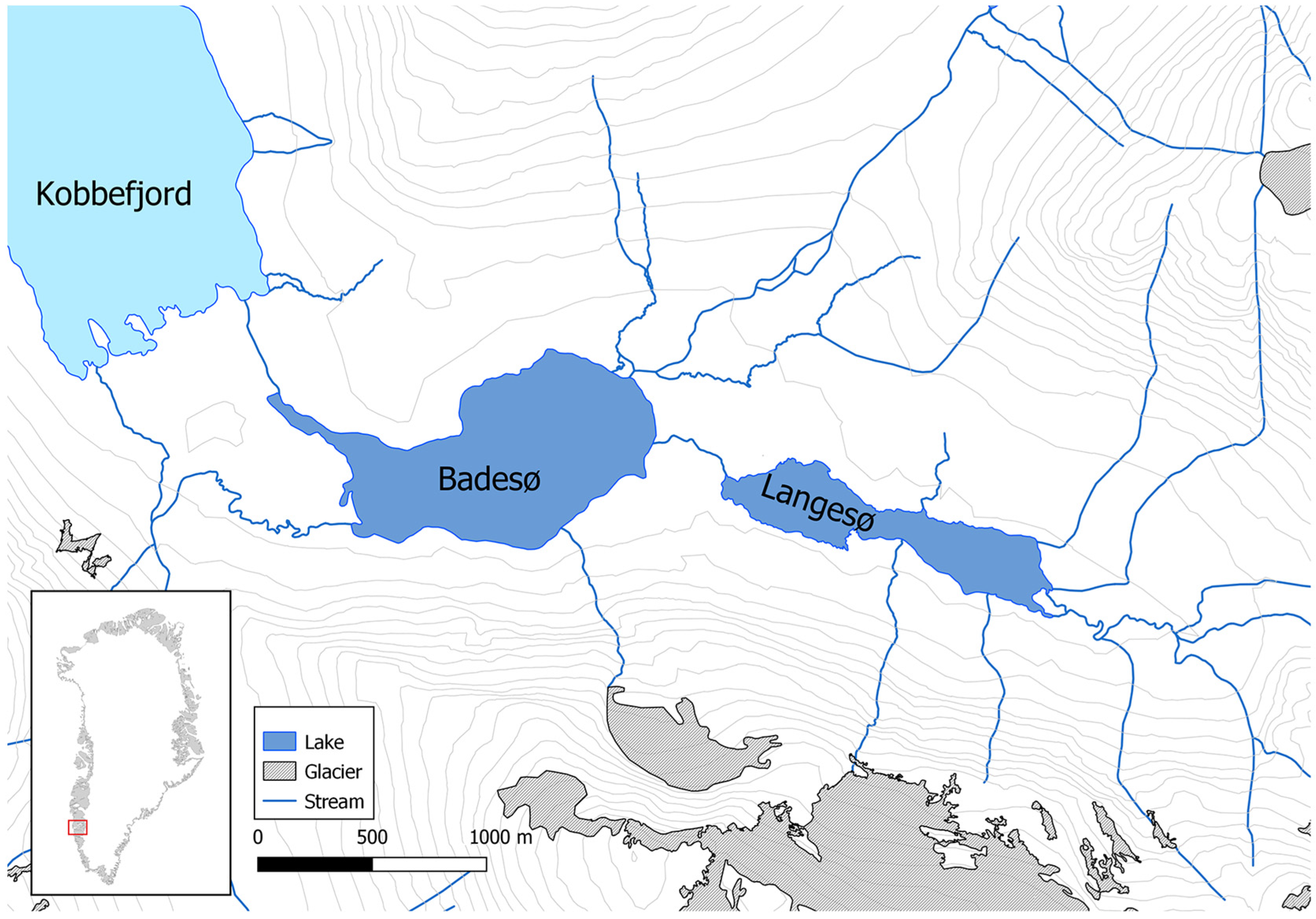






| Badesø | Langesø | |
|---|---|---|
| Surface area (ha) | 80 | 30 |
| Max depth (m) | 38 | 16 |
| Average depth (m) | 9.2 | 5 |
| Elevation (m above sea) | 35 | 40 |
| Total phosphorus (mg/L) | 0.006 | 0.007 |
| Total nitrogen (mg/L) | 0.078 | 0.038 |
| Variables | Coefficients | Std. Error | t-Value | p | ||
|---|---|---|---|---|---|---|
| Badesø | Intercept (2008) | −31.3 | 0.78 | −40 | <0.001 | |
| Fork-length | 5.7 | 0.58 | 9.9 | <0.001 | ||
| 2013 | 0.18 | 0.27 | 0.67 | 0.507 | ||
| 2018 | −1.7 | 0.3 | −6.3 | <0.001 | ||
| 2019 | −0.3 | 0.31 | −0.97 | 0.333 | ||
| Intercept (2008) | −2.7 | 0.33 | −8.09 | <0.001 | ||
| Fork-length | 7.42 | 0.24 | 30.7 | <0.001 | ||
| 2013 | 0.91 | 0.11 | 8.04 | <0.001 | ||
| 2018 | 0.45 | 0.11 | 3.97 | <0.001 | ||
| 2019 | 0.79 | 0.13 | 6.07 | <0.001 | ||
| Langesø | Intercept (2013) | −29.2 | 1.45 | −20.1 | 0.001 | |
| Fork-length | 3.6 | 1.07 | 3.34 | <0.001 | ||
| 2019 | 0.68 | 0.32 | 2.11 | 0.036 | ||
| Intercept (2013) | −0.17 | 0.77 | −0.22 | 0.112 | ||
| Fork-length | 6.34 | 0.57 | 11.19 | <0.001 | ||
| 2019 | 0.27 | 0.17 | 1.58 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthelsen, A.S.; Raundrup, K.; Grønkjær, P.; Jeppesen, E.; Lauridsen, T.L. The Importance of Habitat and Lake Morphometry for the Summer Diet Choice of Landlocked Arctic Char in Two West Greenland Lakes. Water 2023, 15, 2164. https://doi.org/10.3390/w15122164
Berthelsen AS, Raundrup K, Grønkjær P, Jeppesen E, Lauridsen TL. The Importance of Habitat and Lake Morphometry for the Summer Diet Choice of Landlocked Arctic Char in Two West Greenland Lakes. Water. 2023; 15(12):2164. https://doi.org/10.3390/w15122164
Chicago/Turabian StyleBerthelsen, Andreas S., Katrine Raundrup, Peter Grønkjær, Erik Jeppesen, and Torben L. Lauridsen. 2023. "The Importance of Habitat and Lake Morphometry for the Summer Diet Choice of Landlocked Arctic Char in Two West Greenland Lakes" Water 15, no. 12: 2164. https://doi.org/10.3390/w15122164





