Adsorptive Removal of Sb(V) from Wastewater by Pseudo-Boehmite: Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of PB
2.3. Characterization of PB
2.4. Adsorption Experiments
2.4.1. Effect of Solution pH
2.4.2. Kinetic and Isotherms Adsorption Experiments
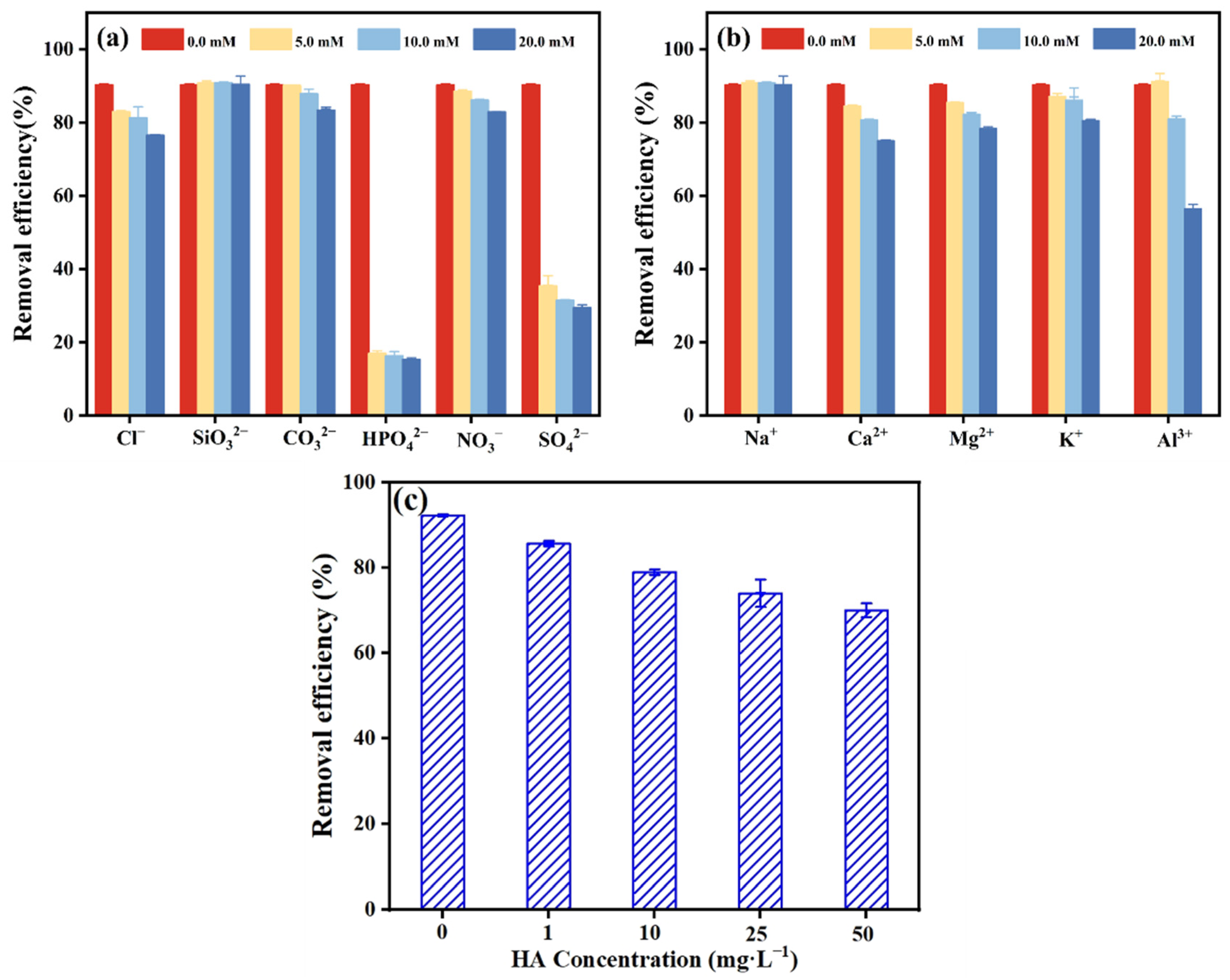
2.4.3. The Coexistence Effect of Common Metal Cation and Anions and Humic Acid
2.5. The Adsorbent Regeneration
3. Results and Discussion
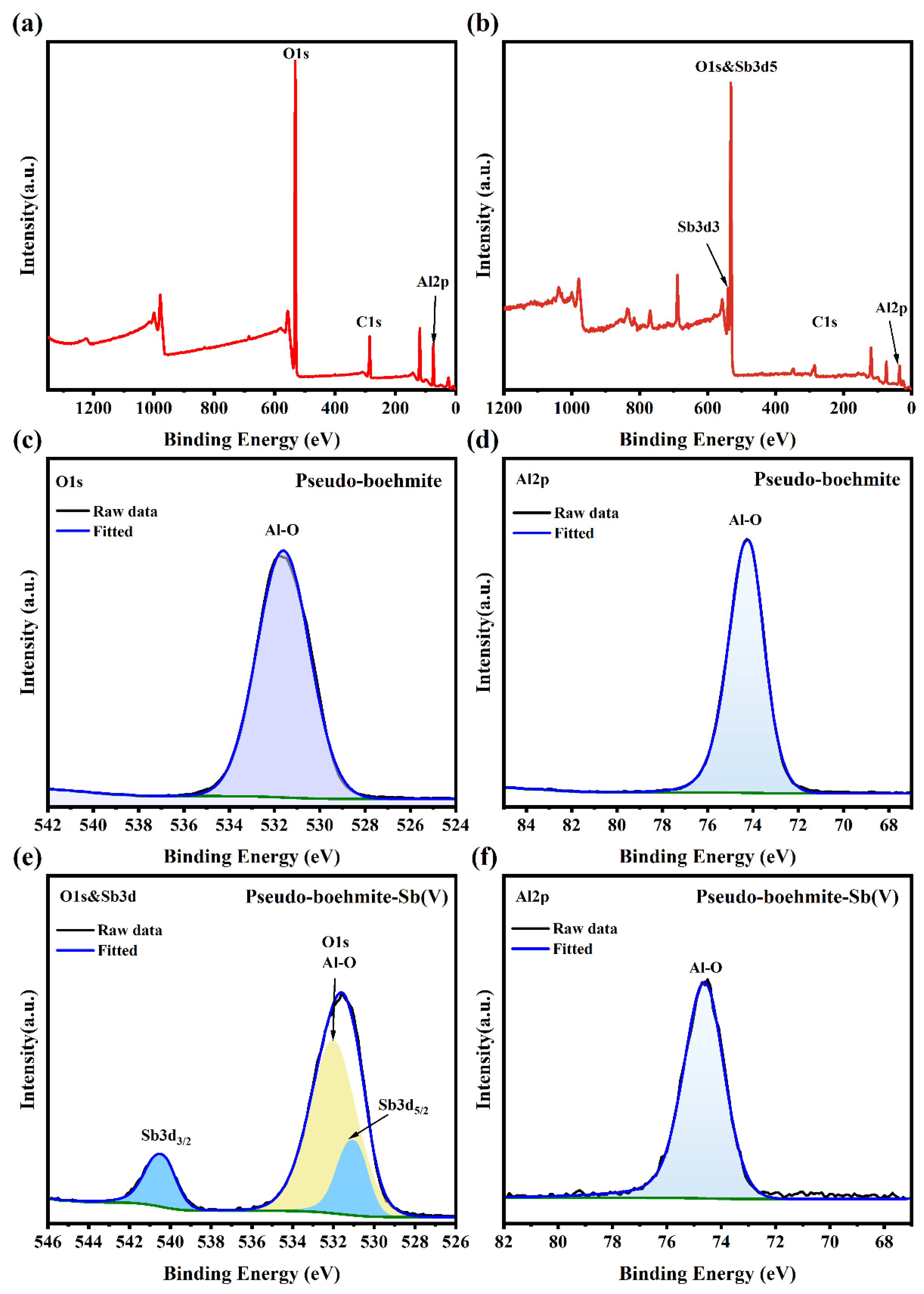
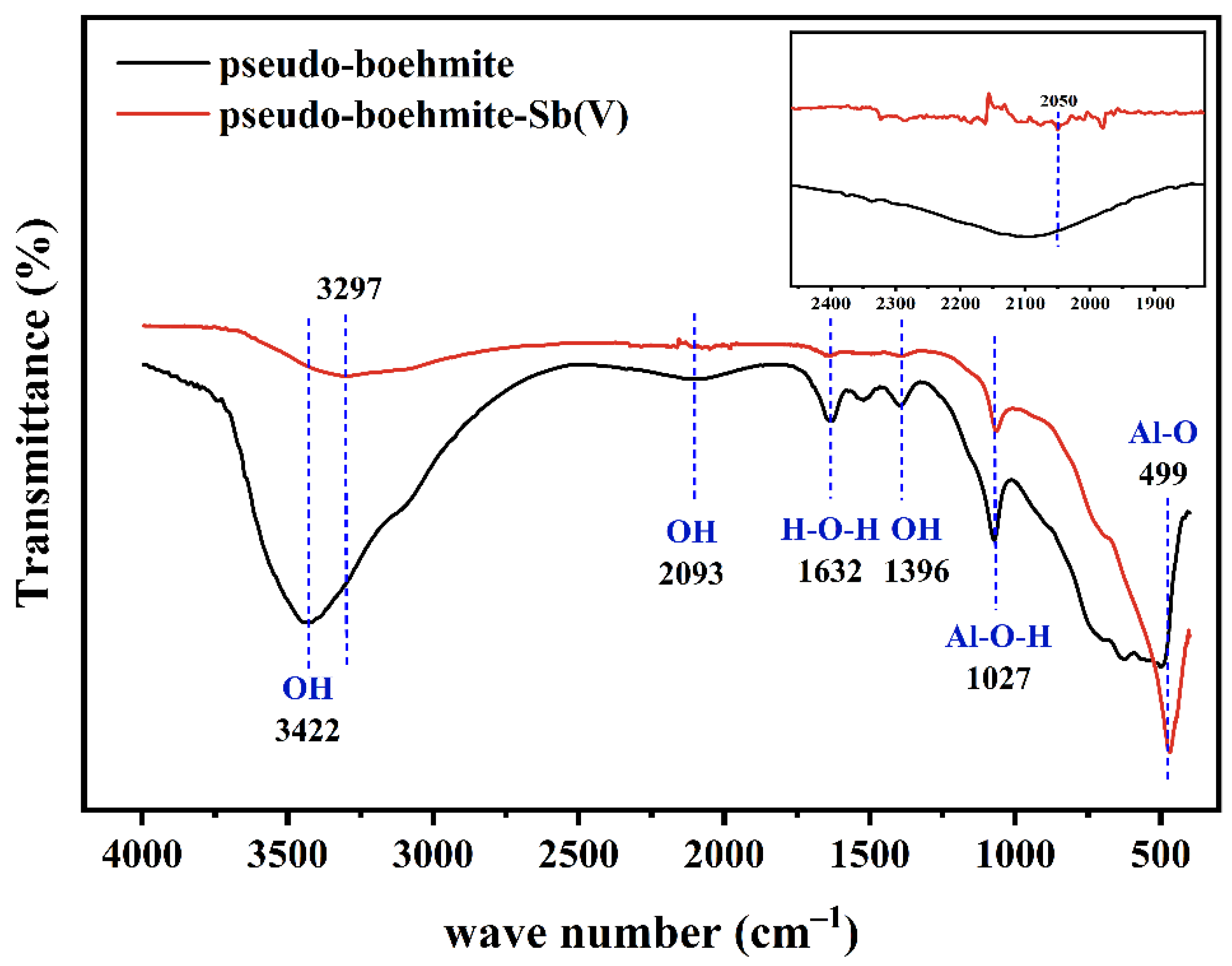
3.1. Characterization of Materials
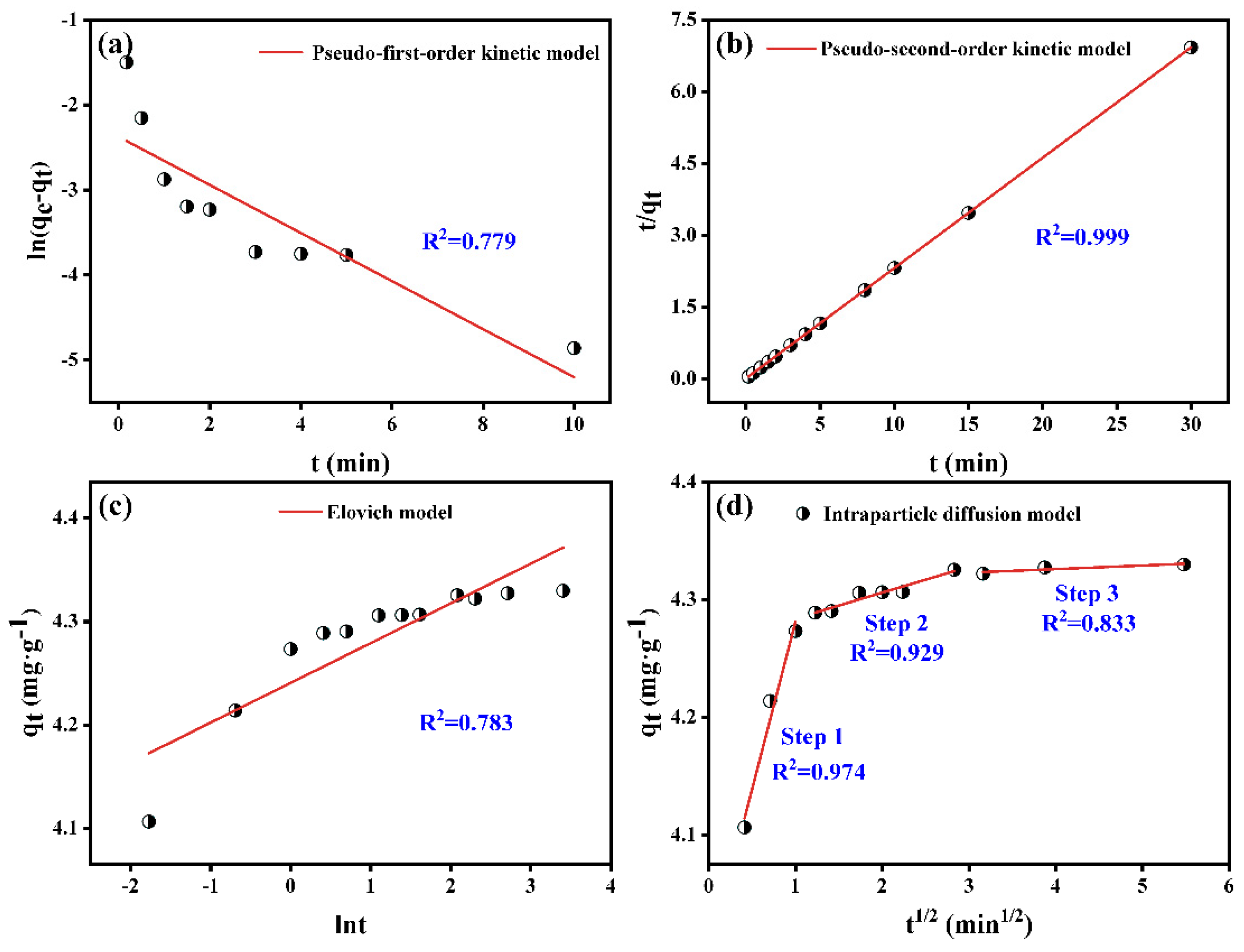
3.2. Adsorption Kinetics
3.3. Adsorption Isotherms
3.4. Effect of pH
3.5. Interference of Co-Existing Ions and Humic Acid
3.6. Removal Mechanisms of Sb(V) by PB
- (1)
- The adsorption kinetic of Sb(V) on PB conformed to the pseudo-second-order model, indicating that the adsorption of Sb(V) was chemisorption. The isothermal model studies demonstrated that Sb(V) adsorption was a homogeneous monolayer and multilayer chemisorption process.
- (2)
- The influence of pH experiments showed that electrostatic attraction was involved in the process of Sb(V) removal by PB.
- (3)
- FTIR and XPS analyses revealed that Sb(V) was adsorbed by surface complexation (Al-O-Sb) and hydrogen bonding.

3.7. Reusability of PB for Sb(V)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lan, B.; Wang, Y.; Wang, X.; Zhou, X.; Kang, Y.; Li, L. Aqueous arsenic (As) and antimony (Sb) removal by potassium ferrate. Chem. Eng. J. 2016, 292, 389–397. [Google Scholar] [CrossRef]
- Wu, T.-L.; Sun, Q.; Fang, G.-D.; Cui, P.-X.; Liu, C.; Alves, M.E.; Qin, W.-X.; Zhou, D.-M.; Shi, Z.-Q.; Wang, Y.-J. Unraveling the effects of gallic acid on Sb(III) adsorption and oxidation on goethite. Chem. Eng. J. 2019, 369, 414–421. [Google Scholar] [CrossRef]
- Zeldin, J.; Tran, T.T.; Yadav, M.; Chaudhary, P.P.; D’Souza, B.N.; Ratley, G.; Ganesan, S.; Myles, I.A. Antimony Compounds Associate with Atopic Dermatitis and Influence Models of Itch and Dysbiosis. Environ. Sci. Technol. Lett. 2023, 10, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Qi, C.; Li, R.; Zhang, W.; Li, J.; Shen, J.; Sun, X. Facile preparation of novel magnetic mesoporous Fe Mn binary oxides from Mn encapsulated carboxymethyl cellulose-Fe(III) hydrogel for antimony removal from water. Sci. Total Environ. 2022, 821, 153529. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Zhang, Z.; Wang, H.; Liu, S.; Feng, W.; Zhao, X.; Giesy, J.P. Synthesis of Fe3O4 magnetic nanoparticles coated with cationic surfactants and their applications in Sb(V) removal from water. Sci. Total Environ. 2020, 710, 136302. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, X.; Guo, X.; He, M. A review of removal technology for antimony in aqueous solution. J. Environ. Sci. 2020, 90, 189–204. [Google Scholar] [CrossRef]
- Salari, K.; Hashemian, S.; Baei, M.T. Sb (V) removal from copper electrorefining electrolyte: Comparative study by different sorbents. Trans. Nonferrous Met. Soc. China 2017, 27, 440–449. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, Z.; He, C.; Lyu, W.; Yan, W.; Yang, L. Enhanced antimonate (Sb(V)) removal from aqueous solution by La-doped magnetic biochars. Chem. Eng. J. 2018, 354, 623–632. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Akram, M.; Khan, S.; Yeom, I.T. Effect of Water Chemistry on Antimony Removal by Chemical Coagulation: Implications of zeta-Potential and Size of Precipitates. Int. J. Mol. Sci. 2019, 20, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Li, H.; Yu, D.; Wang, Y.; Wang, W.; Wu, M. Preparation and selective adsorption of surface-imprinted microspheres based on hyperbranched polyamide–functionalized sodium alginate for the removal of Sb(III). Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124106. [Google Scholar] [CrossRef]
- Du, X.; Qu, F.; Liang, H.; Li, K.; Yu, H.; Bai, L.; Li, G. Removal of antimony (III) from polluted surface water using a hybrid coagulation–flocculation–ultrafiltration (CF–UF) process. Chem. Eng. J. 2014, 254, 293–301. [Google Scholar] [CrossRef]
- Awual, M.R.; Alharthi, N.H.; Okamoto, Y.; Karim, M.R.; Halim, M.E.; Hasan, M.M.; Rahman, M.M.; Islam, M.M.; Khaleque, M.A.; Sheikh, M.C. Ligand field effect for Dysprosium(III) and Lutetium(III) adsorption and EXAFS coordination with novel composite nanomaterials. Chem. Eng. J. 2017, 320, 427–435. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Luo, L.; Zheng, Y.; Huang, L.; Zhang, J.; Yang, Y.; Huang, H. Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: Indispensable role of reactive oxygen species and redox-active moieties. J. Hazard. Mater. 2020, 391, 122057. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, Z.; Zhao, X.; Zhang, H.; Wang, J.; Wu, F.; Giesy, J.P.; Shi, J. Efficient removal of both antimonite (Sb(III)) and antimonate (Sb(V)) from environmental water using titanate nanotubes and nanoparticles. Environ. Sci. Nano 2019, 6, 834–850. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Wang, Y.; Zhang, T.C.; Xiang, G.; Zhang, Y.-X.; Yuan, S. Core–Shell Structured Magnetic γ-Fe2O3@PANI Nanocomposites for Enhanced As(V) Adsorption. Ind. Eng. Chem. Res. 2020, 59, 7554–7563. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, F.; Gong, Y. Oxidation and adsorption of antimony (III) from surface water using novel Al2O3-supported Fe–Mn binary oxide nanoparticles: Effectiveness, dynamic quantitative mechanisms, and life cycle analysis. Environ. Sci. Nano 2020, 7, 3047–3061. [Google Scholar] [CrossRef]
- Ramadugu, S.K.; Mason, S.E. DFT Study of Antimony(V) Oxyanion Adsorption on α-Al2O3(12). J. Phys. Chem. C 2015, 119, 18149–18159. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, A.; Wen, B.; Liu, P.; Zhu, Z.; Finfrock, Z.; Zhou, J. Antimony isotope fractionation during adsorption on aluminum oxides. J. Hazard. Mater. 2022, 429, 128317. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Islam, M.A.; Angove, M.J.; Morton, D.W.; Pramanik, B.K.; Awual, M.R. A mechanistic approach of chromium (VI) adsorption onto manganese oxides and boehmite. J. Environ. Chem. Eng. 2020, 8, 103515. [Google Scholar] [CrossRef]
- Chen, X.; Guan, X.; Zhang, H.; Liu, W.; Han, Y.; Xiong, J.; Zheng, S.; Zhang, Y.; Zhang, X.; Zhao, J.; et al. Green desorption combined with peptization technology for disposing of Cr(VI)-V(V)-containing hazardous aluminum waste to prepare high-valued pseudo-boehmite. Sep. Purif. Technol. 2023, 314, 123655. [Google Scholar] [CrossRef]
- Cao, P.; Gao, D.; Luo, J.; Li, X.; Xing, L.; Jiang, H.; Li, G. Preparation of pseudo-boehmite via carbonation of sodium aluminate lixivium extracted from coal fly ash. Microporous Mesoporous Mater. 2024, 366, 112933. [Google Scholar] [CrossRef]
- Xie, X.; Li, J.; Liu, J.; Chen, Z.; Yuan, X.; Chen, T.; Luo, L. Efficient degradation of chromium picolinate with simultaneous chromium removal from aqueous solutions using the Fenton process. Sep. Purif. Technol. 2023, 317, 123864. [Google Scholar] [CrossRef]
- Panda, A.P.; Jha, U.; Swain, S.K. Synthesis of nanostructured copper oxide loaded boehmite (CuO_Boehmite) for adsorptive removal of As(III/V) from aqueous solution. J. Water Process Eng. 2020, 37, 101506. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, J.; Kumar, A.; Sankararamakrishnan, N. Removal of antimonite (Sb(III)) and antimonate (Sb(V)) using zerovalent iron decorated functionalized carbon nanotubes. RSC Adv. 2016, 6, 95865–95878. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, J.; Ma, J.; Xu, J. In situ growth of hierarchical boehmite on 2024 aluminum alloy surface as superhydrophobic materials. RSC Adv. 2014, 4, 14708–14714. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, W.; Dang, C.; Fan, J.; Zhou, J.; Liu, Z. A facile sol–gel synthesis of chitosan–boehmite film with excellent acid resistance and adsorption performance for Pb(II). Chem. Eng. Res. Des. 2020, 161, 332–339. [Google Scholar] [CrossRef]
- Cai, W.; Li, H.; Zhang, Y. Influences of processing techniques of the H2O2-precipitated pseudoboehmite on the structural and textural properties of γ-Al2O3. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 185–192. [Google Scholar] [CrossRef]
- Panda, A.P.; Giri, M.; Jena, K.K.; Alhassan, S.M.; Kumar, S.A.; Jha, U.; Swain, S.K. Understanding the As(III) oxidative performance of MnO2 polymorphs (α, β, and γ) and synthesis of an efficient nanocomposite of iron ore slime derived 2-line ferrihydrite and γ-MnO2 for sequestration of total arsenic from aqueous solution. Chem. Eng. J. 2022, 442, 136075. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Ge, M.; Lin, M.; Yang, X.; Jing, Y. Insights into adsorption mechanism for fluoride on cactus-like amorphous alumina oxide microspheres. Chem. Eng. J. 2018, 345, 252–259. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Wang, H.; Lv, Z.; Wang, B.; Wang, Y.-N.; Sun, Y.; Tsang, Y.F.; Zhao, J.; Zhan, M. Effective stabilization of antimony in Waste-to-Energy fly ash with recycled laboratory iron–rich residuals. J. Clean. Prod. 2019, 230, 685–693. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Han, K.; Sun, S. Synthesis of nano-zirconium-iron oxide supported by activated carbon composite for the removal of Sb(V) in aqueous solution. RSC Adv. 2021, 11, 31131–31141. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Joshi, T.P.; Liu, R.; Liu, H.; Qu, J. Synthesis of Ce(III)-doped Fe3O4 magnetic particles for efficient removal of antimony from aqueous solution. J. Hazard. Mater. 2017, 329, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Robati, D.; Rajabi, M.; Moradi, O.; Najafi, F.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Kinetics and thermodynamics of malachite green dye adsorption from aqueous solutions on graphene oxide and reduced graphene oxide. J. Mol. Liq. 2016, 214, 259–263. [Google Scholar] [CrossRef]
- Li, L.; Liao, L.; Wang, B.; Li, W.; Liu, T.; Wu, P.; Xu, Q.; Liu, S. Effective Sb(V) removal from aqueous solution using phosphogypsum-modified biochar. Environ. Pollut. 2022, 301, 119032. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; El-Naggar, A.; Niazi, N.K.; Sun, C.; Shaheen, S.M.; Hou, D.; Yang, X.; Tang, Z.; Liu, Z.; et al. Enhanced sorption of trivalent antimony by chitosan-loaded biochar in aqueous solutions: Characterization, performance and mechanisms. J. Hazard. Mater. 2022, 425, 127971. [Google Scholar] [CrossRef]
- Li, X.; Dou, X.; Li, J. Antimony(V) removal from water by iron-zirconium bimetal oxide: Performance and mechanism. J. Environ. Sci. 2012, 24, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, H.; Ren, X.; Zhang, K. Sorption of naphthalene and its hydroxyl substitutes onto biochars in single-solute and bi-solute systems with propranolol as the co-solute. Chem. Eng. J. 2017, 326, 281–291. [Google Scholar] [CrossRef]
- Chen, L.; Cui, W.; Li, J.; Wang, H.; Chen, P.; Zhou, Y.; Dong, F. The high selectivity for benzoic acid formation on Ca2Sb2O7 enables efficient and stable toluene mineralization. Appl. Catal. B Environ. 2020, 271, 118948. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Wang, H.; Liu, S.; Wu, F.; Giesy, J.P. Removal of antimonate (Sb(V)) and antimonite (Sb(III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci. Total Environ. 2018, 644, 1277–1285. [Google Scholar] [CrossRef]
- Xiong, N.; Wan, P.; Zhu, G.; Xie, F.; Xu, S.; Zhu, C.; Hursthouse, A.S. Sb(III) removal from aqueous solution by a novel nano-modified chitosan (NMCS). Sep. Purif. Technol. 2020, 236, 116266. [Google Scholar] [CrossRef]




| Kinetics Type | Equations | Description of Parameters |
|---|---|---|
| Pseudo-first-order kinetics | k1 (mg·L−1), adsorption rate constant; qe (mg·g−1) and qt (mg·g−1), sorption capacities at equilibrium and at any time. | |
| Pseudo-second-order kinetics | k2 (g·mg−1·min−1), adsorption rate constant; qe (mg·g−1) and qt (mg·g−1), sorption capacities at equilibrium and at any time. | |
| Elovich model | t | qt (mg·g−1), sorption capacity at any time; α (mg·mL−1·min−1) and β (mL·mg−1), the initial adsorption coefficient and Elovich model equation constants. |
| Intraparticle diffusion model | k3 (mg·g−1·min−1/2), adsorption rate constant; qt (mg·g−1) sorption capacity at any time; C is a constant. |
| Models | Equations | Description of Parameters |
|---|---|---|
| Langmuir | KL (L·mg−1), adsorption equilibrium constant; qe (mg·g−1) and Ce (mg·L−1), equilibrium sorption capacitiy of adsorbent and equilibrium concentration of adsorbate, respectively. Qmax is calculated saturated adsorption capacity. | |
| Freundlich | KF (L·mg−1) and n, adsorption equilibrium constant, characteristic constant characterizing the properities of adsorption force, respectively; qe (mg·g−1) and Ce (mg·L−1), equilibrium sorption capacitiy of adsorbent and equilibrium concentration of adsorbate, respectively. | |
| Temkin | R, universal gas conctant (8.314 J·mol−1·K−1); kT (L·mg−1) and bT, equilibrium binding constant and Temkin isotherm constant, respectively; qe (mg·g−1) and Ce (mg·L−1), equilibrium sorption capacitiy of adsorbent and equilibrium concentration of adsorbate, respectively. | |
| Dubinin–Radushkevich | β (mol2·kJ−2) and ↋, Dubinin–Radushkevich isotherm constants; T and R, absolute temperature (K) and universal gas conctant (8.314 J·mol−1·K−1), respectively; Ce (mg·L−1), equilibrium concentration of adsorbate. qe (mg·g−1) and qm (mg·g−1), equilibrium sorption capacitiy of adsorbent and theoretical isotherm saturation capacity, respectively. |
| Adsorption Kinetics Models | Parameters | Sb(V) |
|---|---|---|
| Pseudo-first-order kinetic model | k1 (min−1) | 0.651 |
| qe1 (mg·g−1) | 0.093 | |
| R21 | 0.779 | |
| Pseudo-second-order kinetic model | k2 (g·mg−1·min−1) | 13.793 |
| qe2 (mg·g−1) | 4.328 | |
| qe experiment (mg·g−1) | 4.333 | |
| R22 | 0.999 | |
| Elovich model | α (mg·mL−1·min−1) | 0.297 |
| β (mL·mg−1) | 26.001 | |
| R2 | 0.783 | |
| Intraparticle diffusion model | Kd1 (mg·g−1·min−1/2) | 0.284 |
| C1 | 3.997 | |
| R21 | 0.974 | |
| Kd2 (mg·g−1·min−1/2) | 0.022 | |
| C2 | 4.262 | |
| R22 | 0.929 | |
| Kd3 (mg·g−1·min−1/2) | 0.003 | |
| C3 | 4.314 | |
| R23 | 0.833 |
| Isothermal Models | Parameters | Temperature (K) | ||
|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||
| Langmuir model | kL (L·mg−1) | 0.007 | 0.006 | 0.008 |
| qmax (mg·g−1) | 75.25 | 69.92 | 59.77 | |
| R2 | 0.998 | 0.996 | 0.991 | |
| Freundlich model | kf (L·mg−1) | 2.05 | 1.77 | 2.18 |
| n−1 | 0.57 | 0.58 | 0.53 | |
| R2 | 0.976 | 0.985 | 0.965 | |
| Temkin model | kT (L·mg−1) | 13.48 | 12.14 | 11.68 |
| bT | 183.80 | 210.93 | 226.36 | |
| R2 | 0.956 | 0.949 | 0.963 | |
| D-R model | qm (mg·g−1) | 29.18 | 26.56 | 27.64 |
| β (mol2·kJ−2) | 0.004 | 0.003 | 0.003 | |
| R2 | 0.746 | 0.736 | 0.755 | |
| Adsorbents | pH | qe (mg·g−1) | Reference |
|---|---|---|---|
| Fe−Zr amorphous oxides | 7.0 ± 0.2 | 51 | [41] |
| Fe(OH)3 | 4 | 24.47 ± 2.79 | [34] |
| Ce-doped magnetic biochar | 7.5 | 25.0 | [35] |
| Nano zirconium iron oxide based on activated carbon | 5.0 | 11.8 | [36] |
| TiO2 particles | 2.2 ± 0.1 | 12.0 | [16] |
| PB | 5.0 | 75.25 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Mao, Q.; Zhou, Y.; Xie, X.; Luo, L. Adsorptive Removal of Sb(V) from Wastewater by Pseudo-Boehmite: Performance and Mechanism. Water 2024, 16, 1172. https://doi.org/10.3390/w16081172
He Y, Mao Q, Zhou Y, Xie X, Luo L. Adsorptive Removal of Sb(V) from Wastewater by Pseudo-Boehmite: Performance and Mechanism. Water. 2024; 16(8):1172. https://doi.org/10.3390/w16081172
Chicago/Turabian StyleHe, Yating, Qiming Mao, Yaoyu Zhou, Xiande Xie, and Lin Luo. 2024. "Adsorptive Removal of Sb(V) from Wastewater by Pseudo-Boehmite: Performance and Mechanism" Water 16, no. 8: 1172. https://doi.org/10.3390/w16081172




