Comparative Inactivation of the RNA of the Delta and Omicron Variants of SARS-CoV-2 in Wastewater of Five Municipalities in Southeast Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Samples
2.2. Inactivation Experiments
2.3. RNA Extraction and Virus Detection and Quantification
2.4. Inactivation Rate, Calculation of T90 and Statistical Analysis
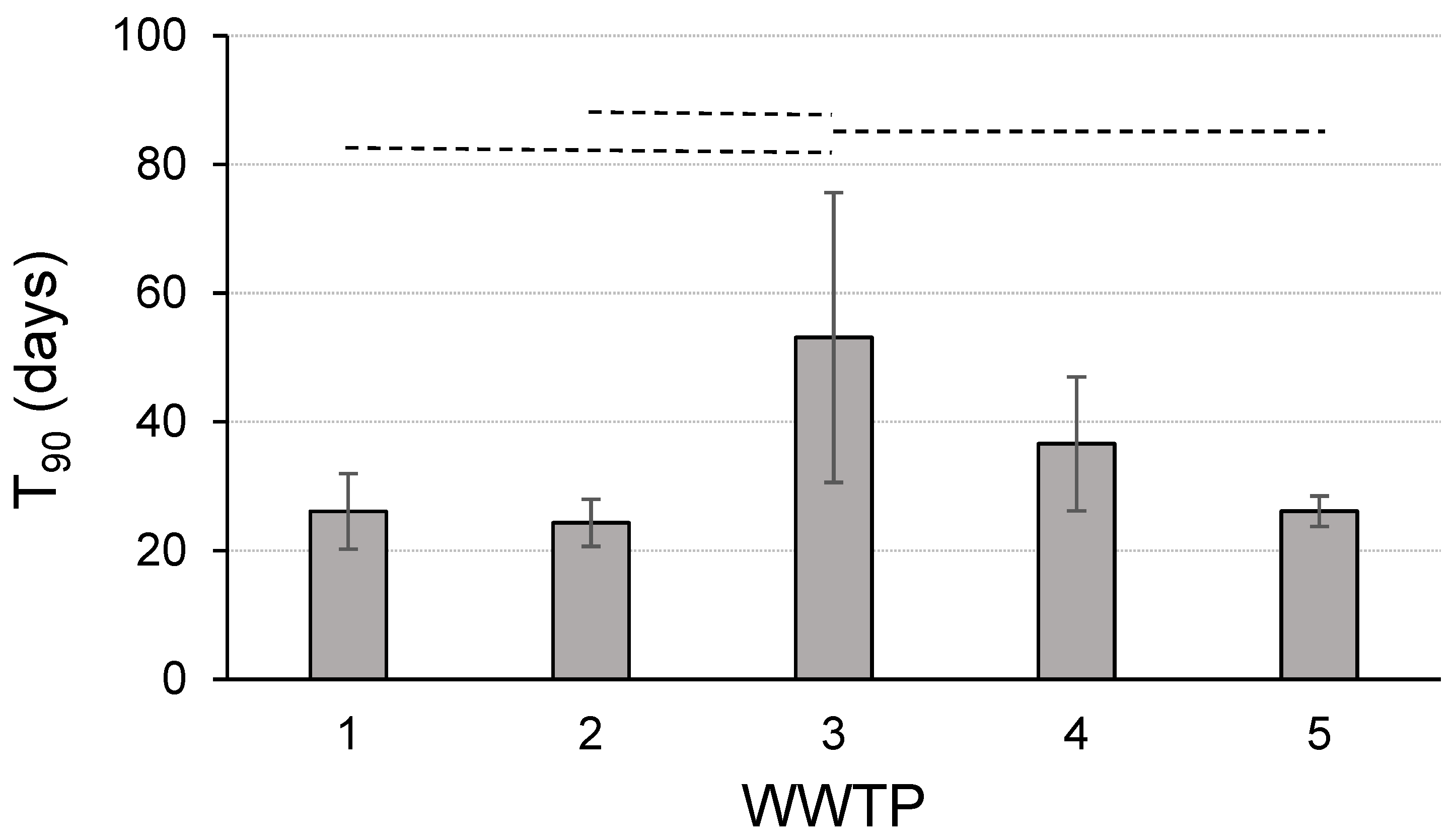
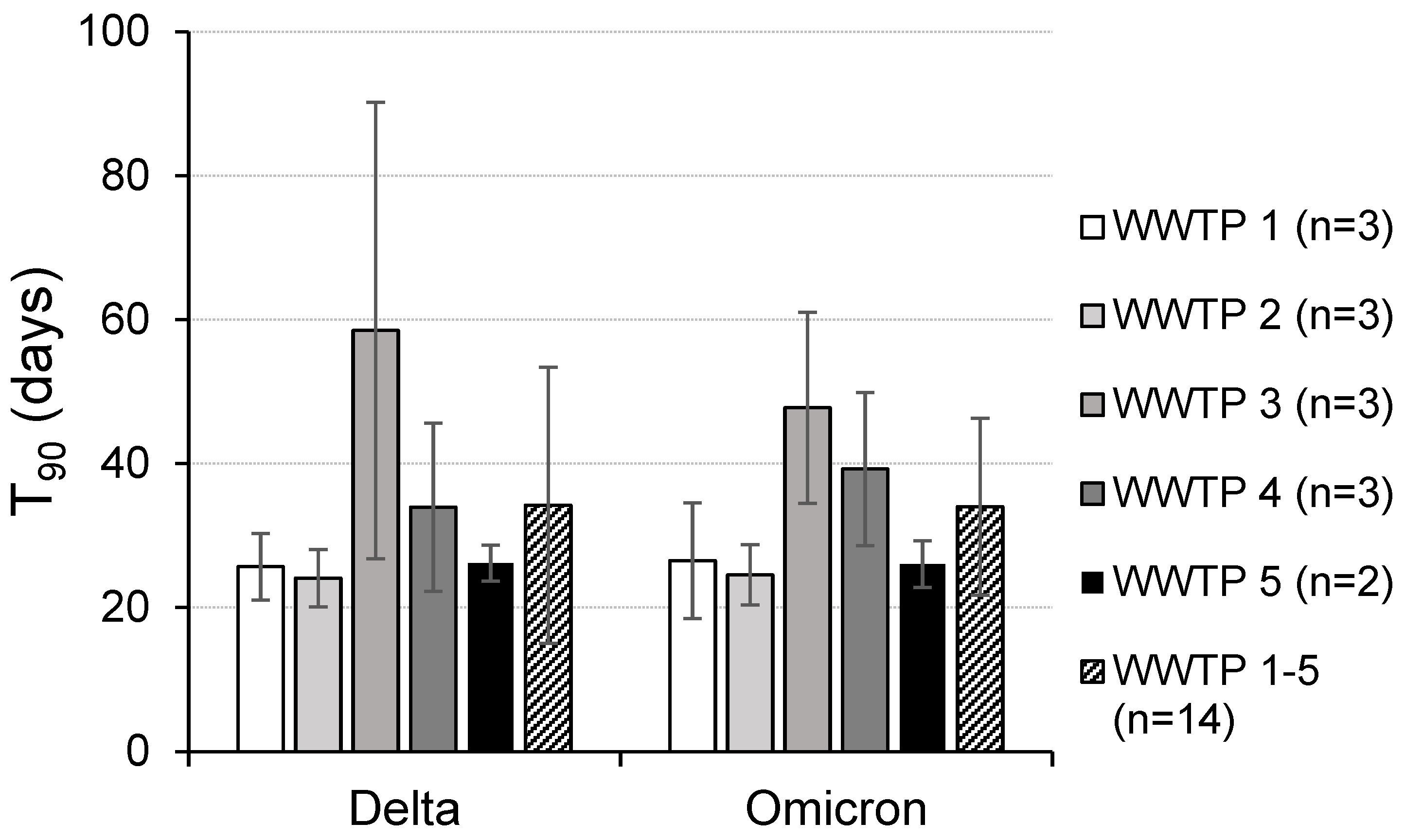
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keshaviah, A.; Diamond, M.B.; Wade, M.J.; Scarpino, S.V. Wastewater monitoring can anchor global disease surveillance systems. Lancet Glob. Health 2023, 11, e976–e981. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Liu, G.X.; Huang, X.L.; Gan, H.T. The importance of fecal nucleic acid detection in patients with coronavirus disease (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gwee, S.X.W.; Ng, J.Q.X.; Lau, N.; Koh, J.; Pang, J. Wastewater surveillance to infer COVID-19 transmission: A systematic review. Sci. Total Environ. 2022, 804, 150060. [Google Scholar] [CrossRef]
- Tiwari, A.; Lipponen, A.; Hokajärvi, A.M.; Luomala, O.; Sarekoski, A.; Rytkönen, A.; Österlund, P.; Al-Hello, H.; Juutinen, A.; Miettinen, I.T.; et al. Detection and quantification of SARS-CoV-2 RNA in wastewater influent in relation to reported COVID-19 incidence in Finland. Water Res. 2022, 215, 118220. [Google Scholar] [CrossRef]
- Helm, B.; Geissler, M.; Mayer, R.; Schubert, S.; Oertel, R.; Dumke, R.; Dalpke, A.; El-Armouche, A.; Renner, B.; Krebs, P. Regional and temporal differences in the relation between SARS-CoV-2 biomarkers in wastewater and estimated infection prevalence—Insights from long-term surveillance. Sci. Total Environ. 2023, 857, 159358. [Google Scholar] [CrossRef]
- Ahmed, W.; Smith, W.J.M.; Tiwari, A.; Bivins, A.; Simpson, S.L. Unveiling indicator, enteric, and respiratory viruses in aircraft lavatory wastewater using adsorption-extraction and Nanotrap Microbiome a Particles workflows. Sci. Total Environ. 2023, 896, 165007. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Smith, W.J.M.; Liu, Y.; Kim, I.; Simpson, S.L.; Thai, P.; Korajkic, A.; Ahmed, W. Comparison of adsorption-extraction (AE) workflows for improved measurements of viral and bacterial nucleic acid in untreated wastewater. Sci. Total Environ. 2024, 908, 167966. [Google Scholar] [CrossRef] [PubMed]
- Atoui, A.; Cordevant, C.; Chesnot, T.; Gassilloud, B. SARS-CoV-2 in the environment: Contamination routes, detection methods, persistence and removal in wastewater treatment plants. Sci. Total Environ. 2023, 881, 163453. [Google Scholar] [CrossRef]
- Mazumder, P.; Dash, S.; Honda, R.; Sonne, C.; Kumar, M. Sewage surveillance for SARS-CoV-2: Molecular detection, quantification, and normalization factors. Curr. Opin. Environ. Sci. Health 2022, 28, 100363. [Google Scholar] [CrossRef]
- Babler, K.M.; Sharkey, M.E.; Abelson, S.; Amirali, A.; Benitez, A.; Cosculluela, G.A.; Grills, G.S.; Kumar, N.; Laine, J.; Lamar, W.; et al. Degradation rates influence the ability of composite samples to represent 24-hourly means of SARS-CoV-2 and other microbiological target measures in wastewater. Sci. Total Environ. 2023, 867, 161423. [Google Scholar] [CrossRef]
- Foladori, P.; Cutrupi, F.; Cadonna, M.; Manara, S. Coronaviruses and SARS-CoV-2 in sewerage and their removal: Step by step in wastewater treatment plants. Environ. Res. 2022, 207, 112204. [Google Scholar] [CrossRef]
- Volz, E. Fitness, growth and transmissibility of SARS-CoV-2 genetic variants. Nat. Rev. Genet. 2023, 24, 724–734. [Google Scholar] [CrossRef]
- Itarte, M.; Bofill-Mas, S.; Martinez-Puchol, S.; Torrell, H.; Cereto, A.; Carrasco, M.; Fores, E.; Canela, N.; Girones, R.; Rusinol, M. Looking for a needle in a haystack. SARS-CoV-2 variant characterization in sewage. Curr. Opin. Environ. Sci. Health 2021, 24, 100308. [Google Scholar] [CrossRef]
- Bobrin, V.A.; Chen, S.R.; Grandes Reyes, C.F.; Smith, T.; Purcell, D.F.J.; Armstrong, J.; McAuley, J.L.; Monteiro, M.J. Surface inactivation of highly mutated SARS-CoV-2 Variants of Concern: Alpha, Delta, and Omicron. Biomacromolecules 2022, 23, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Lai, A.M.Y.; Peiris, M.; Man Poon, L.L. Increased stability of SARS-CoV-2 Omicron variant over ancestral strain. Emerg. Infect. Dis. 2022, 28, 1515–1517. [Google Scholar] [CrossRef]
- Hirose, R.; Itoh, Y.; Ikegaya, H.; Miyazaki, H.; Watanabe, N.; Yoshida, T.; Bandou, R.; Daidoji, T.; Nakaya, T. Differences in environmental stability among SARS-CoV-2 variants of concern: Both omicron BA.1 and BA.2 have higher stability. Clin. Microbiol. Infect. 2022, 28, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Fortmann, J.; Todt, D.; Heinen, N.; Ludwig, A.; Brüggemann, Y.; Elsner, C.; Dittmer, U.; Steinmann, J.; Pfaender, S.; et al. Comparable environmental stability and disinfection profiles of the currently circulating SARS-CoV-2 Variants of Concern B.1.1.7 and B.1.351. J. Infect. Dis. 2021, 224, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Onianwa, O.; Garratt, I.; Carter, J.; Spencer, A.; Verlander, N.Q.; Pottage, T.; Bennett, A.M. Comparison of surface persistence of SARS-CoV-2 Alpha and Delta variants on stainless steel at 4 °C and 24 °C. Appl. Environ. Microbiol. 2022, 88, e0076422. [Google Scholar] [CrossRef]
- Pottage, T.; Onianwa, O.; Atkinson, B.; Spencer, A.; Bennett, A.M. Stability of SARS-CoV-2 variants of concern (Delta and Omicron) on surfaces at room temperature. Virology 2023, 583, 27–28. [Google Scholar] [CrossRef]
- Takeda, Y.; Nikaido, M.; Jamsransuren, D.; Matsuda, S.; Ogawa, H. Virucidal activities of acidic electrolyzed water solutions with different pH values against multiple strains of SARS-CoV-2. Appl. Environ. Microbiol. 2023, 89, e0169922. [Google Scholar] [CrossRef]
- Pinon, A.; Vialette, M. Survival of viruses in water. Intervirology 2018, 61, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahmed, W.; Metcalfe, S.; Smith, W.J.M.; Choi, P.M.; Jackson, G.; Cen, X.; Zheng, M.; Simpson, S.L.; Thomas, K.V.; et al. Impact of sewer biofilms on fate of SARS-CoV-2 RNA and wastewater surveillance. Nat. Water 2023, 1, 272–280. [Google Scholar] [CrossRef]
- Zhang, S.; Sharma, E.; Tiwari, A.; Chen, Y.; Sherchan, S.P.; Gao, S.; Zhou, X.; Shi, J.; Jiang, G. The reduction of SARS-CoV-2 RNA concentration in the presence of sewer biofilms. Water 2023, 15, 2132. [Google Scholar] [CrossRef]
- Ahmed, W.; Bertsch, P.M.; Bibby, K.; Haramoto, E.; Hewitt, J.; Huygens, F.; Gyawali, P.; Korajkic, A.; Riddell, S.; Sherchan, S.P.; et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020, 191, 110092. [Google Scholar] [CrossRef] [PubMed]
- Hokajärvi, A.M.; Rytkönen, A.; Tiwari, A.; Kauppinen, A.; Oikarinen, S.; Lehto, K.M.; Kankaanpää, A.; Gunnar, T.; Al-Hello, H.; Blomqvist, S.; et al. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. Sci. Total Environ. 2021, 770, 145274. [Google Scholar] [CrossRef] [PubMed]
- Bivins, A.; Greaves, J.; Fischer, R.; Yinda, K.C.; Ahmed, W.; Kitajima, M.; Munster, V.J.; Bibby, K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020, 7, 937–942. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.C.; Torres-Franco, A.F.; Lopes, B.C.; Santos, B.S.A.D.S.; Costa, E.A.; Costa, M.S.; Reis, M.T.P.; Melo, M.C.; Polizzi, R.B.; Teixeira, M.M.; et al. Viability of SARS-CoV-2 in river water and wastewater at different temperatures and solids content. Water Res. 2021, 195, 117002. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, S.; Thakali, O.; Ikner, L.A.; Gerba, C.P. Survival of SARS-CoV-2 in wastewater. Sci. Total Environ. 2023, 882, 163049. [Google Scholar] [CrossRef] [PubMed]
- Sala-Comorera, L.; Reynolds, L.J.; Martin, N.A.; O’Sullivan, J.J.; Meijer, W.G.; Fletcher, N.F. Decay of infectious SARS-CoV-2 and surrogates in aquatic environments. Water Res. 2021, 201, 117090. [Google Scholar] [CrossRef]
- Figueroa, A.; Hadengue, B.; Leitao, J.P.; Blumensaat, F. A framework for modelling in-sewer thermal-hydraulic dynamic anomalies driven by stormwater runoff and seasonal effects. Water Res. 2023, 229, 119492. [Google Scholar] [CrossRef]
- Burnet, J.B.; Cauchie, H.M.; Walczak, C.; Goeders, N.; Ogorzaly, L. Persistence of endogenous RNA biomarkers of SARS-CoV-2 and PMMoV in raw wastewater: Impact of temperature and implications for wastewater-based epidemiology. Sci. Total Environ. 2023, 857, 159401. [Google Scholar] [CrossRef] [PubMed]
- Roldan-Hernandez, L.; Graham, K.E.; Duong, D.; Boehm, A.B. Persistence of endogenous SARS-CoV-2 and pepper mild mottle virus RNA in wastewater-settled solids. ACS ES&T Water 2022, 2, 1944–1952. [Google Scholar]
- Sabar, M.A.; Honda, R.; Haramoto, E. CrAssphage as an indicator of human-fecal contamination in water environment and virus reduction in wastewater treatment. Water Res. 2022, 221, 118827. [Google Scholar] [CrossRef]
- Stadtmüller, M.; Laubner, A.; Rost, F.; Winkler, S.; Patrasova, E.; Simunkova, L.; Reinhardt, S.; Beil, J.; Dalpke, A.H.; Yi, B. Emergence and spread of a sub-lineage of SARS-CoV-2 Alpha variant B.1.1.7 in Europe, and with further evolution of spike mutation accumulations shared with the Beta and Gamma variants. Virus Evol. 2022, 8, veac010. [Google Scholar] [CrossRef]
- Dumke, R.; Geissler, M.; Skupin, A.; Helm, B.; Mayer, R.; Schubert, S.; Oertel, R.; Renner, B.; Dalpke, A. Simultaneous detection of SARS-CoV-2 and influenza virus in wastewater of two cities in southeastern Germany, January to May 2022. Int. J. Environ. Res. Public Health 2022, 19, 13374. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, J. Presence and persistence of SARS-CoV-2 in aquatic environments: A mini-review. Curr. Opin. Environ. Sci. Health 2022, 29, 100385. [Google Scholar] [CrossRef]
- Albert, S.; Ruiz, A.; Peman, J.; Salavert, M.; Domingo-Calap, P. Lack of evidence for infectious SARS-CoV-2 in feces and sewage. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2665–2667. [Google Scholar] [CrossRef]
- Robinson, C.A.; Hsieh, H.Y.; Hsu, S.Y.; Wang, Y.; Salcedo, B.T.; Belenchia, A.; Klutts, J.; Zemmer, S.; Reynolds, M.; Semkiw, E.; et al. Defining biological and biophysical properties of SARS-CoV-2 genetic material in wastewater. Sci. Total Environ. 2022, 807, 150786. [Google Scholar] [CrossRef]
- Bertels, X.; Demeyer, P.; van den Bogaert, S.; Boogaerts, T.; van Nuijs, A.L.N.; Delputte, P.; Lahousse, L. Factors influencing SARS-CoV-2 RNA concentrations in wastewater up to the sampling stage: A systematic review. Sci. Total Environ. 2022, 820, 153290. [Google Scholar] [CrossRef]
- Beattie, R.E.; Blackwood, A.D.; Clerkin, T.; Dinga, C.; Noble, R.T. Evaluating the impact of sample storage, handling, and technical ability on the decay and recovery of SARS-CoV-2 in wastewater. PLoS ONE 2022, 17, e0270659. [Google Scholar] [CrossRef]
- Islam, G.; Gedge, A.; Lara-Jacobo, L.; Kirkwood, A.; Simmons, D.; Desaulniers, J.P. Pasteurization, storage conditions and viral concentration methods influence RT-qPCR detection of SARS-CoV-2 RNA in wastewater. Sci. Total Environ. 2022, 821, 153228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ash, K.T.; Joyner, D.C.; Williams, D.E.; Alamilla, I.; McKay, P.J.; Iler, C.; Green, B.M.; Kara-Murdoch, F.; Swift, C.M.; et al. Decay of enveloped SARS-CoV-2 and non-enveloped PMMoV RNA in raw sewage from university dormitories. Front. Microbiol. 2023, 14, 1144026. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dong, Q.; Li, S.; Cheng, Z.; Kang, X.; Ren, D.; Xu, C.; Zhou, X.; Liang, P.; Sun, L.; et al. Persistence of SARS-CoV-2 RNA in wastewater after the end of the COVID-19 epidemics. J. Hazard. Mater. 2022, 429, 128358. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, S.P.; Thakali, O.; Ikner, L.A.; Gerba, C.; Haramoto, E. Survivability of Delta and Omicron variants of SARS-CoV-2 in wastewater. Water Res. 2023, 246, 120644. [Google Scholar] [CrossRef]
- Weidhaas, J.; Aanderud, Z.T.; Roper, D.K.; VanDerslice, J.; Gaddis, E.B.; Ostermiller, J.; Hoffman, K.; Jamal, R.; Heck, P.; Zhang, Y.; et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021, 775, 145790. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geissler, M.; Dumke, R. Comparative Inactivation of the RNA of the Delta and Omicron Variants of SARS-CoV-2 in Wastewater of Five Municipalities in Southeast Germany. Water 2024, 16, 1193. https://doi.org/10.3390/w16081193
Geissler M, Dumke R. Comparative Inactivation of the RNA of the Delta and Omicron Variants of SARS-CoV-2 in Wastewater of Five Municipalities in Southeast Germany. Water. 2024; 16(8):1193. https://doi.org/10.3390/w16081193
Chicago/Turabian StyleGeissler, Michael, and Roger Dumke. 2024. "Comparative Inactivation of the RNA of the Delta and Omicron Variants of SARS-CoV-2 in Wastewater of Five Municipalities in Southeast Germany" Water 16, no. 8: 1193. https://doi.org/10.3390/w16081193





