Investigation of Algal Biotoxin Removal during SWRO Desalination through a Materials Flow Analysis
Abstract
:1. Introduction
2. Materials and Methods
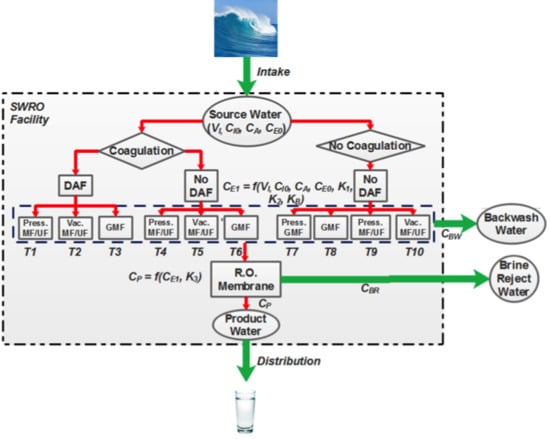
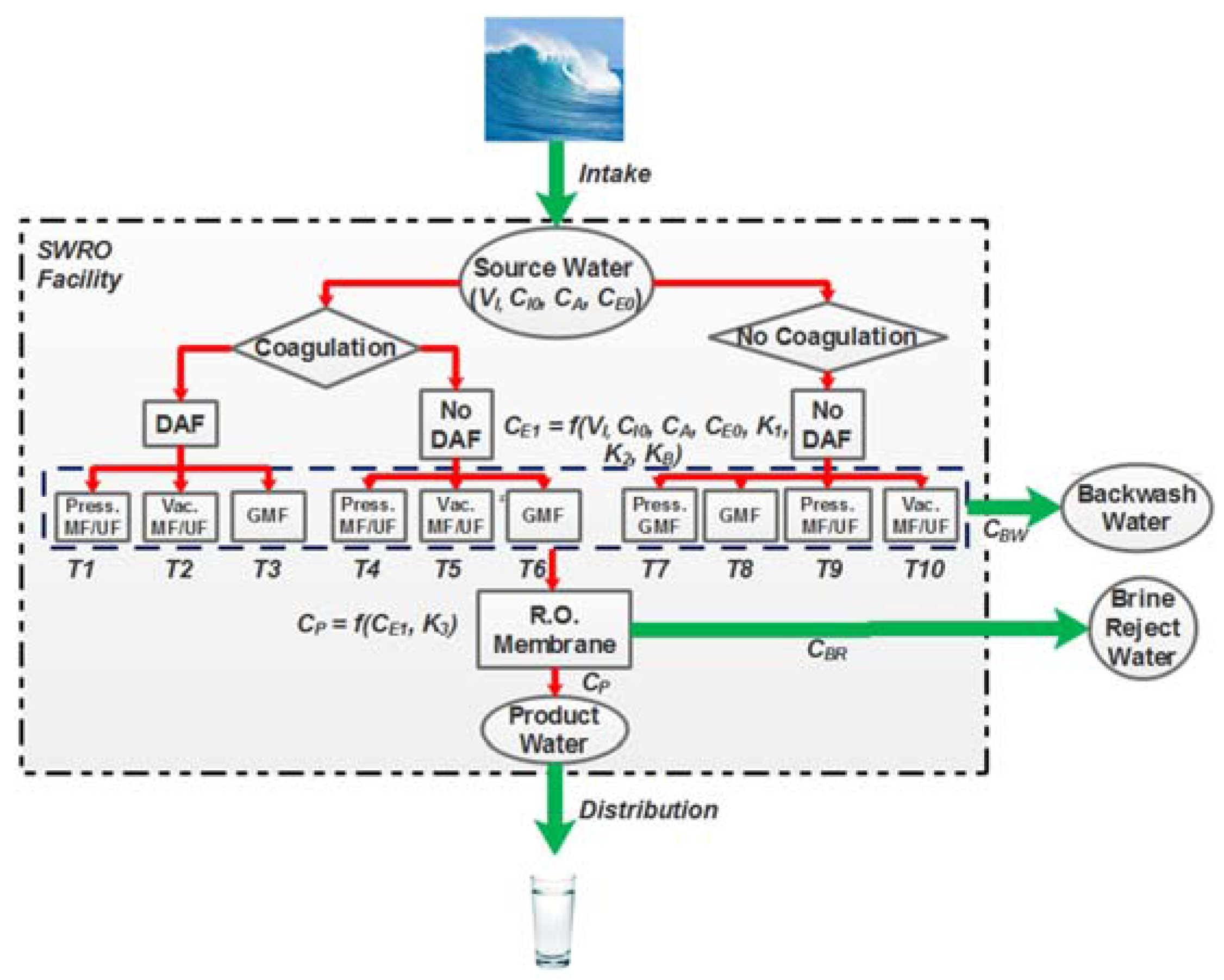
2.1. pMFA Overview and Model Assumptions
2.2. Algal and Toxins Concentrations
2.3. Intracellular and Dissolved Toxin Removal Efficiencies
2.4. pMFA Simulation Algorithm
2.5. Statistical and Sensitivity Analysis
2.6. Determination of Human Health Risks from Marine Algal Toxins
3. Results
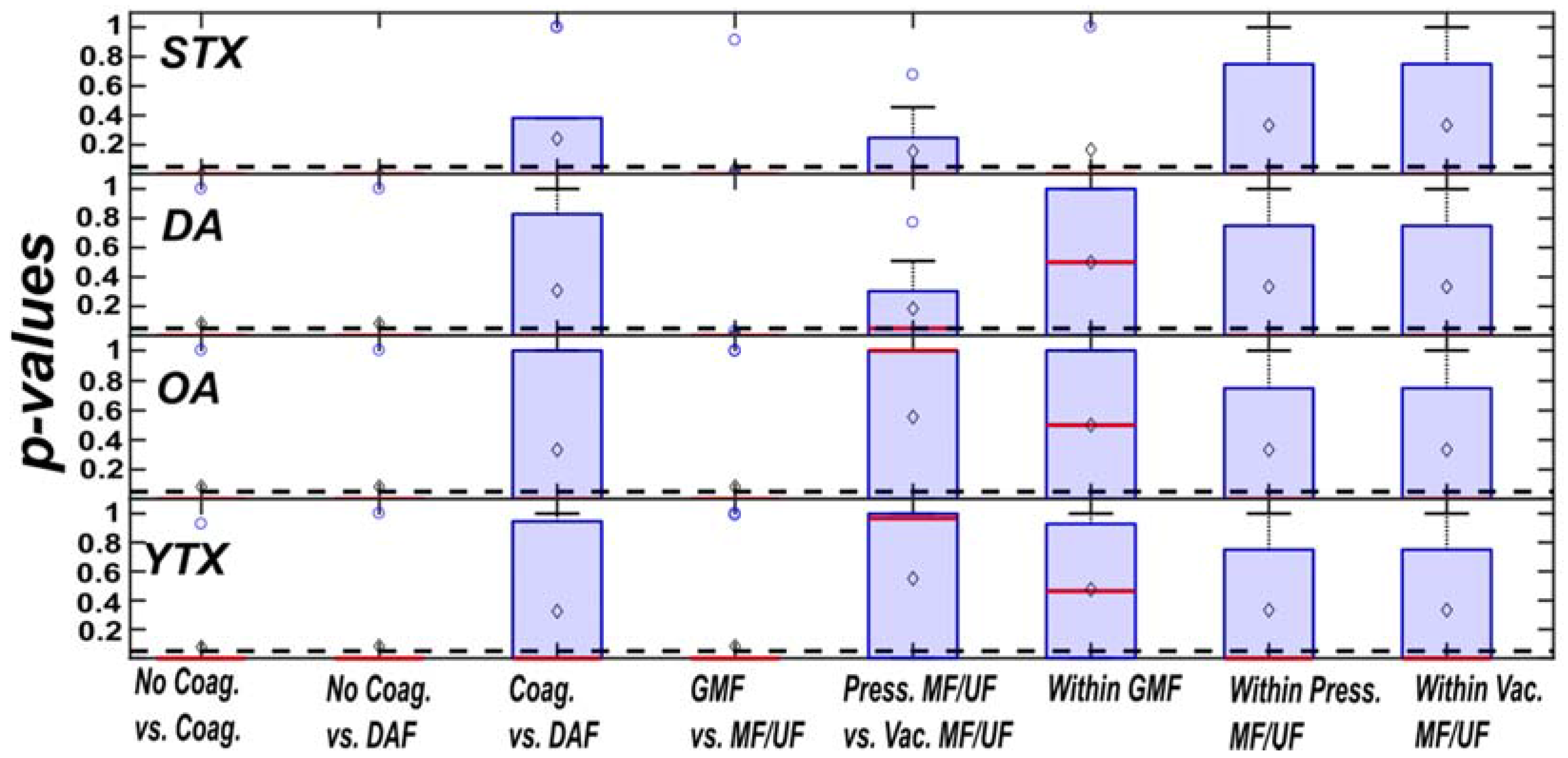
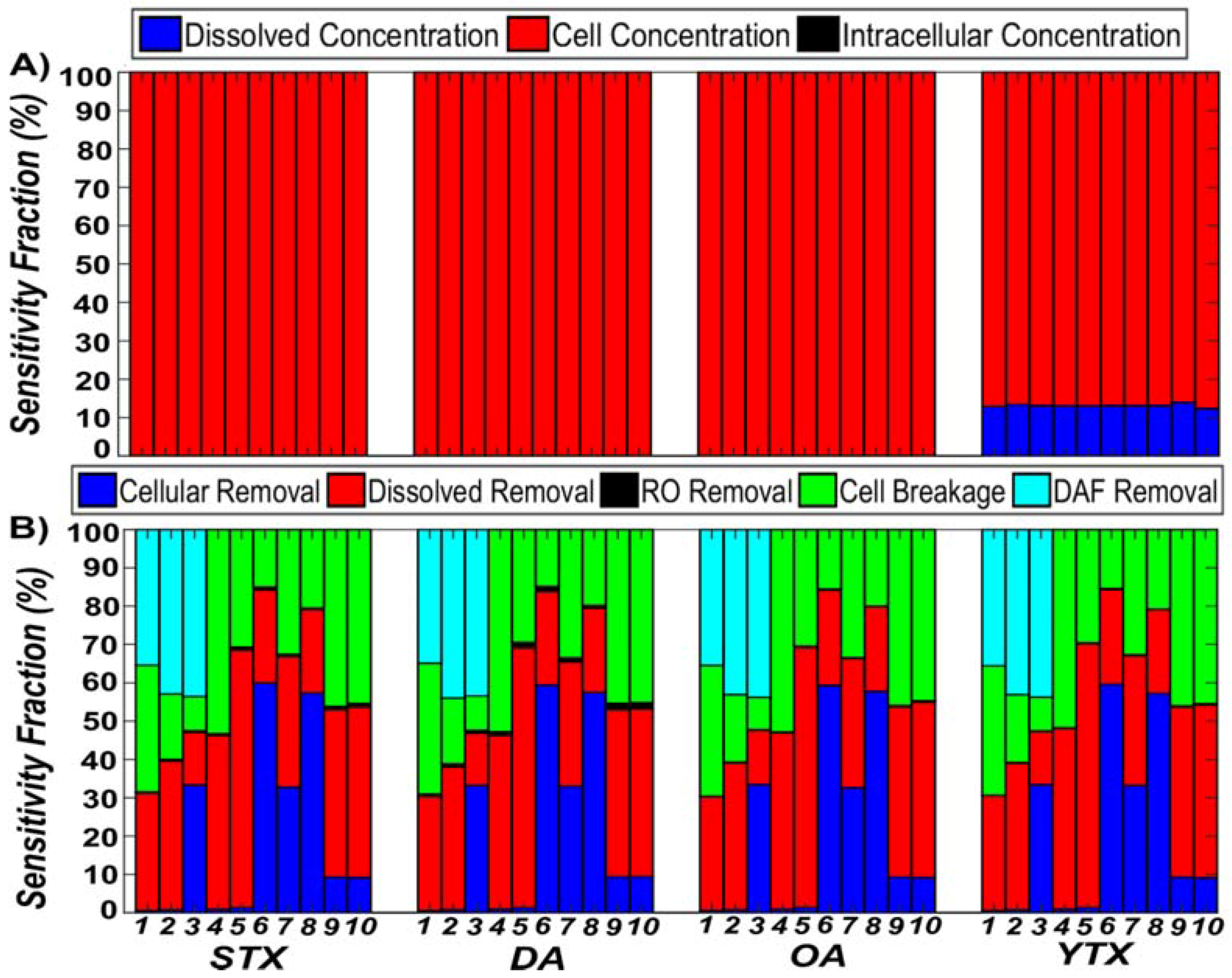
3.1. Comparison of Algal Toxin Removal Efficiencies
3.2. Sensitivity Analysis
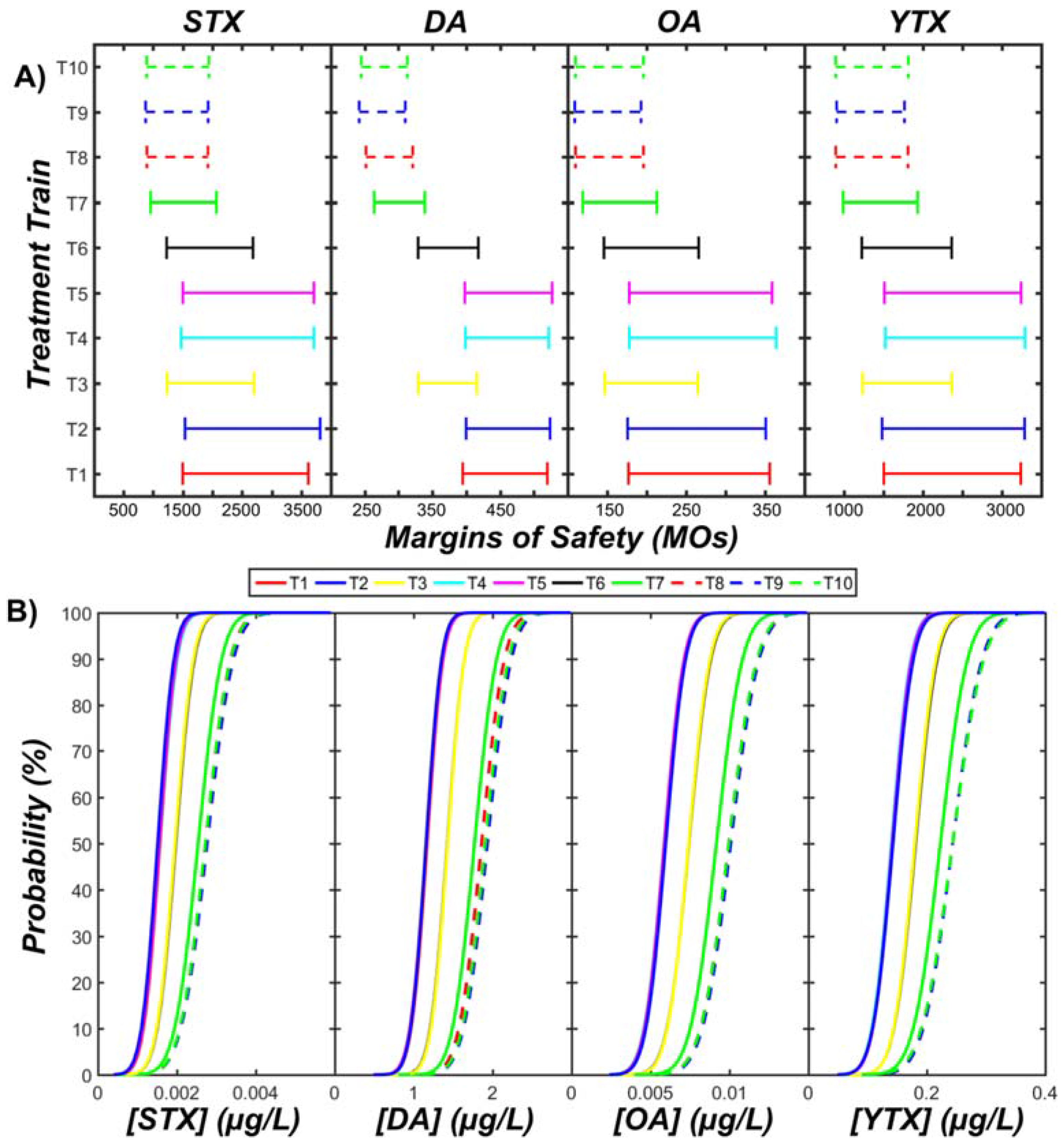
3.3. Acute Human Health Risks
4. Discussion
4.1. Contribution of the Study
4.2. Uncertainty and Variability
4.3. Human Health Effects
5. Conclusions
- A detectable quantity of algal toxins is present in the permeate water, despite almost 99.0–99.9% removal predicted across the RO membranes (in the ng/L to µg/L range);
- A relatively high concentration of algal toxins was predicted for the combined backwash and RO reject waters (in the µg/L to mg/L range);
- MF/UF systems with coagulation generally had the highest predicted toxin removals (and least variability) over all GMF systems/operations (up to 57% of the entire removal across SWRO);
- There is a low to negligible risk of acute intoxication from ingesting desalinated water during algal bloom periods (margins of safety ranged from 100 to 4000).
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SWRO | Seawater Reverse Osmosis |
| RO | Reverse Osmosis |
| HABs | Harmful Algal Blooms |
| pMFA | Probabilistic Materials Flow Analysis |
| MF/UF | Microfiltration/Ultrafiltration |
| GMF | Granular Media Filtration |
| DAF | Dissolved Air Flotation |
| TEP | Transparent Extracellular Particulate |
| MGD | Million Gallons per Day (international unit) |
| SCCOOS | Southern California Coastal Ocean Observing System |
| STX | Saxitoxin |
| DA | Domoic Acid |
| OA | Okadaic Acid |
| YTX | Yessotoxin |
| ECDF | Empirical Cumulative Distribution Function |
| SPATT | Solid Phase Adsorption Toxin Tracking |
| MC-LR | Microcystin-LR |
| ANOVA | Analysis of Variance |
| RfD | Acute Reference Dose |
| EFSA | European Food Safety Administration |
| EPA | Environmental Protection Agency |
| AL | Acceptable Level |
| LOEL | Lowest Observed Effect Level |
| NOAEL | No Observed Adverse Effect Level |
| RSC | Relative Source Contribution |
| MO | Margin of Safety |
| TCD | Toxin Concentration Distribution |
| IQR COV CDF | Interquartile Range Coefficient of Variation Cumulative Probability Distribution Functions |
References
- Voutchkov, N. Desalination Engineering: Planning and Design; McGraw Hill Professional: New York, NY, USA, 2011. [Google Scholar]
- Dawoud, M.A. The role of desalination in augmentation of water supply in GCC countries. Desalination 2005, 186, 187–198. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Schiffler, M. Perspectives and challenges for desalination in the 21st century. Desalination 2004, 165, 1–9. [Google Scholar]
- Eltawil, M.A.; Zhengming, Z.; Yuan, L. A review of renewable energy technologies integrated with desalination systems. Renew. Sustain. Energy Rev. 2009, 13, 2245–2262. [Google Scholar] [CrossRef]
- Subramani, A.; Badruzzaman, M.; Oppenheimer, J.; Jacangelo, J.G. Energy minimization strategies and renewable energy utilization for desalination: A review. Water Res. 2011, 45, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Combined desalination, water reuse, and aquifer storage and recovery to meet water supply demands in the GCC/MENA region. Desalination Water Treat. 2013, 51, 38–43. [Google Scholar] [CrossRef]
- Laycock, M.V.; Anderson, D.M.; Naar, J.; Goodman, A.; Easy, D.J.; Donovan, M.A.; Li, A.; Quilliam, M.A.; Al Jamali, E.; Alshihi, R. Laboratory desalination experiments with some algal toxins. Desalination 2012, 293, 1–6. [Google Scholar] [CrossRef]
- Boerlage, S.; Nada, N. Algal toxin removal in seawater desalination processes. Desalination Water Treat. 2015, 55, 2575–2593. [Google Scholar] [CrossRef]
- Seubert, E.L.; Trussell, S.; Eagleton, J.; Schnetzer, A.; Cetinić, I.; Lauri, P.; Jones, B.H.; Caron, D.A. Algal toxins and reverse osmosis desalination operations: Laboratory bench testing and field monitoring of domoic acid, saxitoxin, brevetoxin and okadaic acid. Water Res. 2012, 46, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.A.; Garneau, M.-È.; Seubert, E.; Howard, M.D.A.; Darjany, L.; Schnetzer, A.; Cetinić, I.; Filteau, G.; Lauri, P.; Jones, B.; et al. Harmful algae and their potential impacts on desalination operations off southern California. Water Res. 2010, 44, 385–416. [Google Scholar] [CrossRef] [PubMed]
- Voutchkov, N. Considerations for selection of seawater filtration pretreatment system. Desalination 2010, 261, 354–364. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Tabatabai, S.A.A.; Dhakal, N.; Amy, G.; Schippers, J.C.; Kennedy, M.D. Algal blooms: An emerging threat to seawater reverse osmosis desalination. Desalination Water Treat. 2015, 55, 2601–2611. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Tabatabai, S.A.A.; Anderson, D.M.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. Seawater reverse osmosis desalination and (harmful) algal blooms. Desalination 2015, 360, 61–80. [Google Scholar] [CrossRef]
- Huang, X.; Min, J.H.; Lu, W.; Jaktar, K.; Yu, C.; Jiang, S.C. Evaluation of methods for reverse osmosis membrane integrity monitoring for wastewater reuse. J. Water Process Eng. 2015, 7, 161–168. [Google Scholar] [CrossRef]
- Meyerhofer, P.; Desormeaux, E.; Luckenbach, H. Seawater Reverse Osmosis Desalination Pilot Test Program Report; Santa Cruz Water District: Santa Cruz, CA, USA, 2010. [Google Scholar]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ. Toxicol. Chem. 2010, 29, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- California Department of Water Resources (CDWR). California Water Plan Update 2013: Volume 3-Resource Management Strategies-Chapter 10 Desalination (Brackish and Seawater); CDWR: Sacramento, CA, USA, 2013.
- Seubert, E.L.; Gellene, A.G.; Howard, M.D.A.; Connell, P.; Ragan, M.; Jones, B.H.; Runyan, J.; Caron, D.A. Seasonal and annual dynamics of harmful algae and algal toxins revealed through weekly monitoring at two coastal ocean sites off southern California, USA. Environ. Sci. Pollut. Res. 2013, 20, 6878–6895. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.I.; Smyth, T.J.; Siddorn, J.R.; Holt, M. How well can we forecast high biomass algal bloom events in a eutrophic coastal sea? Harmful Algae 2008, 8, 70–76. [Google Scholar] [CrossRef]
- Kim, H.-J.; Miller, A.J.; McGowan, J.; Carter, M.L. Coastal phytoplankton blooms in the Southern California Bight. Prog. Oceanogr. 2009, 82, 137–147. [Google Scholar] [CrossRef]
- Schnetzer, A.; Miller, P.E.; Schaffner, R.A.; Stauffer, B.A.; Jones, B.H.; Weisberg, S.B.; DiGiacomo, P.M.; Berelson, W.M.; Caron, D.A. Blooms of Pseudo-nitzschia and domoic acid in the San Pedro Channel and Los Angeles harbor areas of the Southern California Bight, 2003–2004. Harmful Algae 2007, 6, 372–387. [Google Scholar] [CrossRef]
- Trainer, V.L.; Cochlan, W.P.; Erickson, A.; Bill, B.D.; Cox, F.H.; Borchert, J.A.; Lefebvre, K.A. Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae 2007, 6, 449–459. [Google Scholar] [CrossRef]
- Bargu, S.; Powell, C.L.; Wang, Z.; Doucette, G.J.; Silver, M.W. Note on the occurrence of Pseudo-nitzschia australis and domoic acid in squid from Monterey Bay, CA (USA). Harmful Algae 2008, 7, 45–51. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Bill, B.D.; Erickson, A.; Baugh, K.A.; O’Rourke, L.; Costa, P.R.; Nance, S.; Trainer, V.L. Characterization of Intracellular and Extracellular Saxitoxin Levels in Both Field and Cultured Alexandrium spp. Samples from Sequim Bay, Washington. Mar. Drugs 2008, 6, 103–116. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Jester, R.J.; Baugh, K.A.; Lefebvre, K.A. Presence of Alexandrium catenella and paralytic shellfish toxins in finfish, shellfish and rock crabs in Monterey Bay, California, USA. Mar. Biol. 2009, 156, 493. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Suzuki, T.; Selwood, A. Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae 2005, 4, 75–85. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Silver, M.; Kudela, R.M. Yessotoxin detected in mussel (Mytilus californicus) and phytoplankton samples from the U.S. west coast. Harmful Algae 2008, 7, 646–652. [Google Scholar] [CrossRef]
- Kudela, R.M.; Mioni, C.; Peacock, M.; Schraga, T.; Senn, D. Assessing SPATT in San Francisco Bay; SFEI Contract 1051 Final Report; University of California, Santa Cruz (UCSC): Santa Cruz, CA, USA, 2015; Available online: http://sfbaynutrients.sfei.org/sites/default/files/SPATT%20Final%20Report%20May2015.pdf (accessed on 21 August 2017).
- Lane, J.Q.; Roddam, C.M.; Langlois, G.W.; Kudela, R.M. Application of Solid Phase Adsorption Toxin Tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol. Oceanogr. Methods 2010, 8, 645–660. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Graneli, E.; Flynn, K. Chemical and Physical Factors Influencing Toxin Content. In The Ecology of Harmful Algae; Graneli, E., Turner, J., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 229–239. [Google Scholar]
- Leparc, J.; Rapenne, S.; Courties, C.; Lebaron, P.; Croué, J.P.; Jacquemet, V.; Turner, G. Water quality and performance evaluation at seawater reverse osmosis plants through the use of advanced analytical tools. Desalination 2007, 203, 243–255. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Berman-Frank, I.; Liberman, B.; Rahav, E.; Passow, U.; Berman, T. Transparent exopolymer particles: Potential agents for organic fouling and biofilm formation in desalination and water treatment plants. Desalination Water Treat. 2009, 3, 136–142. [Google Scholar] [CrossRef]
- Sabiri, N.E.; Castaing, J.B.; Massé, A.; Jaouen, P. Performance of a sand filter in removal of micro-algae from seawater in aquaculture production systems. Environ. Technol. 2012, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Remize, P.-J.; Laroche, J.-F.; Leparc, J.; Schrotter, J.-C. A pilot-scale comparison of granular media filtration and low-pressure membrane filtration for seawater pretreatment. Desalination Water Treat. 2009, 5, 6–11. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Belkin, N.; Liberman, B.; Berman, T.; Berman-Frank, I. Rapid sand filtration pretreatment for SWRO: Microbial maturation dynamics and filtration efficiency of organic matter. Desalination 2012, 286, 120–130. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Belkin, N.; Liberman, B.; Berman-Frank, I.; Berman, T. Bioflocculation: Chemical free, pre-treatment technology for the desalination industry. Water Res. 2013, 47, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Plantier, S.; Castaing, J.-B.; Sabiri, N.-E.; Massé, A.; Jaouen, P.; Pontié, M. Performance of a sand filter in removal of algal bloom for SWRO pre-treatment. Desalination Water Treat. 2013, 51, 1838–1846. [Google Scholar] [CrossRef]
- Guastalli, A.R.; Simon, F.X.; Penru, Y.; de Kerchove, A.; Llorens, J.; Baig, S. Comparison of DMF and UF pre-treatments for particulate material and dissolved organic matter removal in SWRO desalination. Desalination 2013, 322, 144–150. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. Evaluation of cyanobacterial cells removal and lysis by ultrafiltration. Sep. Purif. Technol. 2010, 70, 345–353. [Google Scholar] [CrossRef]
- Castaing, J.B.; Massé, A.; Séchet, V.; Sabiri, N.-E.; Pontié, M.; Haure, J.; Jaouen, P. Immersed hollow fibres microfiltration (MF) for removing undesirable micro-algae and protecting semi-closed aquaculture basins. Desalination 2011, 276, 386–396. [Google Scholar] [CrossRef]
- Frappart, M.; Massé, A.; Jaffrin, M.Y.; Pruvost, J.; Jaouen, P. Influence of hydrodynamics in tangential and dynamic ultrafiltration systems for microalgae separation. Desalination 2011, 265, 279–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Nan, J.; Gao, S.; Liang, H.; Wang, M.; Li, G. Effect of PAC addition on immersed ultrafiltration for the treatment of algal-rich water. J. Hazard. Mater. 2011, 186, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Min, C.-S.; Lee, S. Application of dissolved air flotation as pretreatment of seawater desalination. Desalination Water Treat. 2011, 33, 261–266. [Google Scholar] [CrossRef]
- Zhu, I.X.; Bates, B.J.; Anderson, D.M. Removal of Prorocentrum minimum from seawater using dissolved air flotation. J. Appl. Water Eng. Res. 2014, 2, 47–56. [Google Scholar] [CrossRef]
- Finley, B.; Paustenbach, D. The Benefits of Probabilistic Exposure Assessment: Three Case Studies Involving Contaminated Air, Water, and Soil. Risk Anal. 1994, 14, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.P. Algebraic sensitivity analysis of environmental models. Environ. Model. Softw. 2008, 23, 963–972. [Google Scholar] [CrossRef]
- Fowle, J.R.; Dearfield, K.L. Risk Characterization Handbook; EPA 100-B-00-002; U.S. EPA Science Policy Council: Washington, DC, USA, 2000.
- Paredes, I.; Rietjens, I.M.C.M.; Vieites, J.M.; Cabado, A.G. Update of risk assessments of main marine biotoxins in the European Union. Toxicon 2011, 58, 336–354. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.A. Drinking water standards and risk assessment. Regul. Toxicol. Pharmacol. 1988, 8, 288–299. [Google Scholar] [CrossRef]
- Donohue, J.; Zavaleta, J.O. Toxicological Basis for Drinking Water Risk Assessment. In Drinking Water Regulation and Health; Pontius, F., Ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 133–146. [Google Scholar]
- Howd, R.A.; Brown, J.P.; Fan, A.M. Risk Assessment for Chemicals in Drinking Water: Estimation of Relative Source Contribution. Toxicologist 2004, 78, 1–10. [Google Scholar]
- Cotruvo, J.; Couper, M.; Cunliffe, D.; Fawell, J.; Giddings, M.; Ohanian, E.; Ong, C.N.; Sanderson, H.; Simizaki, D. Pharmaceuticals in Drinking Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Water Reuse: Potential for Expanding the Nation’s Water Supply through Reuse of Municipal Wastewater; National Research Council; The National Academies Press: Washington, DC, USA, 2012.
- Xu, P.; Drewes, J.E.; Kim, T.-U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Ng, H.Y.; Elimelech, M. Influence of colloidal fouling on rejection of trace organic contaminants by reverse osmosis. J. Membr. Sci. 2004, 244, 215–226. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Petrinic, I.; Luxbacher, T.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Membr. Sci. 2009, 330, 90–103. [Google Scholar] [CrossRef]
- Stewart, J.E.; Marks, L.J.; Gilgan, M.W.; Pfeiffer, E.; Zwicker, B.M. Microbial utilization of the neurotoxin domoic acid: Blue mussels (Mytilus edulis) and soft shell clams (Mya arenaria) as sources of the microorganisms. Can. J. Microbiol. 1998, 44, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Walker, H.W. Effect of Process Variables and Natural Organic Matter on Removal of Microcystin-LR by PAC-UF. Environ. Sci. Technol. 2006, 40, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Gijsbertsen-Abrahamse, A.J.; Schmidt, W.; Chorus, I.; Heijman, S.G.J. Removal of cyanotoxins by ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 276, 252–259. [Google Scholar] [CrossRef]
- Lee, J.; Walker, H.W. Mechanisms and factors influencing the removal of microcystin-LR by ultrafiltration membranes. J. Membr. Sci. 2008, 320, 240–247. [Google Scholar] [CrossRef]
- Babel, S.; Takizawa, S. Microfiltration membrane fouling and cake behavior during algal filtration. Desalination 2010, 261, 46–51. [Google Scholar] [CrossRef]
- Jeong, S.; Bae, H.; Naidu, G.; Jeong, D.; Lee, S.; Vigneswaran, S. Bacterial community structure in a biofilter used as a pretreatment for seawater desalination. Ecol. Eng. 2013, 60, 370–381. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S.; Rice, S.A. Microbial activity in biofilter used as a pretreatment for seawater desalination. Desalination 2013, 309, 254–260. [Google Scholar] [CrossRef]
- Simon, F.X.; Rudé, E.; Llorens, J.; Baig, S. Study of Seawater Biofiltration by Measuring Adenosine Triphosphate (ATP) and Turbidity. Water Air Soil Pollut. 2013, 224, 1568. [Google Scholar] [CrossRef] [Green Version]
- Dehwah, A.H.A.; Al-Mashharawi, S.; Kammourie, N.; Missimer, T.M. Impact of well intake systems on bacterial, algae, and organic carbon reduction in SWRO desalination systems, SAWACO, Jeddah, Saudi Arabia. Desalination Water Treat. 2015, 55, 2594–2600. [Google Scholar] [CrossRef]
- Missimer, T.M.; Ghaffour, N.; Dehwah, A.H.A.; Rachman, R.; Maliva, R.G.; Amy, G. Subsurface intakes for seawater reverse osmosis facilities: Capacity limitation, water quality improvement, and economics. Desalination 2013, 322, 37–51. [Google Scholar] [CrossRef]
- Vandanjon, L.; Rossignol, N.; Jaouen, P.; Robert, J.M.; Quéméneur, F. Effects of shear on two microalgae species. Contribution of pumps and valves in tangential flow filtration systems. Biotechnol. Bioeng. 1999, 63, 1–9. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Subhash, G.; Yao, S.; Bellinger, B.; Gretz, M.R. Investigation of Mechanical Properties of Diatom Frustules Using Nanoindentation. J. Nanosci. Nanotechnol. 2005, 5, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Short, K.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. AFM Nanoindentations of Diatom Biosilica Surfaces. Langmuir 2007, 23, 5014–5021. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.K.L.; Kwok, A.C.M.; Chan, W.K.; Zhang, T.Y.; Wong, J.T.Y. Mechanical Characterization of Cellulosic Thecal Plates in Dinoflagellates by Nanoindentation. J. Nanosci. Nanotechnol. 2007, 7, 452–457. [Google Scholar] [PubMed]
- Ladner, D.A.; Vardon, D.R.; Clark, M.M. Effects of shear on microfiltration and ultrafiltration fouling by marine bloom-forming algae. J. Membr. Sci. 2010, 356, 33–43. [Google Scholar] [CrossRef]
- Michels, M.H.A.; Goot, A.J.; van der Norsker, N.-H.; Wijffels, R.H. Effects of shear stress on the microalgae Chaetoceros muelleri. Bioprocess Biosyst. Eng. 2010, 33, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Manerio, E.; Rodas, V.L.; Costas, E.; Hernandez, J.M. Shellfish consumption: A major risk factor for colorectal cancer. Med. Hypotheses 2008, 70, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Cordier, S.; Monfort, C.; Miossec, L.; Richardson, S.; Belin, C. Ecological Analysis of Digestive Cancer Mortality Related to Contamination by Diarrhetic Shellfish Poisoning Toxins along the Coasts of France. Environ. Res. 2000, 84, 145–150. [Google Scholar] [CrossRef] [PubMed]
- López Rodas, V.; Maneiro, E.; Martínez, J.; Navarro, M.; Costas, E. Harmful algal blooms, red tides and human health: Diarrhetic shellfish poisoning and colorectal cancer. Anales de la Real Academia Nacional de Farmacia 2006, 72, 391–408. [Google Scholar]
- Levin, E.D.; Pizarro, K.; Pang, W.G.; Harrison, J.; Ramsdell, J.S. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicol. Teratol. 2005, 27, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Pang, W.G.; Harrison, J.; Williams, P.; Petro, A.; Ramsdell, J.S. Persistent neurobehavioral effects of early postnatal domoic acid exposure in rats. Neurotoxicol. Teratol. 2006, 28, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Frame, E.R.; Gulland, F.; Hansen, J.D.; Kendrick, P.S.; Beyer, R.P.; Bammler, T.K.; Farin, F.M.; Hiolski, E.M.; Smith, D.R.; Marcinek, D.J. A Novel Antibody-Based Biomarker for Chronic Algal Toxin Exposure and Sub-Acute Neurotoxicity. PLoS ONE 2012, 7, e36213. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Faustman, E.M. Domoic acid as a developmental neurotoxin. Neurotoxicology 2010, 31, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Kendrick, P.S.; Ladiges, W.; Hiolski, E.M.; Ferriss, B.E.; Smith, D.R.; Marcinek, D.J. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae 2017, 64, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Boushey, C.; Tracy, K.; Trainer, V.; Roberts, S.M.; Schluterman, N.; Morris, J.G. The association between razor clam consumption and memory in the CoASTAL Cohort. Harmful Algae 2016, 57, 20–25. [Google Scholar] [CrossRef] [PubMed]

| Environmental Variable | Distribution | Unit | Specified Range or Value | Mean | SD | Reference |
|---|---|---|---|---|---|---|
| Dissolved toxins | ||||||
| DA | Uniform | µg/L | 60–135.6 | - | - | [24,25] |
| STX | Uniform | µg/L | 0.150–0.800 | - | - | [26] |
| OA | Uniform | µg/L | 1.31–4.67 | - | - | [27] |
| YTX | Uniform | µg/L | 23.7–126 | - | - | [27] |
| Algal cell conc. 1 | ||||||
| Alexandrium sp. | ECDF | cells/L | 374–748 | 524 | 205 | |
| Dinophysis sp. | ECDF | cells/L | 123–3886 | 1112 | 1120 | |
| L. polyedrum | ECDF | cells/L | 374–748 | 481 | 183 | |
| Pseudo-nitzschia delicatissima | ECDF | cells/L | 374–48,578 | 15,170 | 35,896 | |
| Pseudo-nitzschia seriata | ECDF | cells/L | 374–563,500 | 22,280 | 92,575 | |
| Intracellular toxin conc. | ||||||
| Alexandrium sp. | Uniform | pg/cell | 57.9 | - | - | [28] |
| Dinophysis sp. | Uniform | pg/cell | 2.7 | - | - | [29] |
| L. polyedrum 2 | Uniform | pg/cell | 0.005 | - | - | [30] |
| Pseudo-nitzschia delicatissima | Uniform | pg/cell | 117 | - | - | [23] |
| Pseudo-nitzschia seriata | Uniform | pg/cell | 117 | - | - | [23] |
| Treatment Trains | Specified Range | Reference |
|---|---|---|
| Cell Removal | ||
| GMF With Coagulation | 79–93% 1 | [36,38,40] |
| GMF Without Coagulation | 48–98% 1 | [37,39,41] |
| GMF Pressurized | 74–99.2% | [16,35,42] |
| MF/UF With Coagulation | 99–99.9% | [16,42] |
| MF/UF Without Coagulation | 95–100% | [38,43,44,45,46] |
| DAF | 43–93% | [42,47,48] |
| RO | - | - |
| Algal Cell Breakage 2 | ||
| GMF With Coagulation | 0–10% | This study |
| GMF Without Coagulation | 0–25% | This study |
| GMF Pressurized | 75–100% | This study |
| MF/UF With Coagulation | 75–100% (pressure driven); 15–35% (submerged) | This study |
| MF/UF Without Coagulation | 50–100% (pressure driven); 15–35% (submerged) | This study |
| DAF | - | - |
| RO | - | - |
| Dissolved toxin removal 3 | ||
| GMF With Coagulation | 0–34% | This study |
| GMF Without Coagulation | 26–50% | This study |
| GMF Pressurized | 6.6–40% | This study |
| MF/UF With Coagulation | 24.7–76.7% | This study |
| MF/UF Without Coagulation | 3–32.7% | This study |
| DAF | - | - |
| RO | 99.4–99.9% | [8,10,16] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manheim, D.C.; Jiang, S.C. Investigation of Algal Biotoxin Removal during SWRO Desalination through a Materials Flow Analysis. Water 2017, 9, 730. https://doi.org/10.3390/w9100730
Manheim DC, Jiang SC. Investigation of Algal Biotoxin Removal during SWRO Desalination through a Materials Flow Analysis. Water. 2017; 9(10):730. https://doi.org/10.3390/w9100730
Chicago/Turabian StyleManheim, Derek C., and Sunny C. Jiang. 2017. "Investigation of Algal Biotoxin Removal during SWRO Desalination through a Materials Flow Analysis" Water 9, no. 10: 730. https://doi.org/10.3390/w9100730
APA StyleManheim, D. C., & Jiang, S. C. (2017). Investigation of Algal Biotoxin Removal during SWRO Desalination through a Materials Flow Analysis. Water, 9(10), 730. https://doi.org/10.3390/w9100730