Inhibition of Carbon Steel Corrosion Using Dextran Derivatives in Circulating Cooling Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
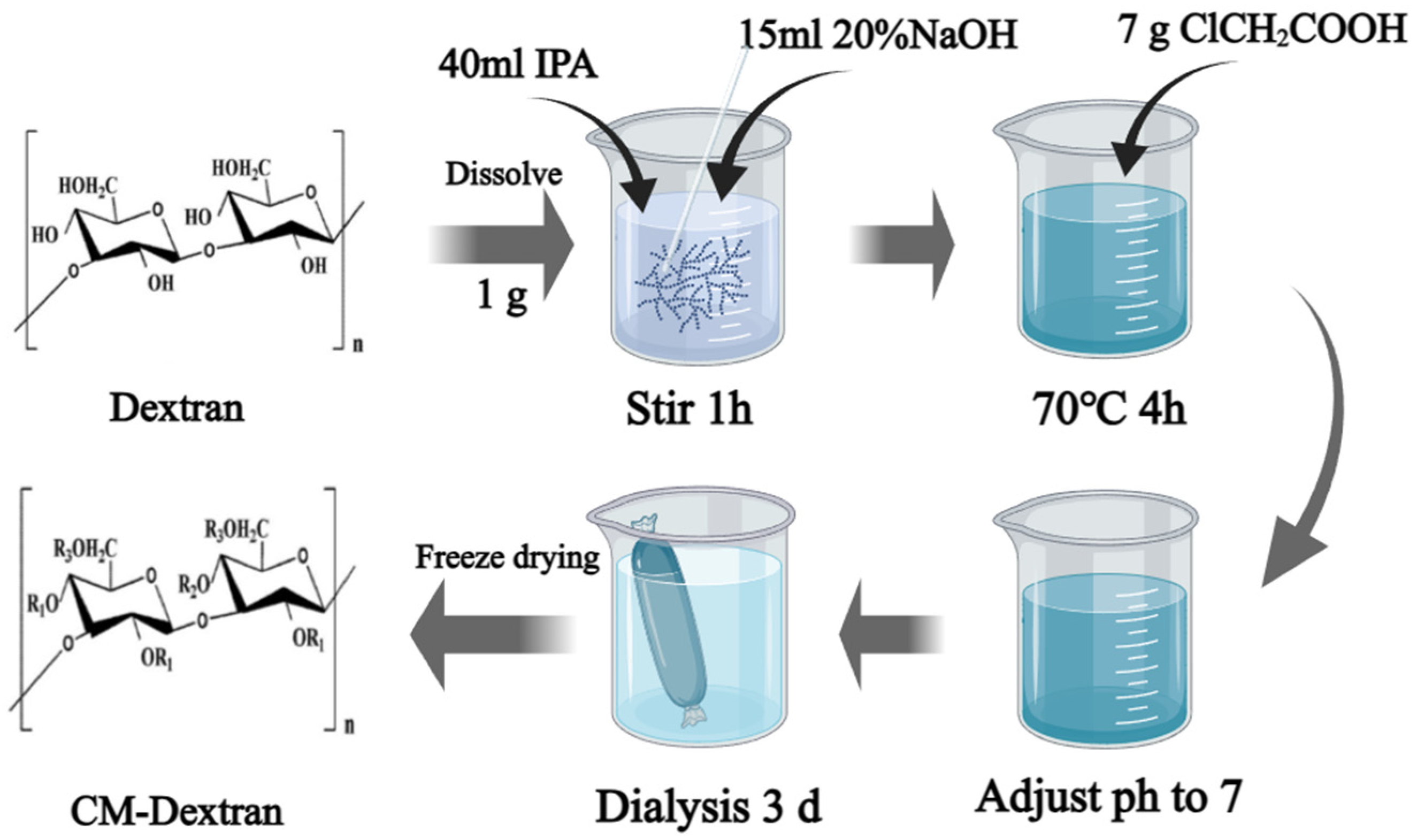
2.2. Preparation of a Modified Dextran Corrosion Inhibitor
2.3. Experimental Setup
2.4. Corrosion Weight Loss
2.5. Electrochemical Testing
2.6. Characterization of the Metal Surface
3. Results
3.1. FT-IR Characterization of Dextran Derivatives
3.2. Corrosion Inhibition Properties of CM-Dextran
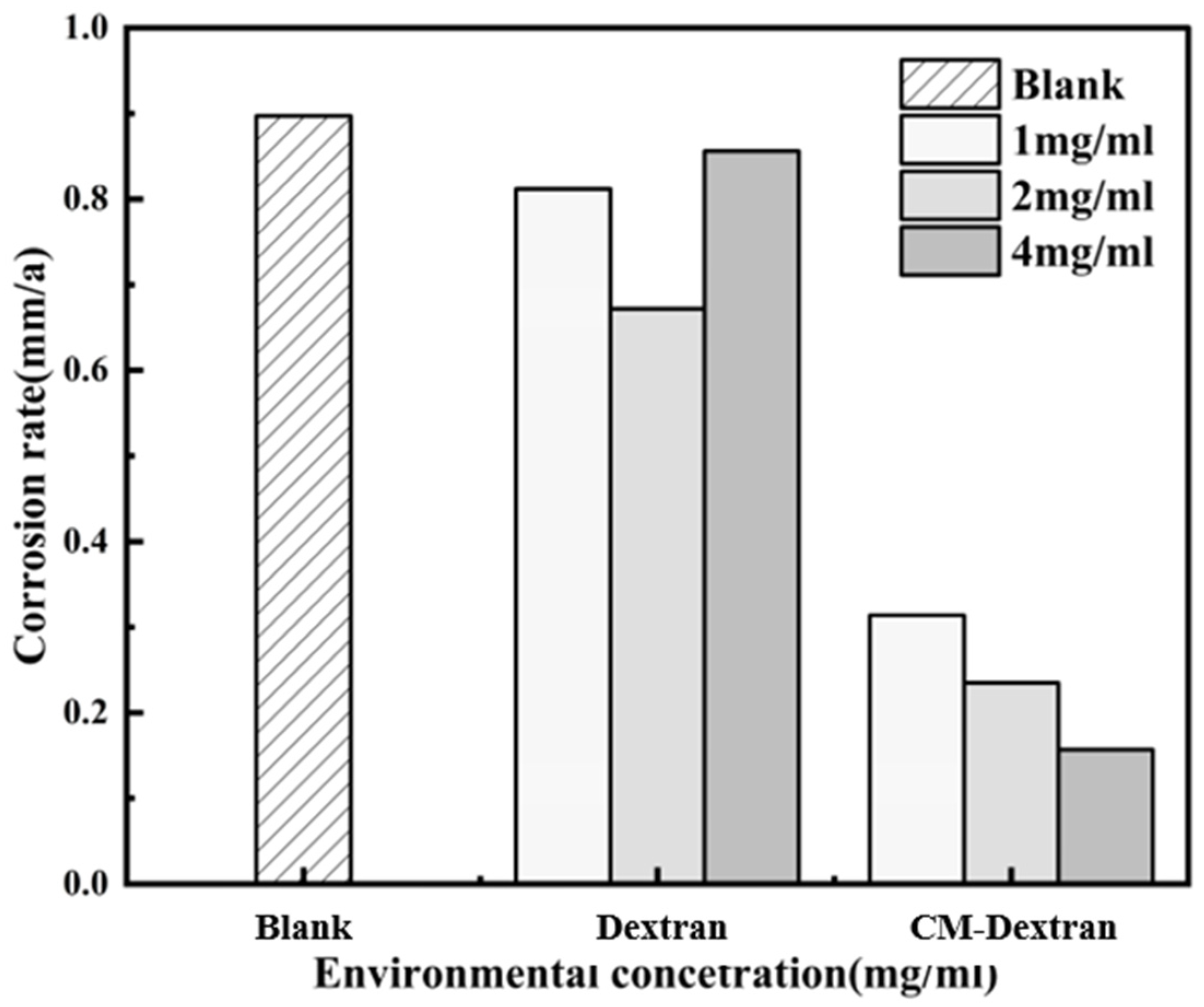
3.2.1. Corrosion Rate Test
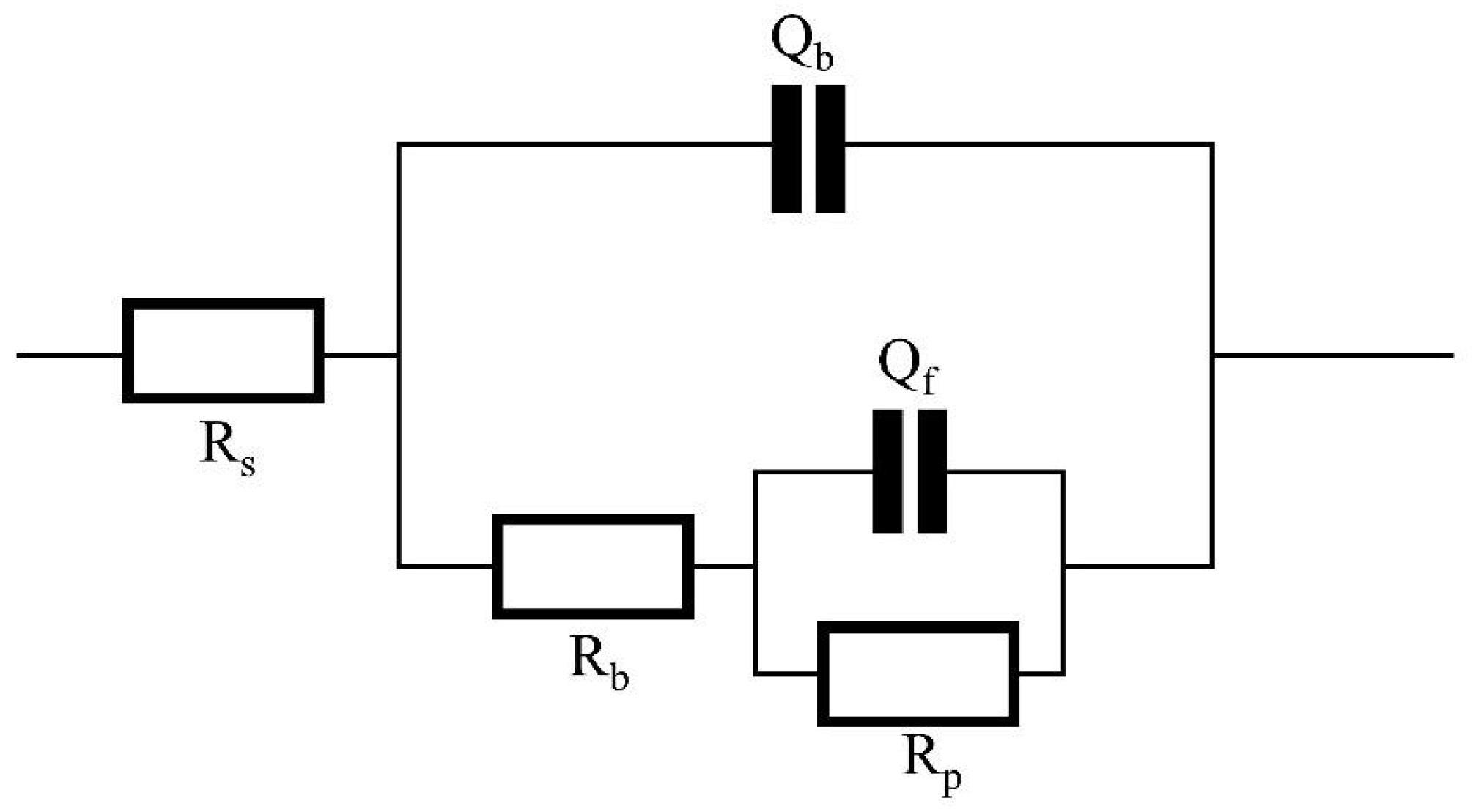
3.2.2. Electrochemical Measurements
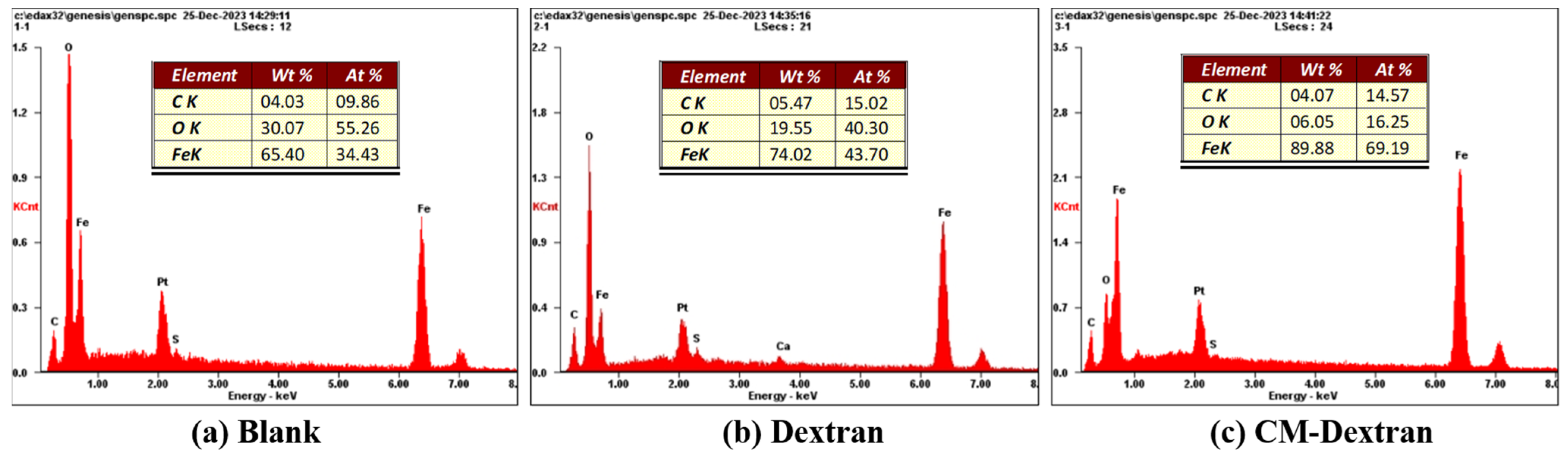
3.3. SEM Analysis of the Carbon Steel Surface
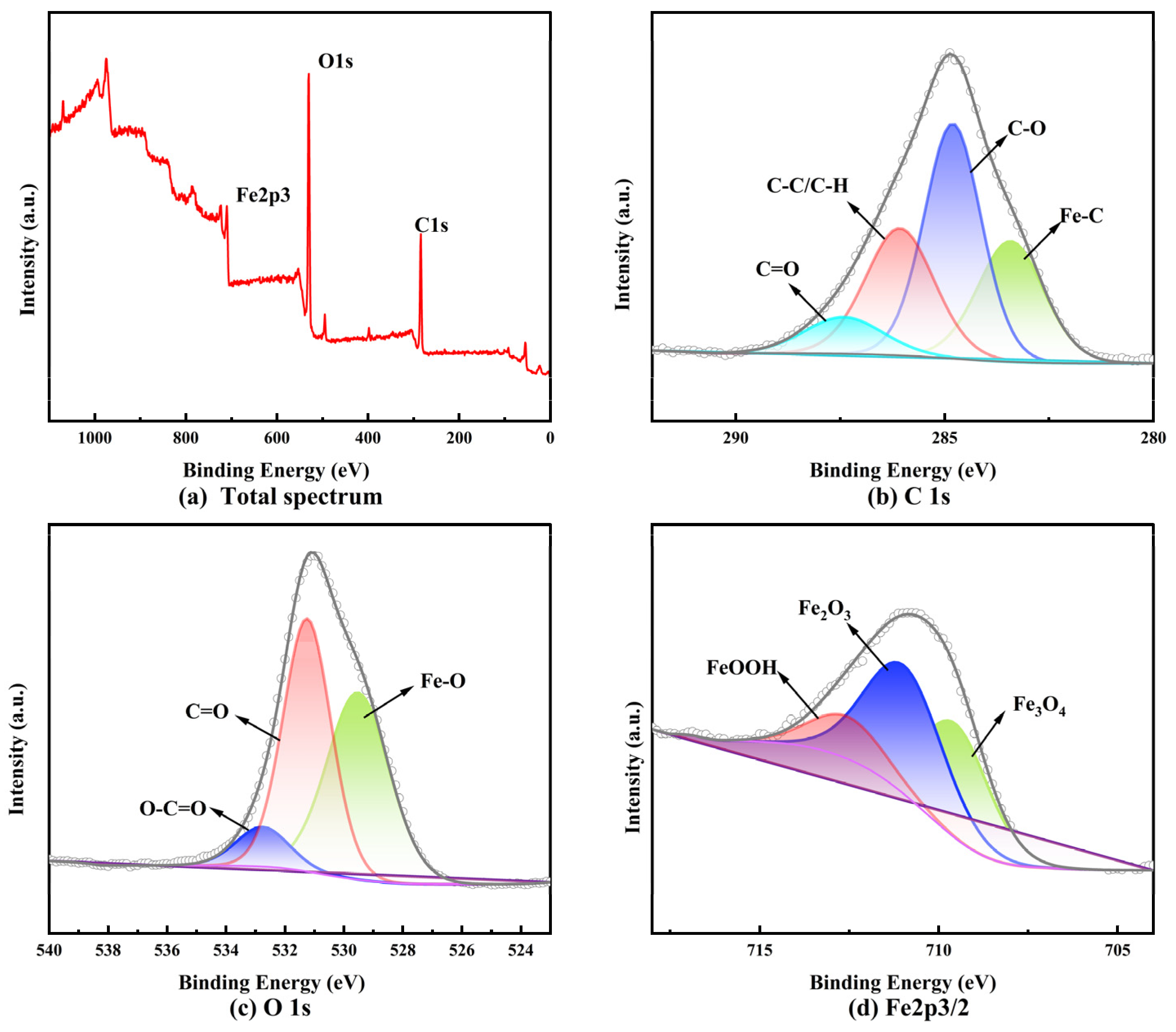
3.4. Analysis of CM-Dextran’s Adsorption Mechanism
4. Discussion
5. Conclusions
- (1)
- Dextran and CM-Dextran are green and environmentally friendly corrosion inhibitors that offer excellent capabilities in inhibiting carbon steel corrosion. In this study, Dextran yielded a corrosion inhibition rate of 25.17% at 2 mg/mL, while CM-Dextran achieved the highest corrosion inhibition rate of 82.52% at 4 mg/mL. Chemical modification did not compromise the green characteristics of the carbon steel. Dextran exhibited low and unstable inhibition efficiency, while modified CM-Dextran enhanced the corrosion inhibition capability by 57.35% and demonstrated long-term stability, thus overcoming natural green corrosion inhibitors’ susceptibility to degradation.
- (2)
- The results of electrochemical kinetics testing indicated that after the addition of CM-Dextran, the corrosion potential range of the carbon steel was between −0.498 and −0.571 V. Compared to the results with the addition of Dextran, the corrosion tendency of carbon steel decreased, and the corrosion current density decreased. Βa was greater than βc, indicating anodic corrosion inhibition capacity. When the environmental concentration reached 4 mg/mL, βa increased by 159 mV·dec−1, effectively inhibiting the metal dissolution reaction on the surface of the carbon steel. Electrochemical impedance spectroscopy and SEM demonstrated that CM-Dextran formed a smooth protective film on the surface of the carbon steel. This film structure impeded the metal dissolution reaction on the surface of the carbon steel, resulting in a 14.96% decrease in surface oxygen content.
- (3)
- CM-Dextran adsorbed Fe2+/Fe3+ with ΔG values of −28.33 kJ mol–1 and −27.85 kJ mol–1, involving both physical and chemical adsorption processes. XPS testing revealed that CM-Dextran adsorbed onto the C–C/C–H, C–O, and C=O sites on the surface of the carbon steel. The surface content of Fe2+ and Fe3+ was 70.81% and 29.19%, respectively, with electrostatic adsorption occurring between Fe2+/Fe3+ and CM-Dextran. Additionally, metal complexes were formed through covalent bonds. These complexes covered the metal surface to form a dense protective film, effectively inhibiting the corrosion process of the carbon steel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Branzoi, F.; Branzoi, V.; Licu, C. Corrosion inhibition of carbon steel in cooling water systems by new organic polymers as green inhibitors. Mater. Corros. 2014, 65, 637–647. [Google Scholar] [CrossRef]
- Seo, B.; Kanematsu, H.; Nakamoto, M.; Miyabayashi, Y.; Tanaka, T. Corrosion resistance and antibacterial activity of a surface coating created by super-spread wetting of liquid copper on laser-ablated carbon steel. Surf. Coat. Technol. 2022, 445, 128706. [Google Scholar] [CrossRef]
- Kokilaramani, S.; Al-Ansari, M.M.; Rajasekar, A.; Al-Khattaf, F.S.; Hussain, A.; Govarthanan, M. Microbial influenced corrosion of processing industry by re-circulating waste water and its control measures—A review. Chemosphere 2021, 265, 129075. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M. The influence of different variables on the electrochemical behavior of mild steel in circulating cooling water containing aggressive anionic species. J. Solid State Electrochem. 2009, 13, 1737–1742. [Google Scholar] [CrossRef]
- Deyab, M. Electrochemical investigations on pitting corrosion inhibition of mild steel by provitamin B5 in circulating cooling water. Electrochim. Acta 2016, 202, 262–268. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Sorour, A.A.; Saji, V.S.; Quraishi, M.A. Green corrosion inhibitors based on biomacromolecules and macrocycles: A review. Sustain. Chem. Pharm. 2023, 36, 101295. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Wan Isahak, W.N.R.; Al-Azzawi, W.K. Sustainable corrosion Inhibitors: A key step towards environmentally responsible corrosion control. Ain Shams Eng. J. 2024, 15, 102672. [Google Scholar] [CrossRef]
- Medupin, R.O.; Ukoba, K.O.; Yoro, K.O.; Jen, T.-C. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: A review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Gerengi, H.; Schaefer, K.; Sahin, H.I. Corrosion-inhibiting effect of Mimosa extract on brass-MM55 corrosion in 0.5 M H2SO4 acidic media. J. Ind. Eng. Chem. 2012, 18, 2204–2210. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Zhao, C.; Wang, R.; Zhou, X.; Sun, Y.; Yan, Z.; Li, X. Study on the corrosion inhibitory performance of Pomacea canaliculata extract as a corrosion inhibitor for carbon steel in acidic environments. J. Mol. Liq. 2024, 394, 123754. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Liu, L.; Zhang, Q.; Wu, X.; Yan, Z.; Sun, Y.; Li, X. Insight into the anti–corrosion behavior of Reineckia Carnea leaves extract as an eco–friendly and high–efficiency corrosion inhibitor. Ind. Crops Prod. 2022, 188, 115640. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Rahsepar, M. The use of green Bistorta Officinalis extract for effective inhibition of corrosion and scale formation problems in cooling water system. J. Alloys Compd. 2019, 770, 669–678. [Google Scholar] [CrossRef]
- van Hijum, S.A.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Cheng, X.; Li, J.; Chen, S.; Dou, W.; Liu, G. Influence of soluble, loosely bound and tightly bound extracellular polymeric substances (EPS) produced by Desulfovibrio vulgaris on EH40 steel corrosion. Corros. Sci. 2023, 221, 111342. [Google Scholar] [CrossRef]
- Bautista, B.E.T.; Wikieł, A.J.; Datsenko, I.; Vera, M.; Sand, W.; Seyeux, A.; Zanna, S.; Frateur, I.; Marcus, P. Influence of extracellular polymeric substances (EPS) from Pseudomonas NCIMB 2021 on the corrosion behaviour of 70Cu–30Ni alloy in seawater. J. Electroanal. Chem. 2015, 737, 184–197. [Google Scholar] [CrossRef]
- Ghafari, M.D.; Bahrami, A.; Rasooli, I.; Arabian, D.; Ghafari, F. Bacterial exopolymeric inhibition of carbon steel corrosion. Int. Biodeterior. Biodegrad. 2013, 80, 29–33. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. (Eds.) Chapter 5—Chitosan. In Polymeric Materials in Corrosion Inhibition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–153. [Google Scholar]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Ni, D.; Xu, W.; Zhang, W.; Mu, W. Microbial dextran-hydrolyzing enzyme: Properties, structural features, and versatile applications. Food Chem. 2024, 437, 137951. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, Y.; Dong, H.; Li, A. Nano-encapsulation of EGCG in dextran 70-stabilized chitosan/SDS nanoparticles: Characterization, permeability behavior and oral bioavailability. Eur. Polym. J. 2024, 205, 112763. [Google Scholar] [CrossRef]
- Luo, X.; Pan, X.; Yuan, S.; Du, S.; Zhang, C.; Liu, Y. Corrosion inhibition of mild steel in simulated seawater solution by a green eco-friendly mixture of glucomannan (GL) and bisquaternary ammonium salt (BQAS). Corros. Sci. 2017, 125, 139–151. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A.; Gerengi, H. Exploration of Dextran for Application as Corrosion Inhibitor for Steel in Strong Acid Environment: Effect of Molecular Weight, Modification, and Temperature on Efficiency. ACS Appl. Mater. Interfaces 2018, 10, 28112–28129. [Google Scholar] [CrossRef]
- Thongchaivetcharat, K.; Jenjob, R.; Seidi, F.; Crespy, D. Programming pH-responsive release of two payloads from dextran-based nanocapsules. Carbohydr. Polym. 2019, 217, 217–223. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Xu, N. Developing two amino acid derivatives as high-efficient corrosion inhibitors for carbon steel in the CO2-containing environment. Ind. Crops Prod. 2023, 201, 116883. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, B. Identification of molecular driving forces involved in the gelation of konjac glucomannan: Effect of degree of deacetylation on hydrophobic association. Carbohydr. Polym. 2011, 86, 865–871. [Google Scholar] [CrossRef]
- Liu, W.; Hu, C.; Liu, Y.; Dai, S.; Lu, W.; lv, X.; Yao, W.; Gao, X. Preparation, characterization, and α-glycosidase inhibition activity of a carboxymethylated polysaccharide from the residue of Sarcandra glabra (Thunb.) Nakai. Int. J. Biol. Macromol. 2017, 99, 454–464. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. (Eds.) Chapter 6—Chitosan derivatives. In Polymeric Materials in Corrosion Inhibition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 155–185. [Google Scholar]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Dextran derivatives as highly efficient green corrosion inhibitors for carbon steel in CO2-saturated oilfield produced water: Experimental and theoretical approaches. Chem. Eng. J. 2021, 424, 130519. [Google Scholar] [CrossRef]
- Ren, J.-L.; Sun, R.-C.; Peng, F. Carboxymethylation of hemicelluloses isolated from sugarcane bagasse. Polym. Degrad. Stab. 2008, 93, 786–793. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Qurashi, A.; Chauhan, D.S.; Quraishi, M.A. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2021, 321, 114385. [Google Scholar] [CrossRef]
- Fan, L.; Wang, L.; Gao, S.; Wu, P.; Li, M.; Xie, W.; Liu, S.; Wang, W. Synthesis, characterization and properties of carboxymethyl kappa carrageenan. Carbohydr. Polym. 2011, 86, 1167–1174. [Google Scholar] [CrossRef]
- Xu, P.; Fu, Q.; Zhao, M. The influence of calcium on copper corrosion and its by-product release in drinking water. RSC Adv. 2023, 13, 17842–17855. [Google Scholar] [CrossRef] [PubMed]
- Číhal, V.R.; Štefec, R. On the development of the electrochemical potentiokinetic method. Electrochim. Acta 2001, 46, 3867–3877. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Tian, J.; Wang, Y.; Xing, L.; Wang, J. Chemical modification, antioxidant and α-amylase inhibitory activities of corn silk polysaccharides. Carbohydr. Polym. 2013, 98, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, G.; Huang, H. Ultrasonic/cellulase-assisted extraction of polysaccharide from Garcinia mangostana rinds and its carboxymethylated derivative. Ultrason. Sonochem. 2023, 99, 106571. [Google Scholar] [CrossRef]
- Luo, Z.-G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.-F.; Liao, B.-K.; Guo, X.-P. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Rbaa, M.; Benhiba, F.; Hssisou, R.; Lakhrissi, Y.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. Green synthesis of novel carbohydrate polymer chitosan oligosaccharide grafted on d-glucose derivative as bio-based corrosion inhibitor. J. Mol. Liq. 2021, 322, 114549. [Google Scholar] [CrossRef]
- Jin, J.; Wu, G.; Zhang, Z.; Guan, Y. Effect of extracellular polymeric substances on corrosion of cast iron in the reclaimed wastewater. Bioresour. Technol. 2014, 165, 162–165. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Gouda, M.; Shalabi, K.; Al-Omair, M.A.; Khalaf, M.M. Superhydrophobic films-based nonanyl carboxy methylcellulose grafted polyacrylamide for AISI-stainless steel corrosion protection: Empirical explorations and computational models. J. Mol. Liq. 2022, 356, 119063. [Google Scholar] [CrossRef]
- Verma, C.; Hussain, C.M. 10—Chitosan and its derivatives as environmental benign corrosion inhibitors: Recent advancements. In Environmentally Sustainable Corrosion Inhibitors; Hussain, C.M., Verma, C., Aslam, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–217. [Google Scholar]
- Wei, G.; Deng, S.; Xu, D.; Xu, J.; Shao, D.; Li, X. Invasive weed of Eupatorium Adenophora Spreng leaves extract as a novel efficient inhibitor for the corrosion of cold rolled steel in chloroacetic acid solution. J. Mol. Liq. 2024, 400, 124501. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Elucidating detailed experimental and fundamental understandings concerning the green organic-inorganic corrosion inhibiting molecules onto steel in chloride solution. J. Mol. Liq. 2019, 290, 111212. [Google Scholar] [CrossRef]
- Jero, D.; Caussé, N.; Marsan, O.; Buffeteau, T.; Chaussec, F.; Buvignier, A.; Roy, M.; Pébère, N. Film-forming amines adsorption and corrosion kinetics on carbon steel surface in neutral solution investigated by EIS and PM-IRRAS analysis. Electrochim. Acta 2023, 443, 141925. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Zeng, J. Role of corrosion products in biofilms in microbiologically induced corrosion of carbon steel. Br. Corros. J. 2013, 35, 131–135. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Zhang, X.; Zhang, X. Effect of cathodic potential and corrosion product on tribocorrosion behavior of S420 steel in the marine environment. Mater. Today Commun. 2024, 38, 108372. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Ding, X.; Xu, H.; Wang, Y.; Zhao, M.; Ren, C.; Zhang, D. Tea polyphenol radical scavenger loaded UV absorber for corrosion resistant and weathering resistant epoxy coating fabrication. Prog. Org. Coat. 2023, 180, 107553. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef] [PubMed]
- Vaszilcsin, C.G.; Putz, M.V.; Kellenberger, A.; Dan, M.L. On the evaluation of metal-corrosion inhibitor interactions by adsorption isotherms. J. Mol. Struct. 2023, 1286, 135643. [Google Scholar] [CrossRef]
- Wang, D.; Gao, L.; Zhang, D.; Yang, D.; Wang, H.; Lin, T. Experimental and theoretical investigation on corrosion inhibition of AA5052 aluminium alloy by l-cysteine in alkaline solution. Mater. Chem. Phys. 2016, 169, 142–151. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Scheerder, J.; Breur, R.; Slaghek, T.; Holtman, W.; Vennik, M.; Ferrari, G. Exopolysaccharides (EPS) as anti-corrosive additives for coatings. Prog. Org. Coat. 2012, 75, 224–230. [Google Scholar] [CrossRef]
- Bhatia, A.K.; Dewangan, S.; Vaidya, N. Chapter 22—Carbohydrates and derivatives as green corrosion inhibitors. In Computational Modelling and Simulations for Designing of Corrosion Inhibitors; Verma, D.K., Verma, C., Aslam, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 435–460. [Google Scholar]







| Element | NaCl | Na2SO4 | NaHCO3 | pH |
|---|---|---|---|---|
| (mg/L) | 58.5 | 213 | 4.2 | 8.3 |
| Inhibitors | Concentration (mg m·L−1) | IE (%) |
|---|---|---|
| Dextran | 1 | 9.58 |
| 2 | 25.17 | |
| 4 | 4.68 | |
| CM-Dextran | 1 | 65.03 |
| 2 | 73.83 | |
| 4 | 82.52 |
| Inhibitors | Concentration (mg m·L−1) | Ecorr (V) | Icorr/mA/cm2 | βa/mV·dec−1 | −βc/mV·dec−1 |
|---|---|---|---|---|---|
| 1 | −0.648 | 0.03259 | 207 | 185 | |
| Dextran | 2 | −0.682 | 0.02882 | 213 | 185 |
| 4 | −0.658 | 0.03753 | 209 | 166 | |
| 1 | −0.571 | 0.02351 | 238 | 181 | |
| CM-Dextran | 2 | −0.542 | 0.02401 | 255 | 175 |
| 4 | −0.498 | 0.01980 | 397 | 133 |
| Inhibitors | Concentration (mg/mL) | Rs/Ω·cm2 | nb | Rb/Ω·cm2 | nf | Rp/Ω·cm2 |
|---|---|---|---|---|---|---|
| Dextran | 1 | 77 | 0.98 | 718 | 0.406 | 993 |
| 2 | 108 | 0.98 | 997 | 0.444 | 1626 | |
| 4 | 110 | 0.97 | 1027 | 0.366 | 1358 | |
| CM-Dextran | 1 | 80 | 0.98 | 450 | 0.662 | 11,130 |
| 2 | 101 | 0.99 | 406 | 0.654 | 14,650 | |
| 4 | 68 | 0.99 | 404 | 0.669 | 17,650 |
| Condition | R2 | Qm (mg/L) | b (mg/L) | ΔG (kJ mol–1) |
|---|---|---|---|---|
| Fe2+ | 0.99 | 65.24 | 1.38 | −28.33 |
| Fe3+ | 0.99 | 155.04 | 1.14 | −27.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Chen, X. Inhibition of Carbon Steel Corrosion Using Dextran Derivatives in Circulating Cooling Water. Water 2024, 16, 1182. https://doi.org/10.3390/w16081182
Xu P, Chen X. Inhibition of Carbon Steel Corrosion Using Dextran Derivatives in Circulating Cooling Water. Water. 2024; 16(8):1182. https://doi.org/10.3390/w16081182
Chicago/Turabian StyleXu, Ping, and Xingrun Chen. 2024. "Inhibition of Carbon Steel Corrosion Using Dextran Derivatives in Circulating Cooling Water" Water 16, no. 8: 1182. https://doi.org/10.3390/w16081182




