Sensitivity of Triops longicaudatus Locomotor Behaviour to Detect Short Low-Level Exposure to Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Triop Rearing
2.2. Chemicals
2.3. The Video-Tracking System and Exposure Experiments
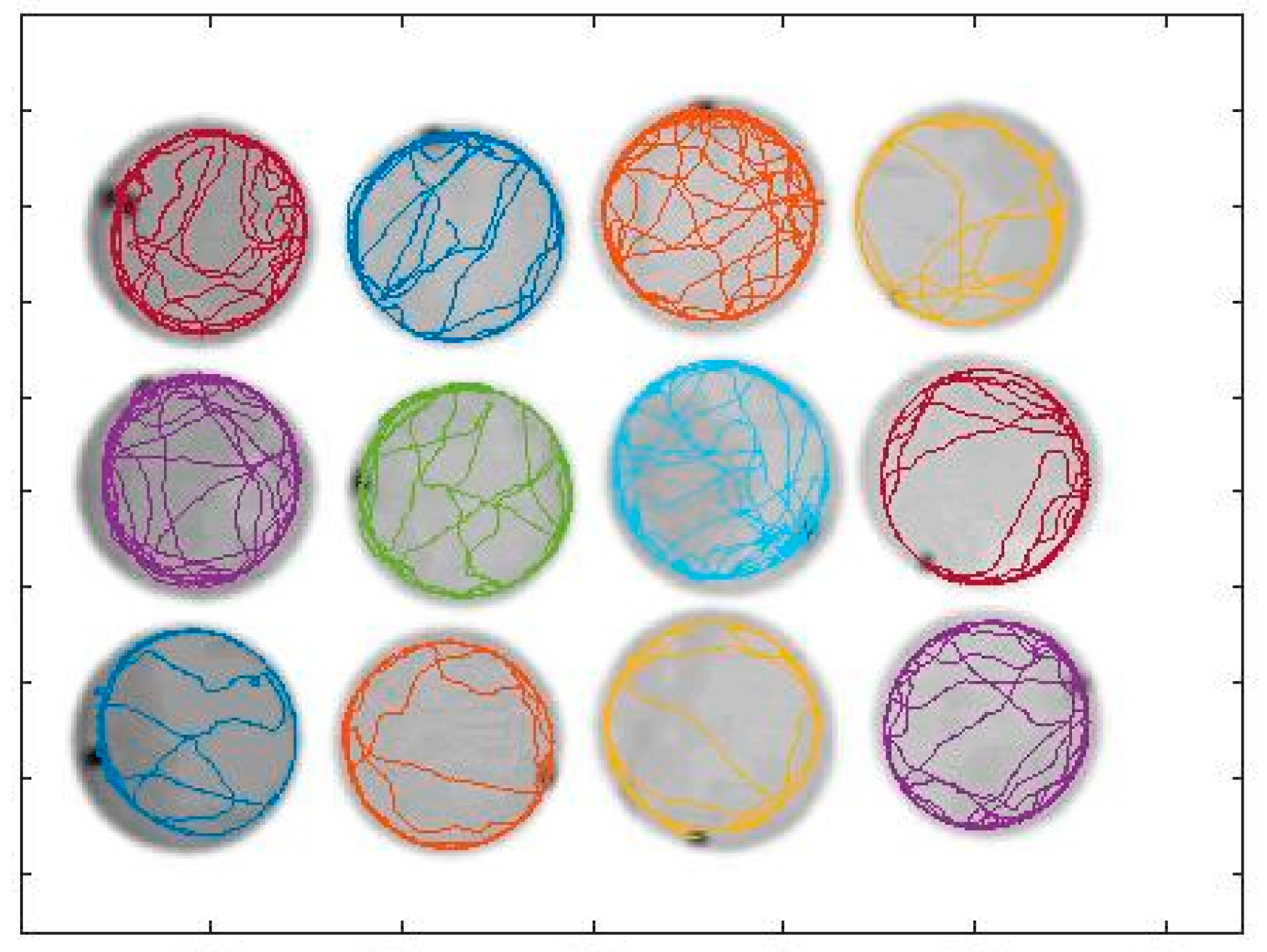
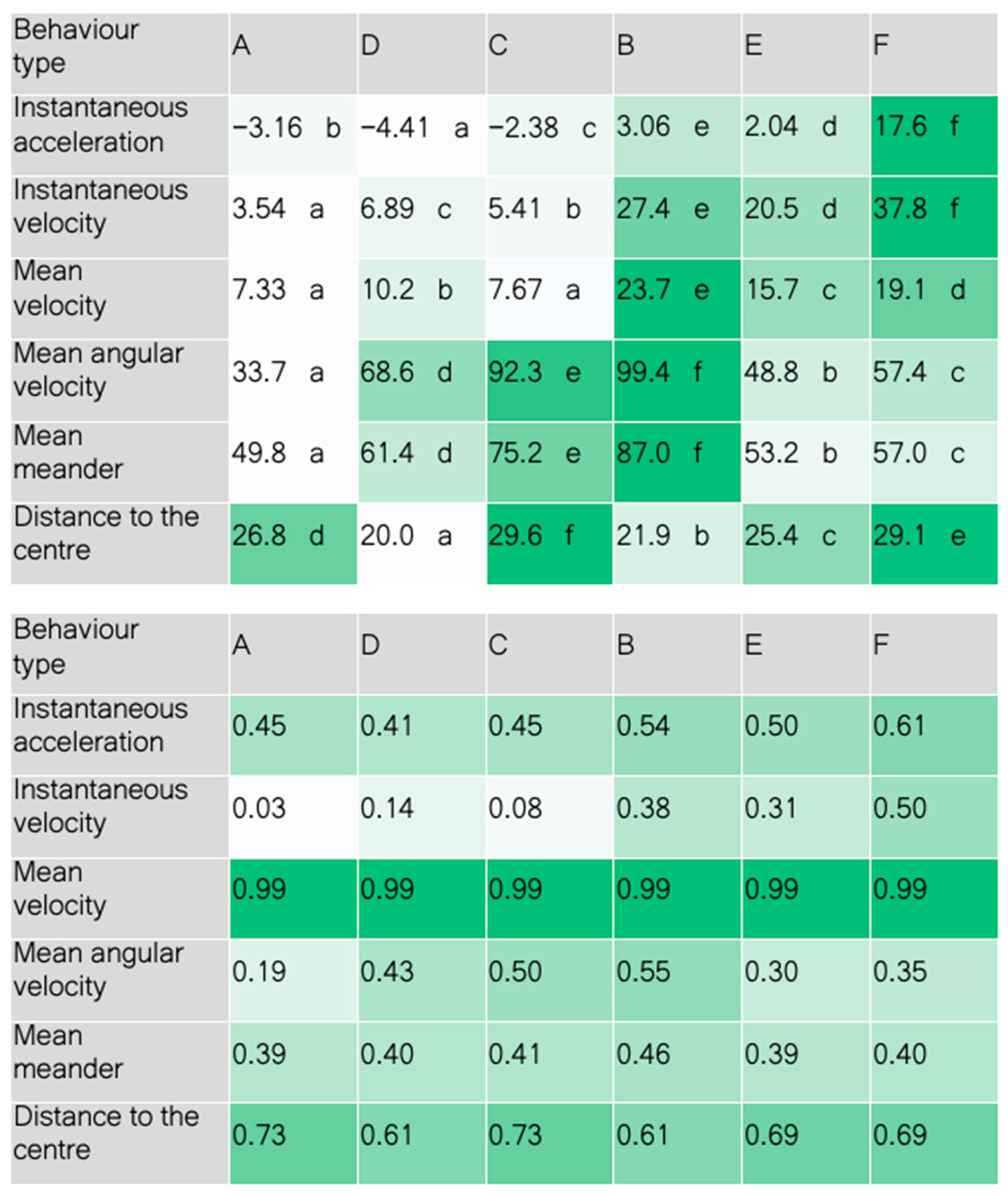
2.4. Data Analysis
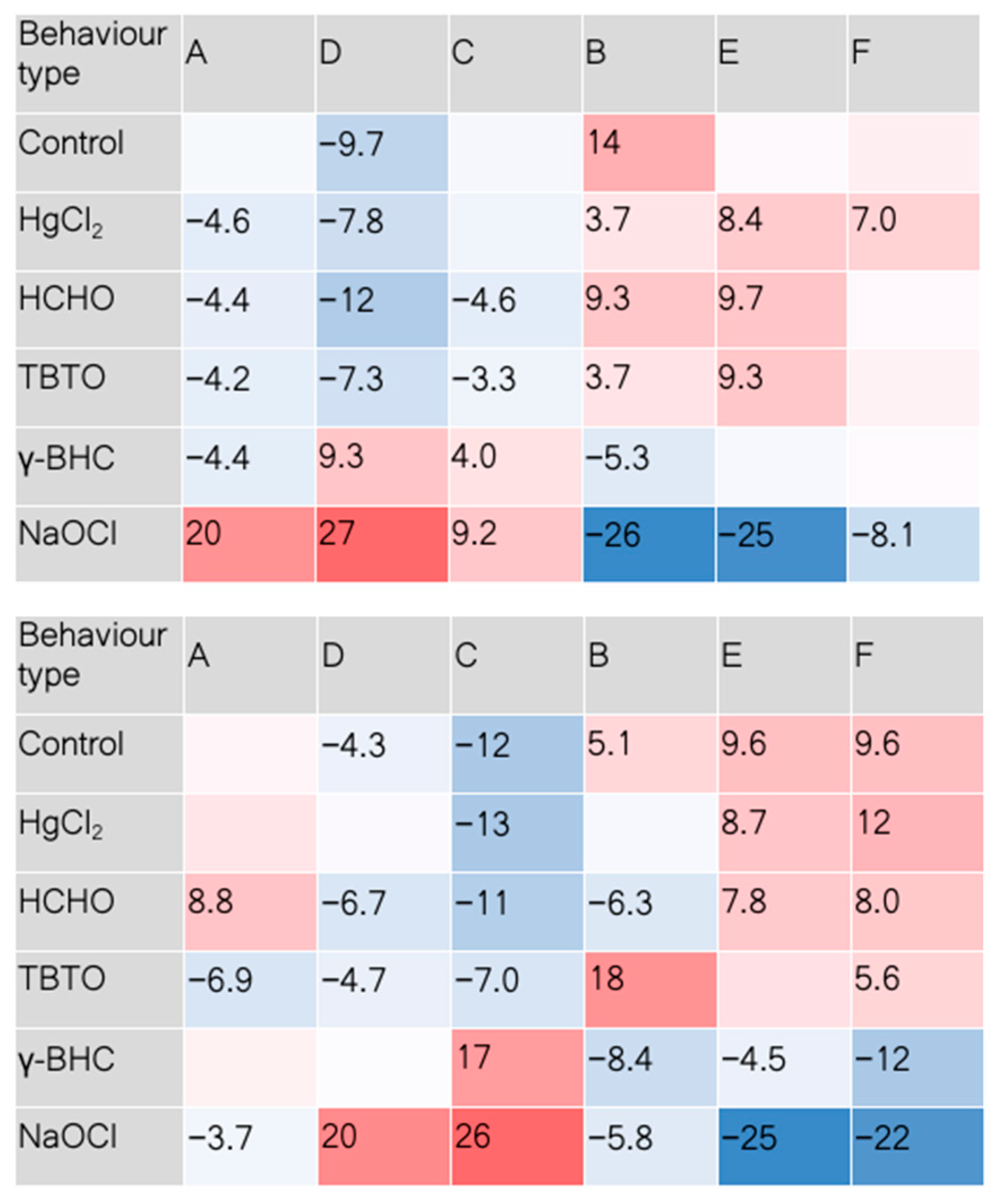
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sassaman, C.; Simovich, M.A.; Fugate, M. Reproductive isolation and genetic differentiation in North American species of Triops (Crustacea: Branchiopoda: Notostraca). Hydrobiologia 1997, 359, 125–147. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Pinceel, T.; Vanhove, M.P.M.; Denis, C.; Jocque, M.; Timms, B.V.; Brendonck, L. Toward a global phylogeny of the “living fossil” crustacean order of the Notostraca. PLoS ONE 2012, 7, e34998. [Google Scholar] [CrossRef] [PubMed]
- Suno-uchi, N.; Sasaki, F.; Chiba, S.; Kawata, M. Morphological stasis and phylogenetic relationships in tadpole shrimps, Triops (Crustacea: Notostraca). Biol. J. Linn. Soc. 1997, 61, 439–457. [Google Scholar]
- Mantovani, B.; Cesari, M.; Luchetti, A.; Scanabissi, F. Mitochondrial and nuclear DNA variability in the living fossil Triops cancriformis (Bosc, 1801) (Crustacea, Branchiopoda, Notostraca). Heredity 2008, 100, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Kang, S.W.; Patnaik, B.B.; Park, S.Y.; Hwang, H.J.; Chung, J.M.; Song, D.K.; Noh, M.Y.; Park, S.H.; Jeon, G.J.; et al. Transcriptome analysis of the tadpole shrimp (Triops longicaudatus) by illumina paired-end sequencing: Assembly, annotation, and marker discovery. Genes 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Fryer, G. Studies on the functional morphology and biology of the Notostraca (Crustacea: Branchiopoda). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 321, 27–124. [Google Scholar]
- Scholnick, D.A.; Snyder, G.K. Response of the tadpole shrimp Triops longicaudatus to hypoxia. Crustaceana 1996, 69, 937–948. [Google Scholar] [CrossRef]
- Tietze, N.S.; Mulla, M.S. Biological control of Culex mosquitoes (Diptera: Culicidae) by the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae). J. Med. Entomol. 1991, 28, 24–31. [Google Scholar] [CrossRef]
- Fry, L.L.; Mulla, M.S.; Adams, C.W. Field Introductions and Establishment of the Tadpole Shrimp, Triops longicaudatus (Notostraca: Triopsidae), a Biological Control Agent of Mosquitos. Biol. Control 1994, 4, 113–124. [Google Scholar] [CrossRef]
- Weeks, S.C. Life-history variation under varying degrees of intraspecific competition in the tadpole shrimp Triops longicaudatus (Leconte). J. Crustac. Biol. 1990, 10, 498–503. [Google Scholar] [CrossRef]
- Sassaman, C. Sex ratio variation in female-biased populations of Notostracans. Hydrobiologia 1991, 212, 169–179. [Google Scholar] [CrossRef]
- Takahashi, F. Triops ssp. [Notostraca: Triopsidae] for the biological control agents of weeds in rice paddies in Japan. Entomophaga 1977, 22, 351–357. [Google Scholar] [CrossRef]
- Carlisle, D.B. Triops (Entomostraca) Eggs Killed Only by Boiling. Science 1968, 161, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Mulla, M.S. Factors affecting egg hatch of the tadpole shrimp, Triops newberryi, a potential biological control agent of immature mosquitoes. Biol. Control 2002, 23, 18–26. [Google Scholar] [CrossRef]
- Møller, O.S.; Olesen, J.; Høeg, J.T. SEM studies on the early larval development of Triops cancriformis (Bosc) (Crustacea: Branchiopoda, Notostraca). Acta Zool. 2003, 84, 267–284. [Google Scholar] [CrossRef]
- Fry-O’Brien, L.; Mulla, M. Optimal conditions for rearing the tadpole shrimp, Triops longicaudatus (Notostraca: Triopsidae), a biological control agent against mosquitoes. J. Am. Mosq. Control Assoc. 1996, 12, 446–453. [Google Scholar] [PubMed]
- Su, T.; Mulla, M.S. Effects of nutritional factors and soil addition on growth, longevity and fecundity of the tadpole shrimp Triops newberryi (Notostraca: Triopsidae), a potential biological control agent of immature mosquitoes. J. Vector Ecol. 2001, 26, 43–50. [Google Scholar] [PubMed]
- Scholnick, D.A. Sensitivity of metabolic rate, growth, and fecundity of tadpole shrimp Triops longicaudatus to environmental variation. Biol. Bull. 1995, 189, 22–28. [Google Scholar] [CrossRef]
- Scott, S.R.; Grigarick, A.A. Laboratory studies of factors affecting egg hatch of Triops longicaudatus (Leconte) (Notostraca: Triopsidae). Hydrobiologia 1979, 63, 145–152. [Google Scholar] [CrossRef]
- Su, T.; Mulla, M.S. Toxicity and effects of microbial mosquito larvicides and larvicidal oil on the development and fecundity of the tadpole shrimp Triops newberryi (Packard) (Notostraca: Triopsidae). J. Vector Ecol. 2005, 30, 107–114. [Google Scholar]
- Su, T.; Jiang, Y.; Mulla, M.S. Toxicity and effects of mosquito larvicides methoprene and surface film (Agnique® MMF) on the development and fecundity of the tadpole shrimp Triops newberryi (Packard) (Notostraca: Triopsidae). J. Vector Ecol. 2014, 39, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Walton, W.E.; Darwazeh, H.A.; Mulla, M.S.; Schreiber, E.T. Impact of selected synthetic pyrethroids and organophosphorous pesticides on the tadpole shrimp, Triops longicaudatus (Le Conte) (Notostraca: Triopsidae). Bull. Environ. Contam. Toxicol. 1990, 45, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tsukimura, B.; Nelson, W.K.; Linder, C.J. Inhibition of ovarian development by methyl farnesoate in the tadpole shrimp, Triops longicaudatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, S.R.; Guilhermino, L.; Guimarães, L. Biochemical and locomotor responses of Carcinus maenas exposed to the serotonin reuptake inhibitor fluoxetine. Chemosphere 2011, 85, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Broly, P.; Deneubourg, J.L. Behavioural contagion explains group cohesion in a social crustacean. PLoS Comput. Biol. 2015, 11, e1004290. [Google Scholar] [CrossRef]
- Oliva Teles, L.; Fernandes, M.; Amorim, J.; Vasconcelos, V. Video-tracking of zebrafish (Danio rerio) as a biological early warning system using two distinct artificial neural networks: Probabilistic neural network (PNN) and self-organizing map (SOM). Aquat. Toxicol. 2015, 165, 241–248. [Google Scholar] [CrossRef]
- Endo, N.; Rahayu, L.P.; Arakawa, T.; Tanaka, T. Video tracking analysis of behavioral patterns during estrus in goats. J. Reprod. Dev. 2016, 62, 115–119. [Google Scholar] [CrossRef]
- Behrend, J.E.; Rypstra, A.L. Contact with a glyphosate-based herbicide has long-term effects on the activity and foraging of an agrobiont wolf spider. Chemosphere 2017, 194, 714–721. [Google Scholar] [CrossRef]
- Ogungbemi, A.O.; Teixido, E.; Massei, R.; Scholz, S.; Küster, E. Optimization of the spontaneous tail coiling test for fast assessment of neurotoxic effects in the zebrafish embryo using an automated workflow in KNIME®. Neurotoxicol. Teratol. 2020, 81, 106918. [Google Scholar] [CrossRef]
- Teixidó, E.; Klüver, N.; Ogungbemi, A.O.; Küster, E.; Scholz, S. Evaluation of Neurotoxic Effects in Zebrafish Embryos by Automatic Measurement of Early Motor Behaviors. Neuromethods 2021, 172, 381–397. [Google Scholar]
- Hellou, J.; Cheeseman, K.; Desnoyers, E.; Johnston, D.; Jouvenelle, M.L.; Leonard, J.; Robertson, S.; Walker, P. A non-lethal chemically based approach to investigate the quality of harbour sediments. Sci. Total Environ. 2008, 389, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.B.I.; Van Lochem, P.B.A.; Gispen, W.H.; Spruijt, B.M. Classification of rat behavior with an image-processing method and a neural network. Behav. Res. Methods Instrum. Comput. 2000, 32, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, B.M.; DeVisser, L. Advanced behavioural screening: Automated home cage ethology. Drug Discov. Today Technol. 2006, 3, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shijun, H.; Yao, H. A study of fish velocity measurement base on video tracking. In Proceedings of the 2012 2nd International Conference on Computer Science and Network Technology, Changchun, China, 29–31 December 2012; pp. 1898–1901. [Google Scholar]
- Amorim, J.; Fernandes, M.; Vasconcelos, V.; Teles, L.O. Evaluation of the sensitivity spectrum of a video tracking system with zebrafish (Danio rerio) exposed to five different toxicants. Environ. Sci. Pollut. Res. 2017, 24, 16086–16096. [Google Scholar] [CrossRef] [PubMed]
- Román, A.C.; Vicente-Page, J.; Pérez-Escudero, A.; Carvajal-González, J.M.; Fernández-Salguero, P.M.; de Polavieja, G.G. Histone H4 acetylation regulates behavioral inter-individual variability in zebrafish. Genome Biol. 2018, 19, 55. [Google Scholar] [CrossRef]
- Hidalgo, E.; Bartolome, R.; Dominguez, C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem. Biol. Interact. 2002, 139, 265–282. [Google Scholar] [CrossRef]
- Islam, R.; Lynch, J.W. Mechanism of action of the insecticides, lindane and fipronil, on glycine receptor chloride channels. Br. J. Pharmacol. 2012, 165, 2707–2720. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H. Neurotoxicity and physiological stress in brain of zebrafish chronically exposed to tributyltin. J. Toxicol. Environ. Health Part A 2020, 84, 20–30. [Google Scholar] [CrossRef]
- Ferreira, N.G.C.; Chessa, A.; Abreu, I.O.; Teles, L.O.; Kille, P.; Carvalho, A.P.; Guimarães, L. Toxic Relationships: Prediction of TBT’s Affinity to the Ecdysteroid Receptor of Triops longicaudatus. Toxics 2023, 11, 937. [Google Scholar] [CrossRef]
- Mohammed, V.S.N.; Sheriff, A.M.; Mohideen, S.A.K.; Azmathullah, M.N. Toxicity of formalin on behaviour and respiration in Danio rerio. Int. J. Environ. Sci. 2012, 2, 1904. [Google Scholar]
- Albrecht, J.; Matyja, E. Glutamate: A potential mediator of inorganic mercury neurotoxicity. Metab. Brain Dis. 1996, 11, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Park, Y.S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466–467, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, A.; Forni, G.; Martelossi, J.; Savojardo, C.; Martelli, P.L.; Casadio, R.; Skaist, A.M.; Wheelan, S.J.; Mantovani, B. Comparative genomics of tadpole shrimps (Crustacea, Branchiopoda, Notostraca): Dynamic genome evolution against the backdrop of morphological stasis. Genomics 2021, 113, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Al-Najjar, Y.; Cramer, E.R.A.; Suhre, K.; Chen, K.C. Development and characterization of microsatellite primers for Triops granarius (Branchiopoda: Notostraca) using MiSeq technology. Mol. Biol. Rep. 2022, 49, 10121–10125. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, L.; Carvalho, A.P.; Ribeiro, P.; Teixeira, C.; Silva, N.; Pereira, A.; Amorim, J.; Oliva-Teles, L. Sensitivity of Triops longicaudatus Locomotor Behaviour to Detect Short Low-Level Exposure to Pollutants. Water 2024, 16, 126. https://doi.org/10.3390/w16010126
Guimarães L, Carvalho AP, Ribeiro P, Teixeira C, Silva N, Pereira A, Amorim J, Oliva-Teles L. Sensitivity of Triops longicaudatus Locomotor Behaviour to Detect Short Low-Level Exposure to Pollutants. Water. 2024; 16(1):126. https://doi.org/10.3390/w16010126
Chicago/Turabian StyleGuimarães, Laura, António Paulo Carvalho, Pedro Ribeiro, Cláudia Teixeira, Nuno Silva, André Pereira, João Amorim, and Luís Oliva-Teles. 2024. "Sensitivity of Triops longicaudatus Locomotor Behaviour to Detect Short Low-Level Exposure to Pollutants" Water 16, no. 1: 126. https://doi.org/10.3390/w16010126





