Root Development of Transplanted Cotton and Simulation of Soil Water Movement under Different Irrigation Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.1.1. Experimental Site
2.1.2. Agronomic Practices
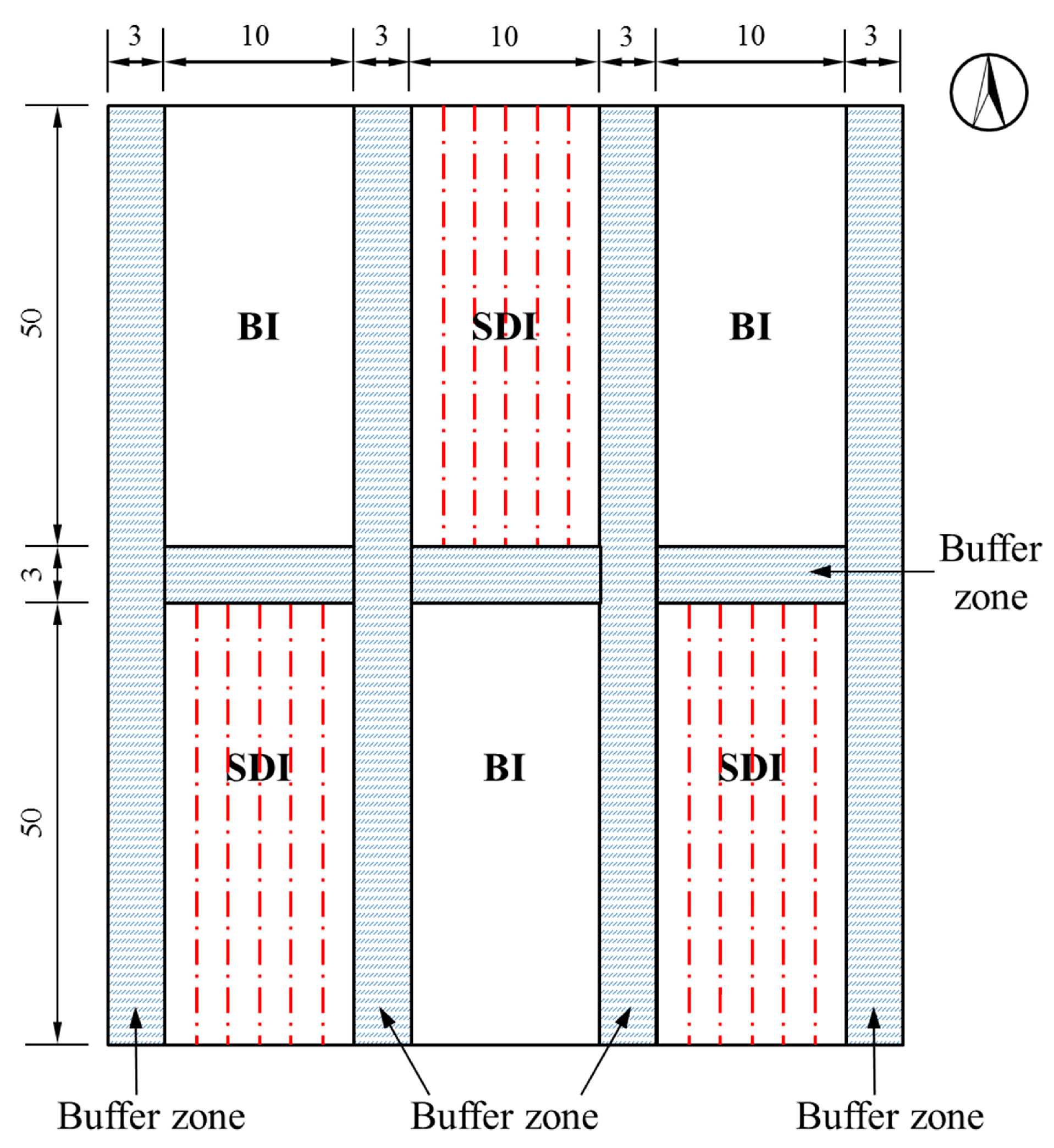
2.1.3. Experimental Design
2.2. Measurement Methods
2.2.1. Root Spatial Distribution
2.2.2. Dynamic Growth of the Root System
2.2.3. Leaf Area and Plant Height
2.2.4. Soil Water Content (SWC)
2.3. Numerical Modelling
2.3.1 Water Flow Equations
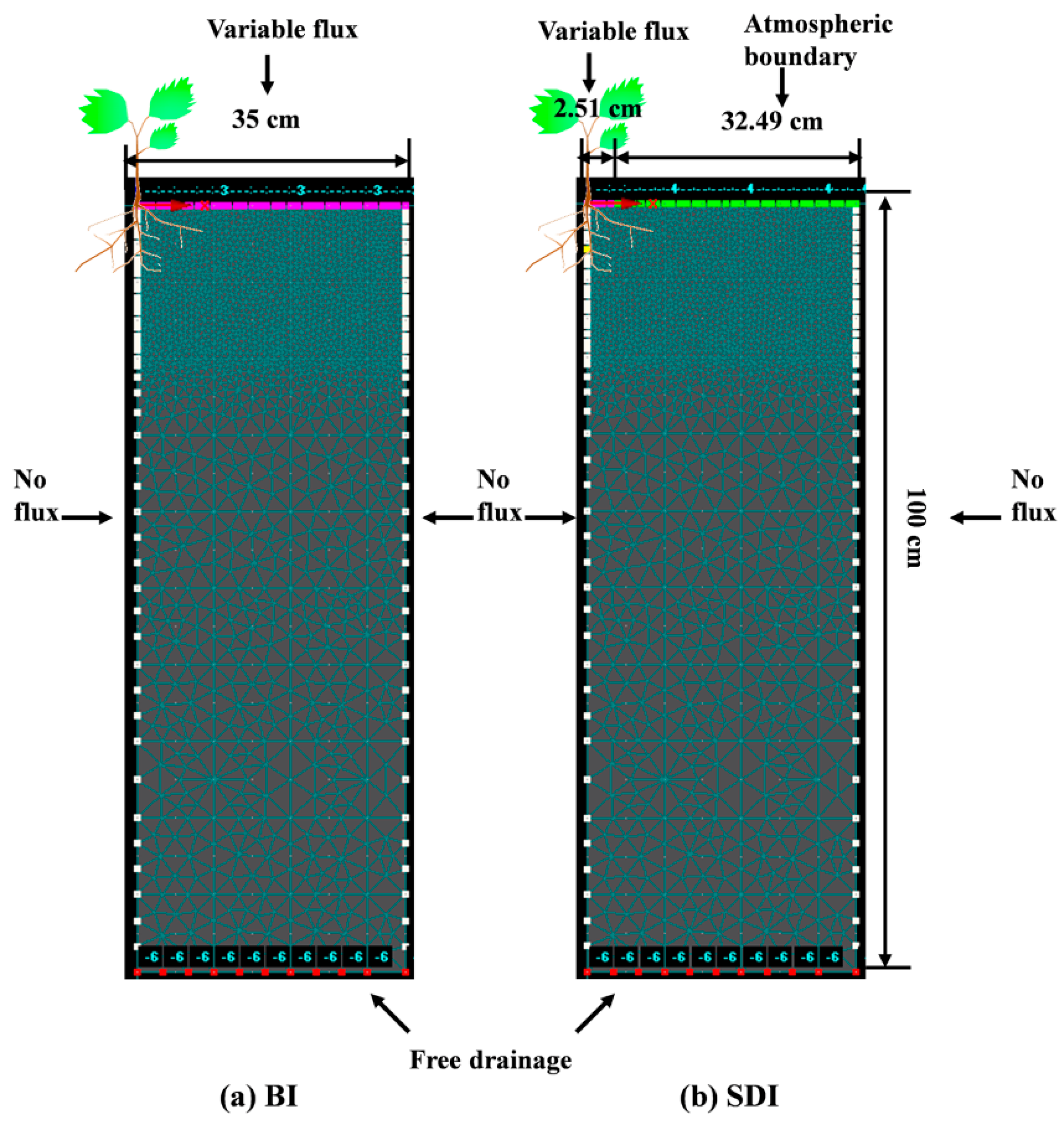
2.3.2. Domain and Boundary Conditions
2.3.3. Initial Conditions and Temporal and Spatial Discretization
2.3.4. Estimating Evaporation and Transpiration
2.3.5. Root Water Uptake
2.3.6. Model Calibration and Verification
2.4. Statistical Analysis and Criteria of Model Evaluation
3. Results
3.1. Changes in Spatial Distribution of Root Length and Biomass
3.1.1. RLD Distribution
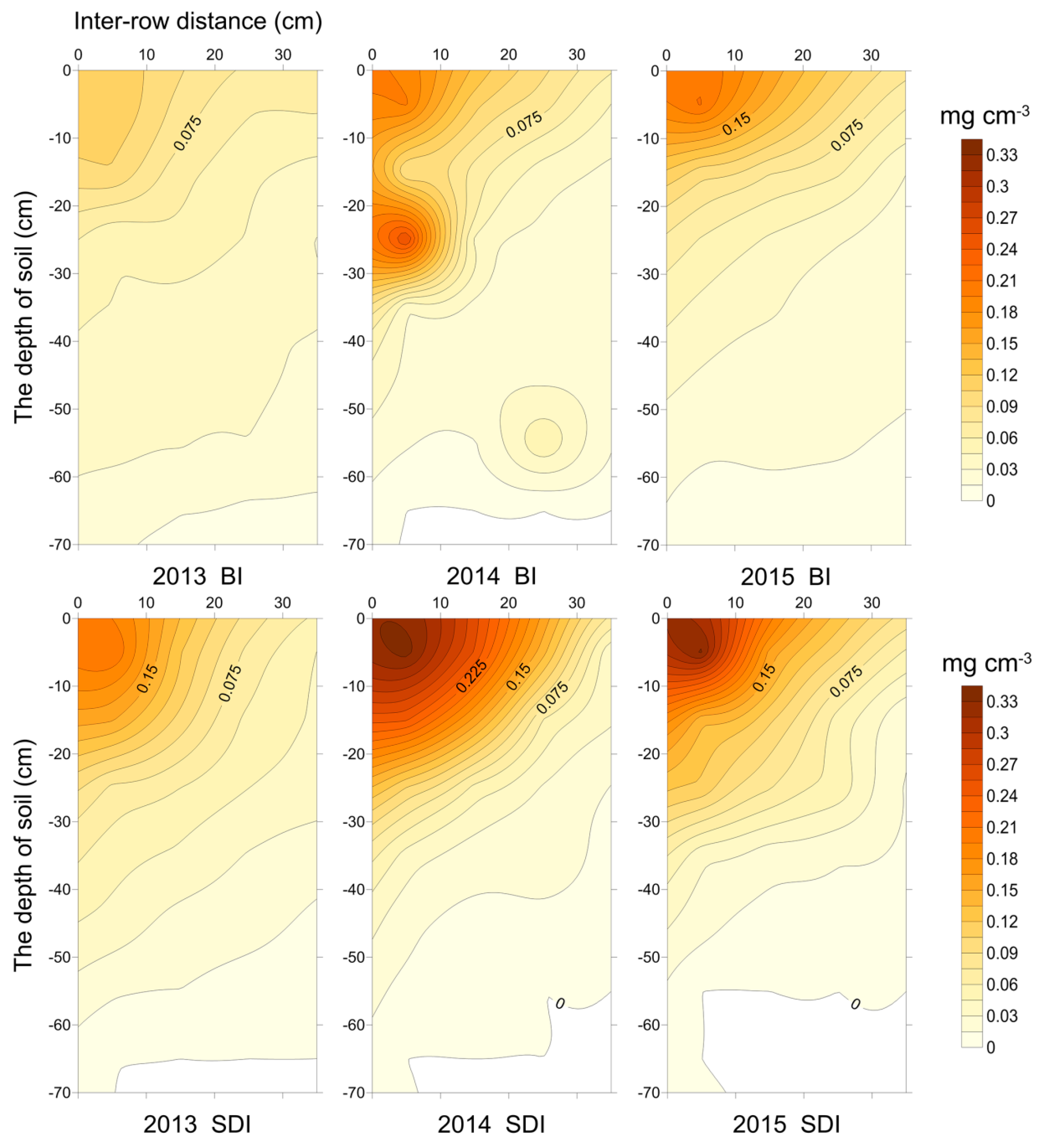
3.1.2. RBD Distribution
3.2. Growth of Root System
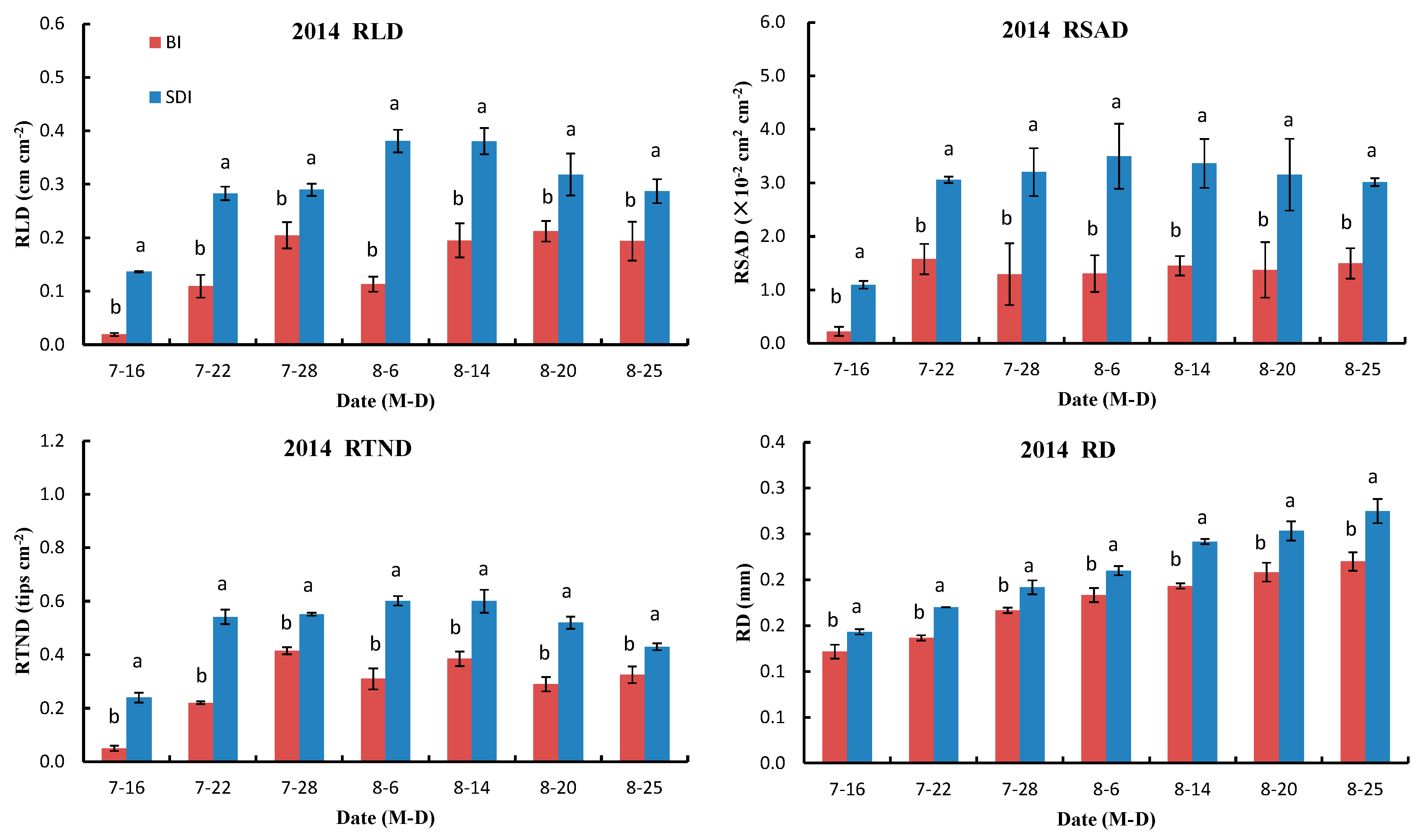
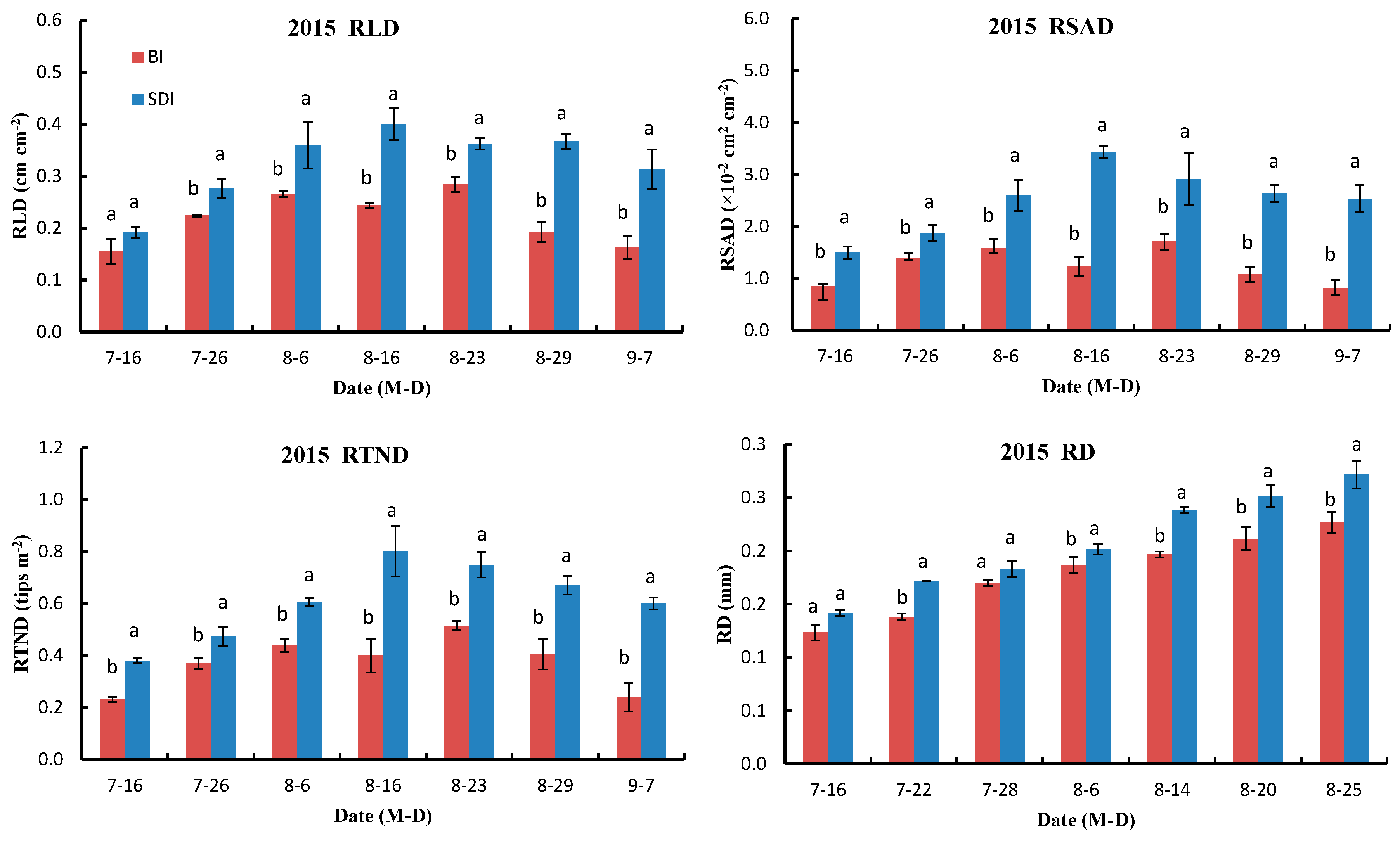
3.2.1. Variations in RLD, RSAD, RTND and Root Diameter (RD)
3.2.2. Two-Dimensional Model of RLD Parameters
3.3. Simulation of Soil Water Movement
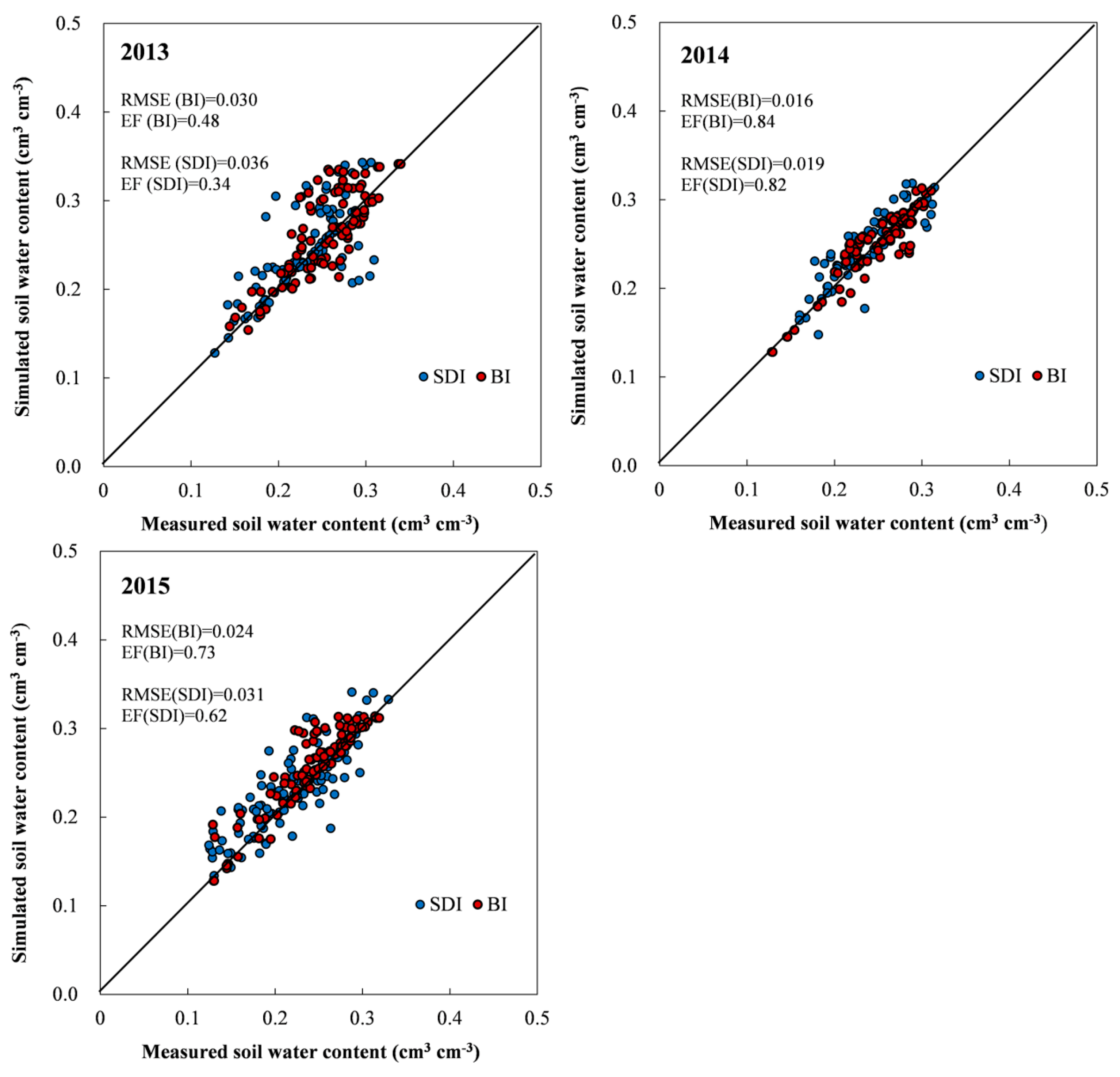
3.3.1. Model Evaluation
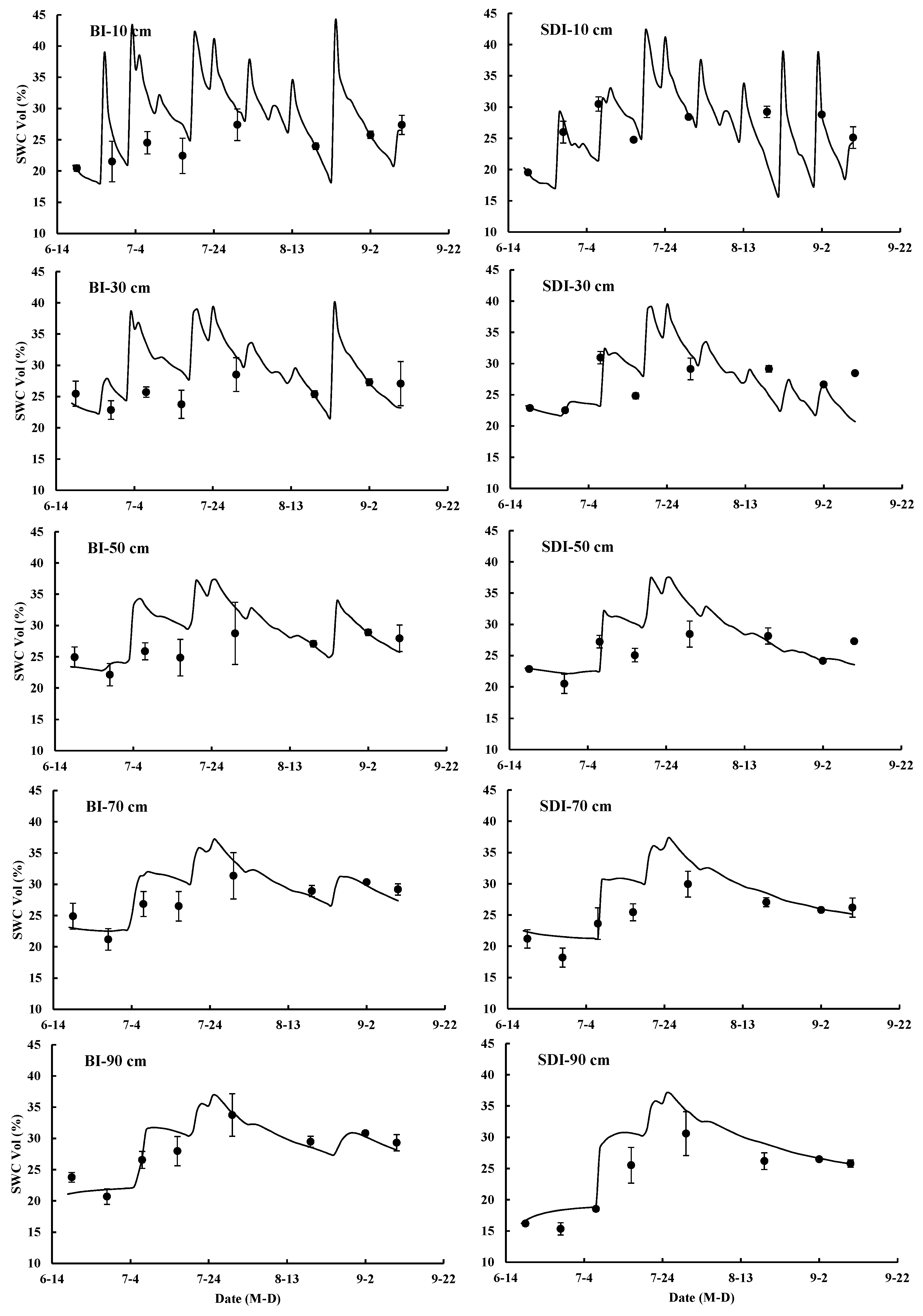
3.3.2. Simulation of Soil Water Movement
4. Discussion
4.1. Root Characteristics of Transplanted Cotton
4.2. Effects of Soil Water Movement on Root Development of Transplanted Cotton under Different Irrigation Methods
4.3. Modeling Soil Water Movement under Different Irrigation Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, H.Z.; Li, W.J.; Tang, W.; Li, Z.H.; Zhang, D.W.; Niu, Y.H. Yield, quality and leaf senescence of cotton grown at varying planting dates and plant densities in the Yellow River Valley of China. Field Crops Res. 2006, 98, 106–115. [Google Scholar] [CrossRef]
- CRI (Cotton Research Institute Chinese Academy of Agricultural Sciences). Cultivation of Cotton in China; Shanghai Science and Technology Press: Shanghai, China, 2013. [Google Scholar]
- Dong, H.Z.; Li, W.J.; Tang, W.; Li, Z.H.; Zhang, D.M. Enhanced plant growth, development and fiber yield of Bt transgenic cotton by an integration of plastic mulching and seedling transplanting. Ind. Crops Prod. 2007, 26, 298–306. [Google Scholar] [CrossRef]
- Dong, H.Z.; Li, W.J.; Tang, W.; Li, Z.H.; Zhang, D.M. Increased yield and revenue with a seedling transplanting system for hybrid seed production in Bt cotton. J. Agron. Crop Sci. 2005, 191, 116–124. [Google Scholar] [CrossRef]
- Zhang, L.; van der Werf, W.; Zhang, S.; Li, B.; Spiertz, J.H.J. Growth, yield and quality of wheat and cotton in relay strip intercropping systems. Field Crops Res. 2007, 103, 178–188. [Google Scholar] [CrossRef]
- Zhang, L.; van der Werf, W.; Bastiaans, L.; Zhang, S.; Li, B.; Spiertz, J.H.J. Light interception and utilization in relay intercrops of wheat and cotton. Field Crops Res. 2008, 107, 29–42. [Google Scholar] [CrossRef]
- Dai, J.L.; Dong, H.Z. Intensive cotton farming technologies in China: Achievements, challenges and countermeasures. Field Crops Res. 2014, 155, 99–110. [Google Scholar] [CrossRef]
- Luo, H.H.; Tao, X.P.; Hu, Y.Y.; Zhang, Y.L.; Zhang, W.F. Response of cotton root growth and yield to root restriction under various water and nitrogen regimes. J. Plant Nutr. Soil Sci. 2015, 178, 384–392. [Google Scholar] [CrossRef]
- Xu, X.; Huang, G.H.; Qu, Z.Y.; Pereira, L.S. Assessing the groundwater dynamics and impacts of water saving in the Hetao Irrigation District, Yellow River basin. Agric. Water Manag. 2010, 98, 301–313. [Google Scholar] [CrossRef]
- Mao, S.C.; Li, P.C.; Han, Y.C.; Wang, G.P.; Li, Y.B.; Wang, X.H. Preliminary Observation on Morphological Parameters of Root System of the Root-naked Transplanting Cotton (Gossypium hirsutum L.). Cotton Sci. 2008, 20, 76–78. [Google Scholar]
- Hulugalle, N.R.; Broughton, K.J.; Tan, D.K.Y. Fine root production and mortality in irrigated cotton, maize and sorghum sown in vertisols of northern New South Wales, Australia. Soil Tillage Res. 2015, 146, 313–322. [Google Scholar] [CrossRef]
- Hu, X.T.; Chen, H.; Wang, J.; Meng, X.B.; Chen, F.H. Effects of Soil Water Content on Cotton Root Growth and Distribution Under Mulched Drip Irrigation. Agric. Sci. China 2009, 8, 709–716. [Google Scholar] [CrossRef]
- Ning, S.R.; Shi, J.C.; Zuo, Q.; Wang, S.; Ben-Gal, A. Generalization of the root length density distribution of cotton under film mulched drip irrigation. Field Crops Res. 2015, 177, 125–136. [Google Scholar] [CrossRef]
- Hodgson, A.S.; Constable, G.A.; Duddy, G.R.; Daniells, I.G. A comparison of drip and furrow irrigated cotton on a cracking clay soil. 2. Water use efficiency, waterlogging, root distribution and soil structure. Irrig. Sci. 1990, 11, 143–148. [Google Scholar] [CrossRef]
- Rao, S.S.; Tanwar, S.P.S.; Regar, P.L. Effect of deficit irrigation, phosphorous inoculation and cycocel spray on root growth, seed cotton yield and water productivity of drip irrigated cotton in arid environment. Agric. Water Manag. 2016, 169, 14–25. [Google Scholar] [CrossRef]
- Sampathkumar, T.; Pandian, B.J.; Mahimairaja, S. Soil moisture distribution and root characters as influenced by deficit irrigation through drip system in cotton–maize cropping sequence. Agric. Water Manag. 2012, 103, 43–53. [Google Scholar] [CrossRef]
- Min, W.; Guo, H.J.; Zhou, G.W.; Zhang, W.; Ma, L.J.; Ye, J.; Hou, Z.N. Root distribution and growth of cotton as affected by drip irrigation with saline water. Field Crops Res. 2014, 169, 1–10. [Google Scholar] [CrossRef]
- Li, X.Y.; Shi, H.B.; Šimůnek, J.; Gong, X.W.; Peng, Z.Y. Modeling soil water dynamics in a drip-irrigated intercropping field under plastic mulch. Irrig. Sci. 2015, 33, 289–302. [Google Scholar] [CrossRef]
- Provenzano, G. Using HYDRUS-2D simulation model to evaluate wetted soil volume in subsurface drip irrigation systems. J. Irrig. Drain. Eng. 2007, 133, 342–349. [Google Scholar] [CrossRef]
- Šimůnek, J.; Sejna, M.; Genuchten, M.T.V. The HYDRUS Software Package for Simulating Two-and Three-Dimensional Movement of Water, Heat, and Multiple Solutes in Variable-Saturated Media; User Manual, Version 1.0; PC Progress: Prague, Czech Republic, 2006. [Google Scholar]
- Bufon, V.B.; Lascano, R.J.; Bednarz, C.; Booker, J.D.; Gitz, D.C. Soil water content on drip irrigated cotton: Comparison of measured and simulated values obtained with the Hydrus 2-D model. Irrig. Sci. 2012, 30, 259–273. [Google Scholar] [CrossRef]
- Kandelous, M.M.; ŠImůNek, J. Numerical simulations of water movement in a subsurface drip irrigation system under field and laboratory conditions using HYDRUS-2D. Agric. Water Manag. 2010, 97, 1070–1076. [Google Scholar] [CrossRef]
- Phogat, V.; Mahadevan, M.; Skewes, M.; Cox, J.W. Modelling soil water and salt dynamics under pulsed and continuous surface drip irrigation of almond and implications of system design. Irrig. Sci. 2012, 30, 315–333. [Google Scholar] [CrossRef]
- Han, M.; Zhao, C.Y.; Feng, G.; Yan, Y.Y.; Sheng, Y. Evaluating the Effects of Mulch and Irrigation Amount on Soil Water Distribution and Root Zone Water Balance Using HYDRUS-2D. Water 2015, 7, 2622–2640. [Google Scholar] [CrossRef]
- Trout, T.J.; ŠImůNek, J.; Shouse, P.J.; Skaggs, T.H. Comparison of HYDRUS-2D Simulations of Drip Irrigation with Experimental Observations. J. Irrig. Drain. Eng. 2004, 130, 304–310. [Google Scholar]
- Mguidiche, A.; Provenzano, G.; Douh, B.; Khila, S.; Rallo, G.; Boujelben, A. Assessing Hydrus-2D to Simulate Soil Water Content (SWC) and Salt Accumulation Under an SDI System: Application to a Potato Crop in a Semi-Arid Area of Central Tunisia. Irrig. Drain. 2015, 64, 263–274. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wu, P.T.; Zhao, X.N.; Wang, Z.K. Simulation of soil water dynamics for uncropped ridges and furrows under irrigation conditions. Can. J. Soil Sci. 2017, 93, 85–98. [Google Scholar] [CrossRef]
- Chen, L.J.; Feng, Q.; Li, F.R.; Li, C.S. Simulation of soil water and salt transfer under mulched furrow irrigation with saline water. Geoderma 2014, 241–242, 87–96. [Google Scholar] [CrossRef]
- Crevoisier, D.; Popova, Z.; Mailhol, J.C.; Ruelle, P. Assessment and simulation of water and nitrogen transfer under furrow irrigation. Agric. Water Manag. 2008, 95, 354–366. [Google Scholar] [CrossRef]
- Kang, Y.H.; Wang, R.S.; Wan, S.Q.; Hu, W.; Jiang, S.F.; Liu, S.P. Effects of different water levels on cotton growth and water use through drip irrigation in an arid region with saline ground water of Northwest China. Agric. Water Manag. 2012, 109, 117–126. [Google Scholar] [CrossRef]
- Cetin, O.; Bilgel, L. Effects of different irrigation methods on shedding and yield of cotton. Agric. Water Manag. 2002, 54, 1–15. [Google Scholar] [CrossRef]
- Ibragimov, N.; Evett, S.R.; Esanbekov, Y.; Kamilov, B.S.; Mirzaev, L.; Lamers, J.P.A. Water use efficiency of irrigated cotton in Uzbekistan under drip and furrow irrigation. Agric. Water Manag. 2007, 90, 112–120. [Google Scholar] [CrossRef]
- Guan, H.J.; Li, J.S.; Li, Y.F. Effects of drip system uniformity and irrigation amount on cotton yield and quality under arid conditions. Agric. Water Manag. 2013, 124, 37–51. [Google Scholar] [CrossRef]
- Lv, G.H.; Kang, Y.H.; Li, L.; Wan, S.Q. Effect of irrigation methods on root development and profile soil water uptake in winter wheat. Irrig. Sci. 2010, 28, 387–398. [Google Scholar] [CrossRef]
- Salgado, E.; Cautín, R. Avocado root distribution in fine and coarse-textured soils under drip and microsprinkler irrigation. Agric. Water Manag. 2008, 95, 817–824. [Google Scholar] [CrossRef]
- Kage, H.; Kochler, M.; Stutzel, H. Root growth of cauliflower (Brassica oleracea L. botrytis) under unstressed conditions: Measurement and modelling. Plant Soil 2000, 223, 131–145. [Google Scholar]
- Jose, S.; Gillespie, A.R.; Seifert, J.R.; Pope, P.E. Comparison of minirhizotron and soil core methods for quantifying root biomass in a temperate alley cropping system. Agrofor. Syst. 2001, 52, 161–168. [Google Scholar] [CrossRef]
- Li, C.X.; Zhou, X.G.; Sun, J.S.; Wang, H.Z.; Gao, Y. Dynamics of root water uptake and water use efficiency under alternate partial root-zone irrigation. Desalin. Water Treat. 2014, 52, 2805–2810. [Google Scholar] [CrossRef]
- Bragg, P.L.; Govi, G.; Cannell, R.Q. A comparison of methods, including angled and vertical minirhizotrons, for studying root growth and distribution in a spring oat crop. Plant Soil 1983, 73, 435–440. [Google Scholar] [CrossRef]
- Johnson, M.G.; Tingey, D.T.; Phillips, D.L.; Storm, M.J. Advancing fine root research with minirhizotrons. Environ. Exp. Bot. 2001, 45, 263–289. [Google Scholar] [CrossRef]
- Li, C.X.; Sun, J.S.; Li, F.S.; Zhou, X.G.; Li, Z.Y.; Qiang, X.M.; Guo, D.D. Response of root morphology and distribution in maize to alternate furrow irrigation. Agric. Water Manag. 2011, 98, 1789–1798. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration–Guidelines for Computing Crop Water Requirements–FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Weitz, A.M.; Grauel, W.T.; Keller, M.; Veldkamp, E. Calibration of time domain reflectometry technique using undisturbed soil samples from humid tropical soils of volcanic origin. Water Resour. Res. 1997, 33, 1241–1249. [Google Scholar] [CrossRef]
- Richards, L.A. Capillary conduction of liguids through porous mediums. Physics 1931, 1, 318–333. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Allen, R.G.; Smith, M.; Wright, J.L.; Raes, D.; Pereira, L.S. FAO-56 Dual Crop Coefficient Method for Estimating Evaporation from Soil and Application Extensions. J. Irrig. Drain. Eng. 2005, 131, 2–13. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of field water use and crop yield. Soil Sci. 1982, 129, 193. [Google Scholar]
- Vrugt, J.A.; Hopmans, J.W.; Šimunek, J. Calibration of a Two-Dimensional Root Water Uptake Model. Fluid Phase Equilib. 2001, 65, 1027–1037. [Google Scholar] [CrossRef]
- Schaap, M.G.; Leij, F.J.; Genuchten, M.T.V. A computer program for estimating soil hydraulic parameters with hierarchical pedotransfer functions. J. Hydrol. 2001, 251, 163–176. [Google Scholar] [CrossRef]
- Forkutsa, I.; Sommer, R.; Shirokova, Y.I.; Lamers, J.P.A.; Kienzler, K.; Tischbein, B.; Martius, C.; Vlek, P.L.G. Modeling irrigated cotton with shallow groundwater in the Aral Sea Basin of Uzbekistan: II. Soil salinity dynamics. Irrig. Sci. 2009, 27, 319–330. [Google Scholar] [CrossRef]
- Paredes, P.; de Melo-Abreu, J.P.; Alves, I.; Pereira, L.S. Assessing the performance of the FAO AquaCrop model to estimate maize yields and water use under full and deficit irrigation with focus on model parameterization. Agric. Water Manag. 2014, 144, 81–97. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. Asabe 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Li, H.J.; Yi, J.; Zhang, J.G.; Zhao, Y.; Si, B.C.; Hill, R.L.; Cui, L.; Liu, X.Y. Modeling of Soil Water and Salt Dynamics and Its Effects on Root Water Uptake in Heihe Arid Wetland, Gansu, China. Water 2015, 7, 2382–2401. [Google Scholar] [CrossRef]
- Qu, L.Y.; Quoreshi, A.M.; Koike, T. Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant Soil 2003, 255, 293–302. [Google Scholar] [CrossRef]
- Lv, G.H.; Song, J.Q.; Bai, W.B.; Wu, Y.F.; Liu, Y.; Kang, Y.H. Effects of different irrigation methods on micro-environments and root distribution in winter wheat fields. J. Integr. Agric. 2015, 14, 1658–1672. [Google Scholar]
- Zaman, W.Z.; Arshad, M.; Saleem, A. Distribution of nitrate-nitrogen in the soil profile under different irrigation methods. Int. J. Agric. Biol. 2001, 3, 208–209. [Google Scholar]
- Home, P.G.; Panda, R.K.; Kar, S. Effect of method and scheduling of irrigation on water and nitrogen use efficiencies of Okra (Abelmoschus esculentus). Agric. Water Manag. 2002, 55, 159–170. [Google Scholar] [CrossRef]
- Yu, Y.X.; Zhao, C.Y.; Zhao, Z.M.; Yu, B.; Zhou, T.H. Soil respiration and the contribution of root respiration of cotton (Gossypium hirsutum L.) in arid region. Acta Ecol. Sin. 2015, 35, 17–21. [Google Scholar] [CrossRef]
- Elmaloglou, S.; Diamantopoulos, E. Simulation of soil water dynamics under subsurface drip irrigation from line sources. Water Resour. Manag. 2013, 27, 4131–4148. [Google Scholar] [CrossRef]





| Depth (cm) | Soil Texture a | Particle Size Distribution (g 100 g−1) | Bulk Density (Mg m−3) | Field Capacity (cm3 cm−3) | ||
|---|---|---|---|---|---|---|
| <0.002 mm | 0.02–0.002 mm | 2.0–0.02 mm | ||||
| 0–20 | loam | 4 | 43 | 53 | 1.56 | 0.34 |
| 20–40 | silt loam | 7 | 45 | 48 | 1.58 | 0.31 |
| 40–60 | silt loam | 6 | 48 | 46 | 1.54 | 0.33 |
| 60–80 | silt loam | 5 | 47 | 48 | 1.42 | 0.28 |
| 80–100 | sandy loam | 2 | 17 | 81 | 1.45 | 0.29 |
| Practices | 2013 | 2014 | 2015 |
|---|---|---|---|
| Sowing | 11 May | 6 May | 6 May |
| Transplantation | 13 June | 6 June | 10 June |
| Fertilizer application | 13 June 25 June | 6 June 7 July | 10 June 8 July |
| Spraying of pesticides | 15 July 2 August | 30 June 25 July 5 August | 1 July 30 July |
| Hand cutting of plant growth | 30 July | 25 July | 25 July |
| Spraying of mepiquat chloride | 25 July 6 August | 27 July 5 August | 27 July 6 August |
| Pruning | 30 July 13 August | 23 July 6 August | 24 July 7 August |
| Harvest | 4 October 20 October | 26 September 13 October | 27 September 15 October |
| Irrigation Method | Design Specifications | Field Surface Slope | Irrigation Flow | Equipment Specifications |
|---|---|---|---|---|
| BI | Border width, 2.1 m; border length, 50 m; 3 rows of cotton per plot | 0.002 | Flow per unit width into the furrow, 4 L s−1 m−1 | - |
| SDI | Laying length, 25 m; tube spacing, 0.7 m | - | Emitter flow, 2.0 L h−1 | Inter-tube dripper with 16 mm diameter; emitters 0.2 m apart; working pressure of 0.1 MPa |
| Depth (cm) | (cm3 cm−3) | (cm3 cm−3) | (cm3 Day−1) | n | l | |
|---|---|---|---|---|---|---|
| 0~20 | 0.127 | 0.467 | 27.6 | 0.012 | 1.54 | 0.5 |
| 20~40 | 0.096 | 0.417 | 9.7 | 0.017 | 1.31 | 0.5 |
| 40~60 | 0.095 | 0.424 | 4.7 | 0.022 | 1.21 | 0.5 |
| 60~80 | 0.107 | 0.487 | 12.6 | 0.009 | 1.46 | 0.5 |
| 80~100 | 0.066 | 0.501 | 84.7 | 0.012 | 1.88 | 0.5 |
| Soil Depth (cm) | BI | SDI | ||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2013 | 2014 | 2015 | |
| 0–10 | 0.47 b | 0.30 c | 0.44 bc | 0.67 a | 0.47 b | 0.64 a |
| 10–20 | 0.44 b | 0.31 c | 0.37 bc | 0.58 a | 0.40 b | 0.45 a |
| 20–30 | 0.40 bc | 0.26 d | 0.29 cd | 0.55 a | 0.37 bcd | 0.43 ab |
| 30–40 | 0.34 a | 0.16 b | 0.20 b | 0.38 a | 0.17 b | 0.13 b |
| 40–50 | 0.29 a | 0.14 b | 0.13 b | 0.31 a | 0.07 b | 0.06 b |
| 50–60 | 0.25 a | 0.06 bc | 0.09 b | 0.11 b | 0.01 c | - |
| 60–70 | 0.12 a | - | 0.03 b | - | - | - |
| Soil Depth (cm) | BI | SDI | ||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2013 | 2014 | 2015 | |
| 0–10 | 0.082 d | 0.108 c | 0.129 c | 0.111 c | 0.191 a | 0.164 b |
| 10–20 | 0.064 cd | 0.056 d | 0.066 bcd | 0.087 b | 0.123 a | 0.079 bc |
| 20–30 | 0.044 a | 0.087 a | 0.038 a | 0.054 a | 0.050 a | 0.065 a |
| 30–40 | 0.037 a | 0.023 ab | 0.028ab | 0.036 a | 0.024 ab | 0.021 b |
| 40–50 | 0.032 a | 0.024 ab | 0.021 bc | 0.024 b | 0.014 c | 0.015 c |
| 50–60 | 0.030 a | 0.026 ab | 0.017 abc | 0.012 bcd | 0.005 cd | - |
| 60–70 | 0.015 a | - | 0.008 b | - | - | - |
| Growth Period | Irrigation Method | px | pz | x* | z* |
|---|---|---|---|---|---|
| Seedling stage | BI | 1.03 | 1.39 | 10 | 12.73 |
| SDI | 0.02 | 0.03 | 5 | 2.12 | |
| Squaring stage | BI | 1.019 | 1.39 | 10 | 17.68 |
| SDI | 0.04 | 0.09 | 5 | 4.95 | |
| Bloom and boll-forming stage | BI | 0.719 | 1.39 | 15 | 21.21 |
| SDI | 0.11 | 0.21 | 5 | 5.66 | |
| Boll opening stage | BI | 1.019 | 1.59 | 15 | 24.04 |
| SDI | 0.22 | 0.35 | 5 | 6.36 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, H.; Sun, C.; Gao, Y.; Gong, X.; Sun, J.; Wang, W. Root Development of Transplanted Cotton and Simulation of Soil Water Movement under Different Irrigation Methods. Water 2017, 9, 503. https://doi.org/10.3390/w9070503
Zhang H, Liu H, Sun C, Gao Y, Gong X, Sun J, Wang W. Root Development of Transplanted Cotton and Simulation of Soil Water Movement under Different Irrigation Methods. Water. 2017; 9(7):503. https://doi.org/10.3390/w9070503
Chicago/Turabian StyleZhang, Hao, Hao Liu, Chitao Sun, Yang Gao, Xuewen Gong, Jingsheng Sun, and Wanning Wang. 2017. "Root Development of Transplanted Cotton and Simulation of Soil Water Movement under Different Irrigation Methods" Water 9, no. 7: 503. https://doi.org/10.3390/w9070503





