Treatment of Rural Wastewater Using a Spiral Fiber Based Salinity-Persistent Sequencing Batch Biofilm Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculation and Synthetic Wastewater
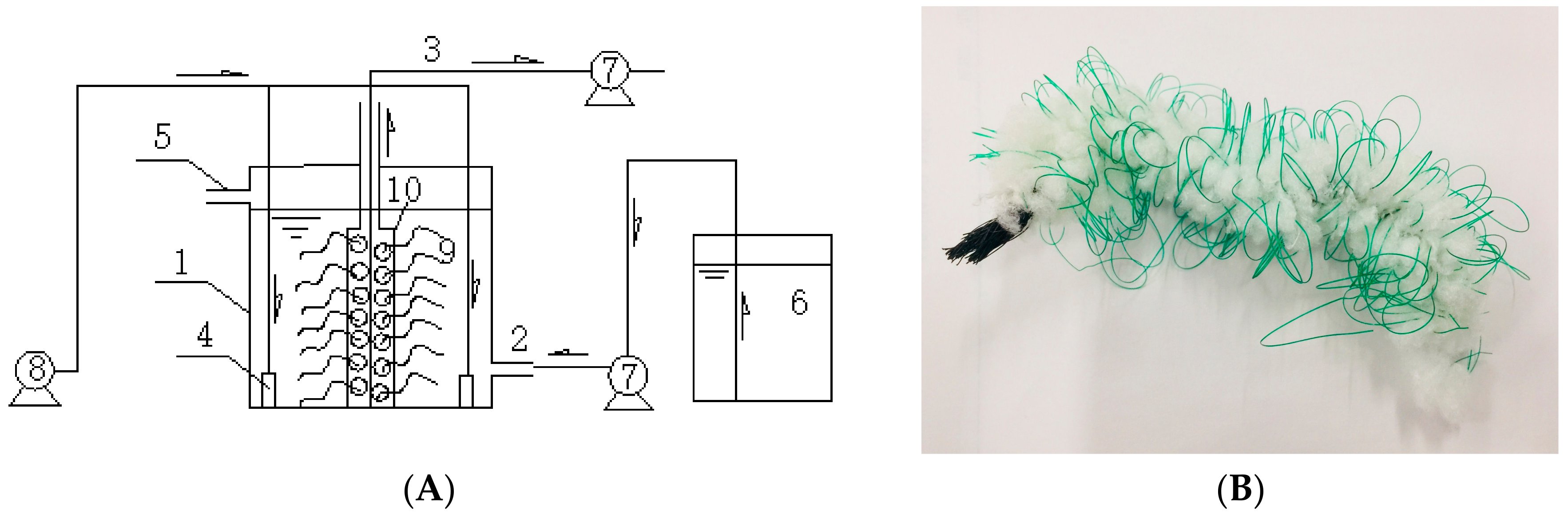
2.2. The SBBR System
2.3. Operating Conditions
2.4. Analytical Methods
3. Results
3.1. SBBR Performance during Start-Up Period
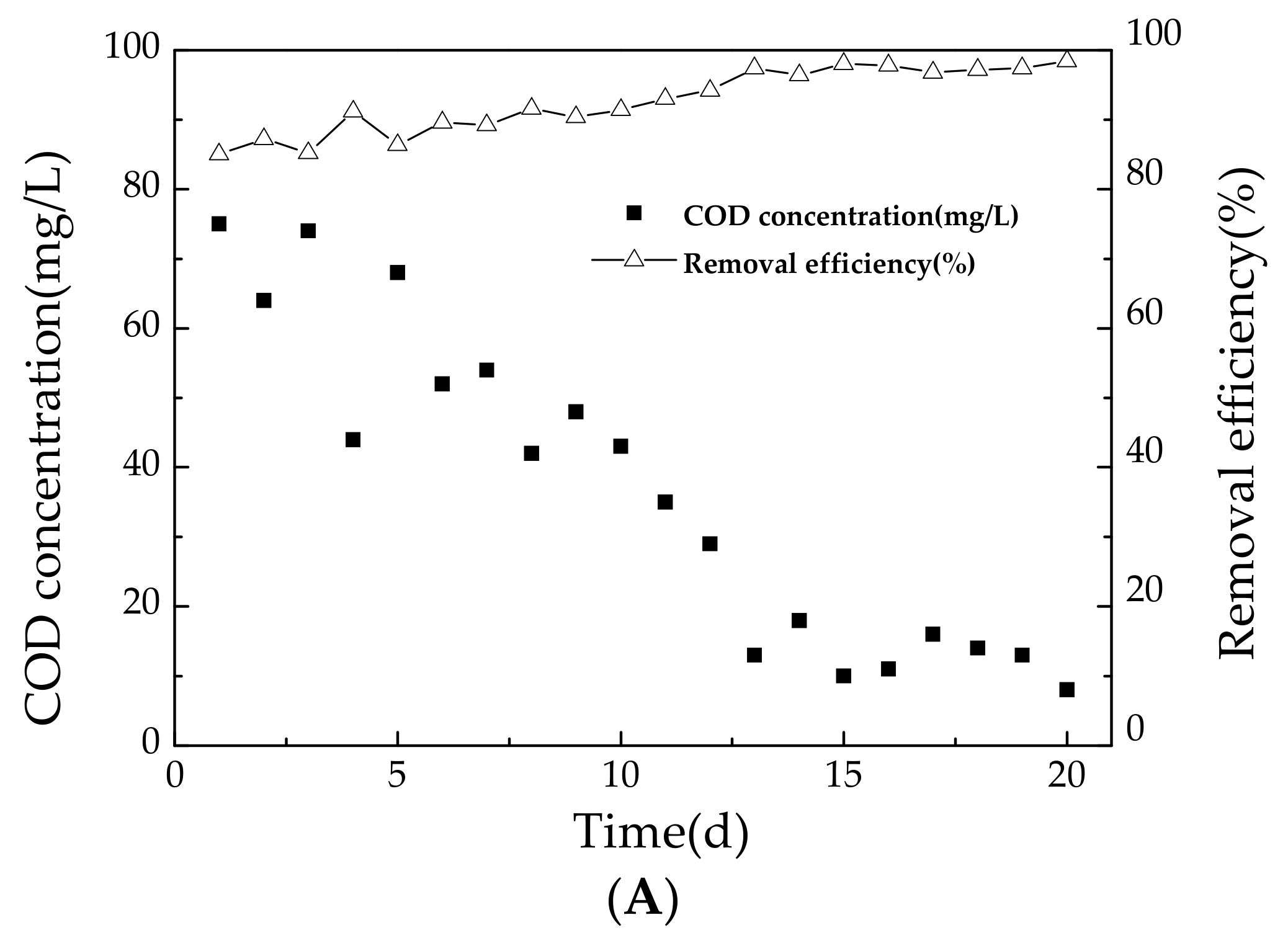
3.1.1. Variation of COD during Start-Up Period
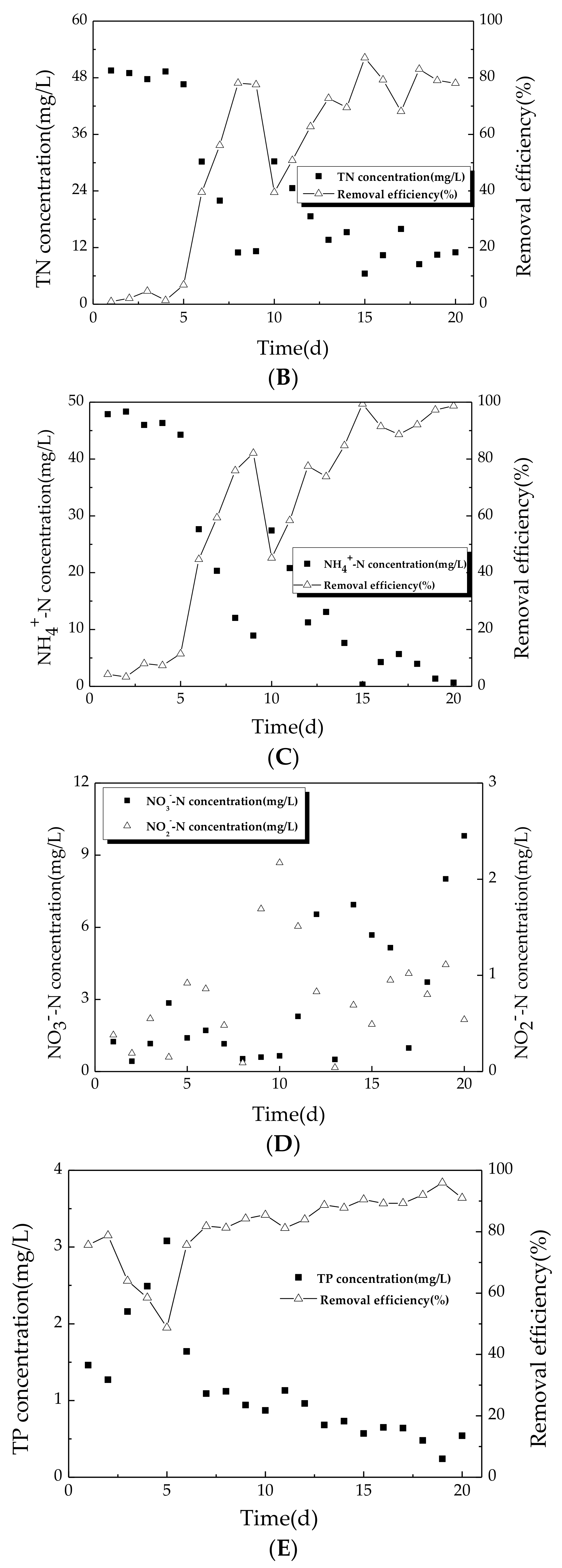
3.1.2. Variation of Nitrogen during Start-Up Period
3.1.3. Variation of TP during Start-Up Period
3.2. SBBR Performance during Operating Conditions
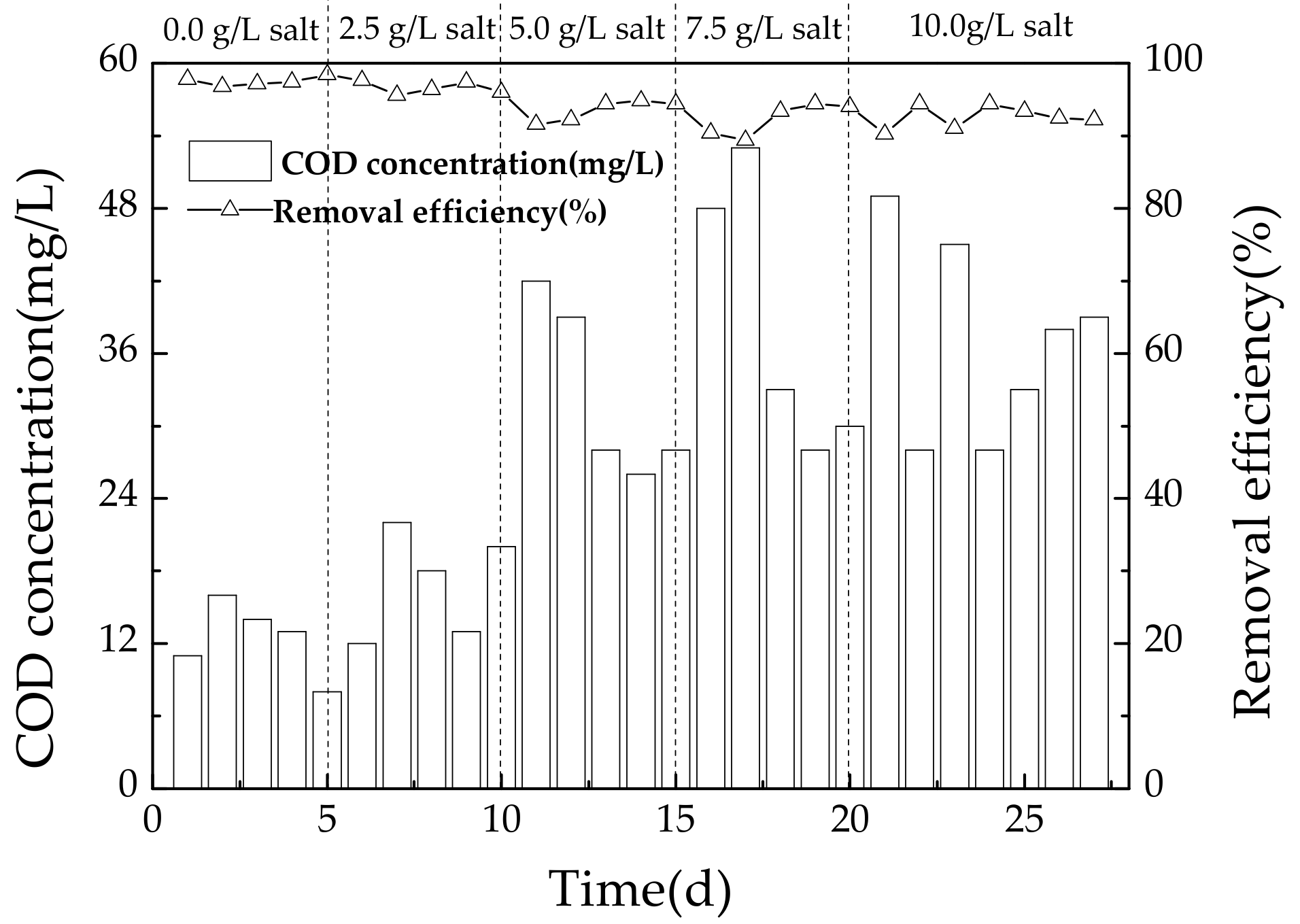
3.2.1. Variation of COD during Operating Conditions
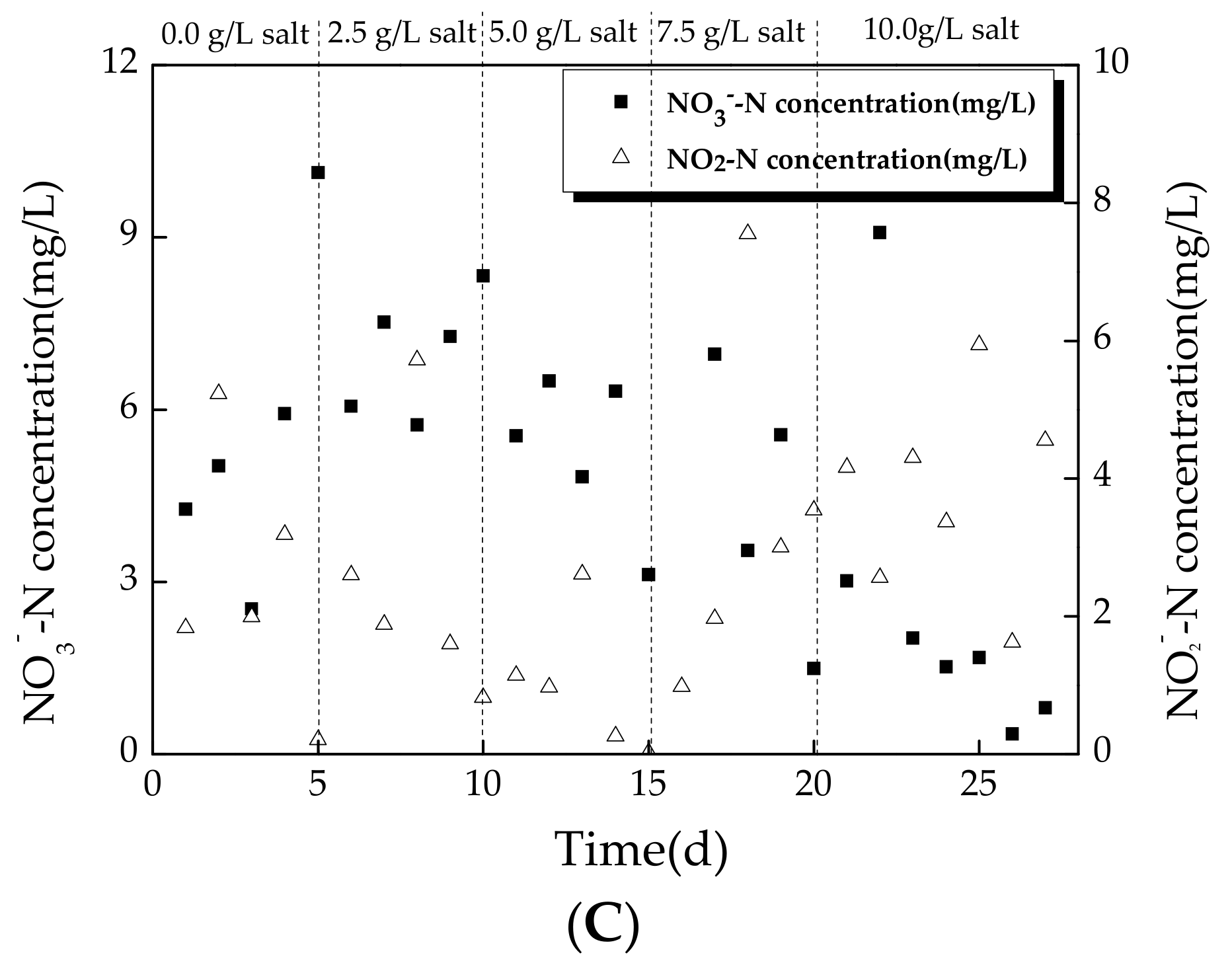
3.2.2. Variation of Nitrogen during Operating Conditions
3.2.3. Variation of TP during Operating Conditions
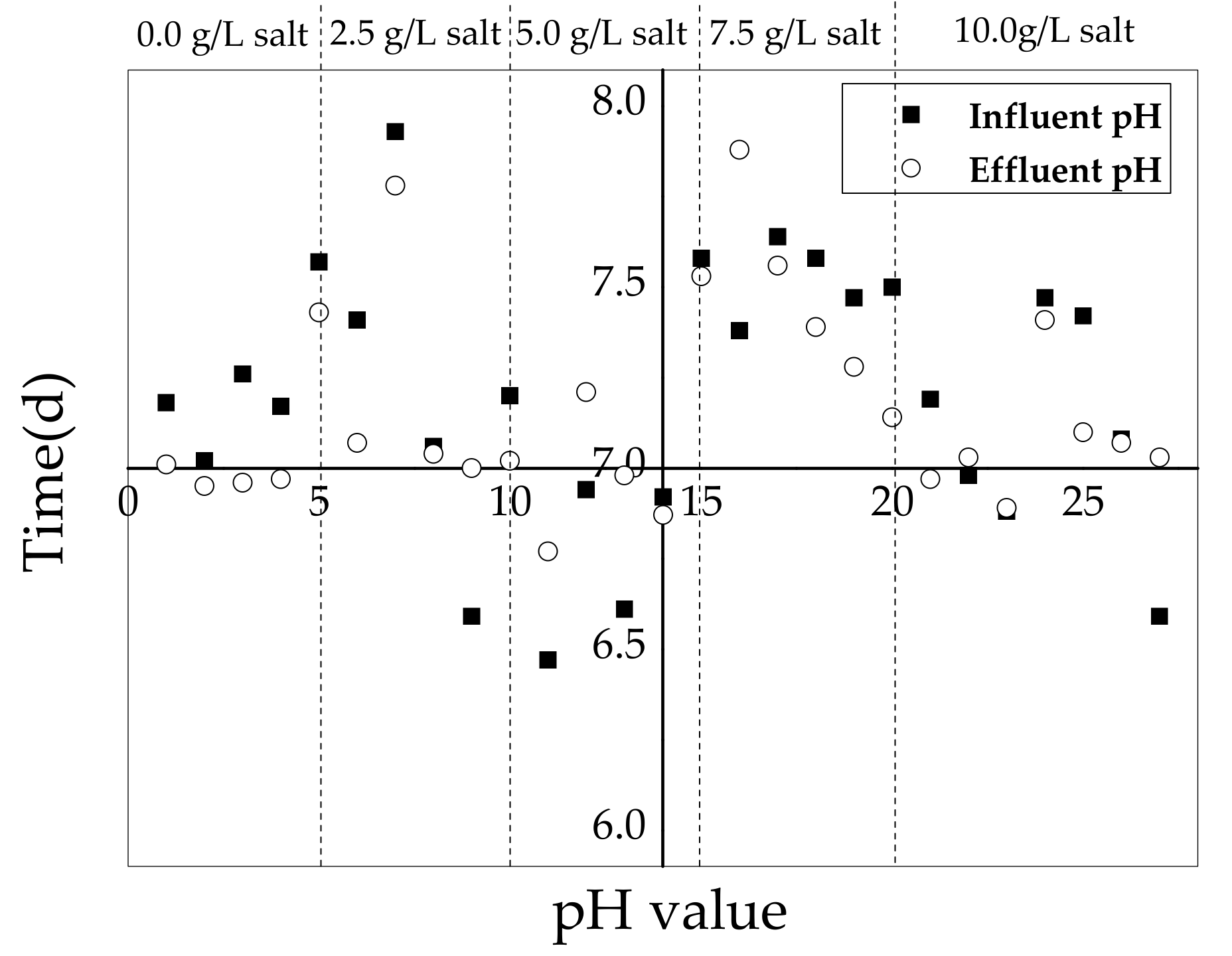
3.3. pH Variation
4. Discussion
4.1. Start-Up of Bioreactors
4.2. Effect of Salinity on COD Removal
4.3. Effect of Salinity on Nitrogen Removal
4.4. Effect of Salinity on Phosphorus Removal
4.5. Comparisons with Other SBBRs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Massoud, M.A.; Tarhini, A.; Nasr, J.A. Decentralized approaches to wastewater treatment and management: Applicability in developing countries. J. Environ. Manag. 2009, 90, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Feng, C.P.; Zhao, Y.X.; Hao, C.B. Denitrification of nitrate contaminated groundwater with a fiber-based biofilm reactor. Bioresour. Technol. 2009, 100, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.R.; Chung, J.H.; Heo, Y.R.; Kang, S.T.; Lee, S.M.; Shin, H.S. Full-Scale implementation of a vertical membrane bioreactor for simultaneous removal of organic matter and nutrients from municipal wastewater. Water 2015, 7, 1164–1172. [Google Scholar] [CrossRef]
- Wan, D.J.; Liu, H.J.; Qu, J.H.; Lei, P.J.; Xiao, S.H.; Hou, Y.N. Using the combined bioelectrochemical and sulfur autotrophic denitrification system for groundwater denitrification. Bioresour. Technol. 2009, 100, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.; Healy, M.G.; Prendergast, J. Nitrification in a vertically moving biofilm system. J. Environ. Manag. 2006, 79, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Keller-Lehmann, B.; Zeng, R.J.; Yuan, Z.J.; Keller, E. Review: Optimisation of poly-b-hydroxyalknoate analysis using gas chromatography for enhanced biological phosphorus removal systems. J. Chromatogr. A 2005, 1070, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.A.; Shane, A.S. Wastewater treatment and reuse: Past, present, and future. Water 2015, 7, 4887–4895. [Google Scholar]
- Zhang, Z.Y.; Zhou, J.T.; Wang, J.; Guo, H.Y.; Tong, J. Integration of nitrification and denitrifying dephosphatation in airlift loop sequencing batch biofilm reactor. Process Biochem. 2006, 41, 599–608. [Google Scholar] [CrossRef]
- Fu, B.; Liao, X.Y.; Ding, L.L.; Ren, H.Q. Characterization of microbial community in an aerobic moving bed biofilm reactor applied for simultaneous nitrification and denitrification. World J. Microbial. Biotechnol. 2010, 2, 1981–1990. [Google Scholar] [CrossRef]
- Brito, A.G.; Rodrigues, A.C.; Melo, F.L. Feasibility of a pulsed sequencing batch reactor with anaerobic aggregated biomass for the treatment of low strength wastewaters. Water Sci. Technol. 1997, 35, 193–198. [Google Scholar]
- Laconi, D.C.; Bonemazzi, F.; Lopez, A.; Ramadori, R. Integration of chemical and biological oxidation in a SBBR for tannery wastewater treatment. Water Sci. Technol. 2004, 50, 107–114. [Google Scholar]
- Ferraina, A.R.; Hu, Z.Q.; Ericson, F.J.; MacKey, A.A.; Smets, F.B. Biomass characteristics in three sequencing batch reactors treating a wastewater containing synthetic organic chemicals. Water Res. 2005, 39, 710–720. [Google Scholar]
- Zhang, Z.J.; Chen, S.H.; Wu, P.; Lin, L.F.; Luo, H.Y. Start-up of the Canon process from activated sludge under salt stress in a sequencing batch biofilm reactor (SBBR). Bioresour. Technol. 2010, 101, 6309–6314. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, B.G.; Jin, Y.X.; Lei, Z.F.; Feng, C.P.; Ding, D.H.; Hu, W.W.; Chen, N.; Suemura, T. Behavior of total phosphorus removal in an intelligent controlled sequencing batch biofilm reactor for municipal wastewater treatment. Bioresour. Technol. 2013, 132, 190–196. [Google Scholar] [CrossRef] [PubMed]
- She, Z.L.; Zhao, L.T.; Zhang, X.L.; Jin, C.J.; Gu, L.; Yang, S.Y.; Zhao, Y.G.; Gao, M.C. Partial nitrification and denitrification in a sequencing batch reactor treating high-salinity wastewater. Chem. Eng. J. 2016, 288, 207–215. [Google Scholar] [CrossRef]
- Rodrigues, J.A.D.; Ratusznei, S.M.; Camargo, E.F.M.; Zaiat, M. Influence of the agitation rate on the performance of an anaerobic sequencing batch reactor containing granulated biomass treating low strength wastewater. Adv. Environ. Res. 2003, 7, 405–410. [Google Scholar] [CrossRef]
- Dincer, A.R.; Kargi, F. Salt inhibition kinetics in nitrification of synthetic saline wastewater. Enzym. Microb. Technol. 2001, 28, 661–665. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Fontaine, J.; Laruelle, F.; Yolande, D.; Anissa, A.L.H. Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Sci. Total Environ. 2015, 533, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.H.; Yan, L.; Wang, Z.; Miao, Y.; Shen, K.; Peng, S.; Ma, Y.; Tao, L.W.; Li, A.M. 454 pyrosequencing analysis on microbial diversity of an expanded granular sludge bed reactor treating high NaCl and nitrate concentration wastewater. Biotechnol. Bioprocess Eng. 2014, 19, 183–190. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhan, X.M.; Feng, Y.C.; Qian, Y. Effect of salinity variations on the performance of activated sludge system. Biomed. Environ. Sci. 2005, 18, 5–8. [Google Scholar] [PubMed]
- Gao, F.; Zhang, H.M.; Yang, F.L.; Qian, G.H.; Li, H.G.; Zhang, R. Study of an innovative anaerobic (A)/Oxic (O)/aerobic (A) bioreactor based on denitrification-anammox technology treating low C/N municipal sewage. Chem. Eng. J. 2013, 232, 65–73. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Alonso-Molina, J.L.; Pastor-Alcaniz, L. Biological treatment performance of hypersaline wastewaters with high phenols concentration from table olive packaging industry using sequencing batch reactors. J. Ind. Eng. Chem. 2016, 43, 44–52. [Google Scholar] [CrossRef]
- Sun, C.; Leiknes, T.; Jan, W.; Bernt, T. Salinity effect on a biofilm-MBR process for shipboard wastewater treatment. Sep. Purif. Technol. 2010, 72, 380–387. [Google Scholar] [CrossRef]
- Uygur, A. Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem. 2006, 41, 61–66. [Google Scholar] [CrossRef]
- Zhai, S.Y.; Ji, M.; Zhao, Y.X.; Pavlostathis, S.G.; Zhao, Q. Effects of salinity and COD/N on denitrification and bacterial community in dicyclic-type electrode based biofilm reactor. Chemosphere 2018, 192, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Park, H.; Park, J.; Zhang, F.S.; Chen, C.; Li, X.K.; Zhao, D.; Zhao, F.B. Effect of different salinity adaptation on the performance and microbial community in a sequencing batch reactor. Bioresour. Technol. 2016, 216, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Olivier, L.; Vasudevan, N.; Margarita, T.; Moletta, R. Anaerobic digestion of tannery soak liquor with an aerobic post-treatment. Water Res. 2006, 40, 1492–1500. [Google Scholar]
- Ge, S.J.; Peng, Y.Z.; Wang, S.Y.; Guo, J.H.; Ma, B.; Zhang, L.; Cao, X. Enhanced nutrient removal in a modified step feed process treating municipal wastewater with different inflow distribution ratios and nutrient ratios. Bioresour. Technol. 2010, 101, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Lins, P.; Reitschuler, C.; Illmer, P. Development and evaluation of inocula combating high acetate concentrations during the start-up of an anaerobic digestion. Bioresour. Technol. 2012, 104, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sukru, A.; Erdal, S. Influence of salinity on partial nitrification in a submerged biofilter. Bioresour. Technol. 2012, 118, 24–29. [Google Scholar]
- Wang, Z.W.; Mark, C.M.; van Loosdrecht, M.C.M.; Pascal, E.S. Gradual adaptation to salt and dissolved oxygen: Strategies to minimize adverse effect of salinity on aerobic granular sludge. Water Res. 2017, 124, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.X.; Ding, D.H.; Feng, C.P.; Tong, S.; Suemura, T.; Zhang, F. Performance of sequencing batch biofilm reactors with different control systems in treating synthetic municipal wastewater. Bioresour. Technol. 2012, 104, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Karg, F.; Dincer, A.R. Use of halophilic bacteria in biological treatment of saline wastewater by fed-batch operation. Water Environ. Res. 2000, 72, 170–174. [Google Scholar] [CrossRef]
- Uygur, A.; Kargi, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym. Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Eldon, R.R.; Sung, J.K.; Hung, S.P. Effect of COD/N ratio and salinity on the performance of sequencing batch reactors. Bioresour. Technol. 2008, 99, 839–846. [Google Scholar]
- Jang, D.; Hwang, Y.; Shin, H.; Lee, W. Effects of salinity on the characteristics of biomass and membrane fouling in membrane bioreactors. Bioresour. Technol. 2013, 141, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Yan, X.; Ma, Y.F.; Tian, C.X.; Ding, J.C. Impact of salinity on treatment of saline wastewater by sequencing batch biofilm reactor process. J. Cent. South Univ. Technol. 2014, 21, 1989–1994. [Google Scholar] [CrossRef]
- Liu, S.T.; Yang, F.L.; Gong, Z.; Su, Z.C. Assessment of the positive effect of salinity on the nitrogen removal performance and microbial composition during the start-up of CANON process. Appl. Microbial. Biotechnol. 2008, 80, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, F.L.; Fu, Z.M.; Wang, T.; Lei, R.B. Simultaneous nitrogen and phosphorus removal by a novel sequencing batch moving bed membrane bioreactor for wastewater treatment. J. Hazard. Mater. 2010, 175, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; Bassin, J.P.; de Kreuk, M.K.; Kleerebezem, R.; van Loosdrecht, M.C.M. Evaluating the main and side effects of high salinity on aerobic granular sludge. Appl. Microbiol. Biotechnol. 2014, 98, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, J.; Xie, H.J.; Tan, L.R.; Luo, J.N.; Tao, Z.Y.; Wang, S.J. Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors. Water 2017, 9, 477–488. [Google Scholar] [CrossRef]
- Welles, L.; Lopez-Vazquez, C.M.; Hooijmans, C.M.; van Loosdrecht, M.C.M.; Brdjanovic, D. Impact of salinity on the anaerobic metabolism of phosphate-accumulating organisms (PAO) and glycogen-accumulating organisms (GAO). Appl. Microbiol. Biotechnol. 2014, 98, 7609–7622. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.H.; Feng, C.P.; Jin, Y.X.; Hao, C.B.; Zhao, Y.X.; Suemura, T. Domestic sewage treatment in a Sequencing Batch Biofilm Reactor (SBBR) with an intelligent controlling system. Desalination 2011, 276, 260–265. [Google Scholar] [CrossRef]
- Wang, Z.C.; Gao, M.C.; She, Z.L.; Wang, S.; Jin, C.J.; Zhao, Y.G.; Yang, S.Y.; Guo, L. Effects of salinity on performance, extracellular polymeric substances and microbial community of an aerobic granular sequencing batch reactor. Sep. Purif. Technol. 2015, 144, 223–231. [Google Scholar] [CrossRef]


| Parameter | COD (mg/L) | NH4+-N (mg/L) | TN (mg/L) | TP (mg/L) | pH |
|---|---|---|---|---|---|
| Values | 500.0 ± 60.0 | 50.0 ± 2.5 | 50.0 ± 2.5 | 5.9 ± 0.5 | 7.4 ± 0.5 |
| Salinity | Acclima-Tion Period (d) | TN (Total Nitrogen) | TP (Total Phosphorus) | COD (Chemical Oxygen Demand) | NH4+-N (Ammonia Nitrogen) | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial Con. (mg/L) | Rem-Oval Effic. | Initial Con. (mg/L) | Rem-Oval Effic. | Initial Con. (mg/L) | Rem-Oval Effic. | Initial Con. (mg/L) | Rem-Oval Effic. | |||
| 0.00 wt % | - | - | - | 6 | 92% | 370 | 86% | - | - | [14] |
| 0.00 wt % | 7 | 35 | 90% | 5.5 | 93% | 350 | 85% | 35 | 92% | [32] |
| 0.00 wt % | 45 | 50 | 80% | 2.5 | 90% | 500 | 93% | 50 | 85% | [44] |
| 0.65 wt % | 37 | 150 | 27% | - | - | - | - | 150 | 35% | [13] |
| 0.75 wt % | 15 | 50 | 85% | 6 | 60% | 500 | 97% | 50 | 92% | This study |
| 1.00 wt % | 80 | - | - | - | - | 1300 | 90% | 70 | 90% | [43] |
| 1.40 wt % | 34 | - | - | - | - | 500 | 75% | 30 | 63% | [37] |
| 2.00 wt % | 40 | - | - | 25 | 95% | 1000 | 93% | 50 | 96% | [26] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.-X.; Chao, C.-F.; Zhai, S.-Y.; Wang, Z.-J.; Ji, M. Treatment of Rural Wastewater Using a Spiral Fiber Based Salinity-Persistent Sequencing Batch Biofilm Reactor. Water 2017, 9, 970. https://doi.org/10.3390/w9120970
Zhao Y-X, Chao C-F, Zhai S-Y, Wang Z-J, Ji M. Treatment of Rural Wastewater Using a Spiral Fiber Based Salinity-Persistent Sequencing Batch Biofilm Reactor. Water. 2017; 9(12):970. https://doi.org/10.3390/w9120970
Chicago/Turabian StyleZhao, Ying-Xin, Chun-Fang Chao, Si-Yuan Zhai, Zi-Jian Wang, and Min Ji. 2017. "Treatment of Rural Wastewater Using a Spiral Fiber Based Salinity-Persistent Sequencing Batch Biofilm Reactor" Water 9, no. 12: 970. https://doi.org/10.3390/w9120970





