Stress and Welfare of African Catfish (Clarias gariepinus Burchell, 1822) in a Coupled Aquaponic System
Abstract
:1. Introduction
2. Materials and Methods
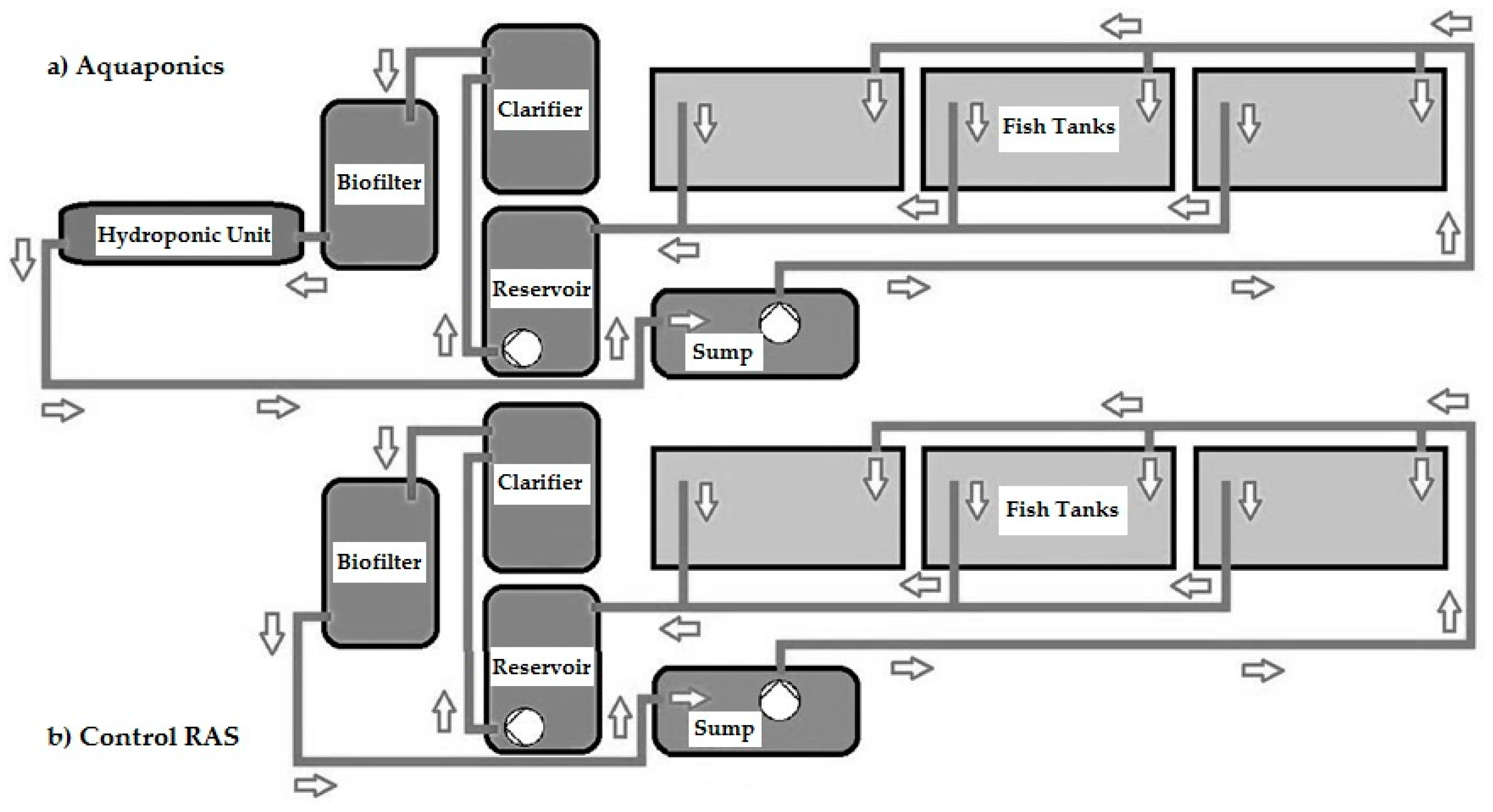
2.1. Experimental Systems
2.2. Process Water
2.3. Hydroponics
LA: Leaf area; L: Leaf length; W: Leaf width
2.4. Fish Cultivation
2.5. Sampling and Data Analyses
2.6. Statistical Analyses
3. Results
3.1. System Characteristics and Water Quality
3.2. Plant Growth
3.3. Fish Growth
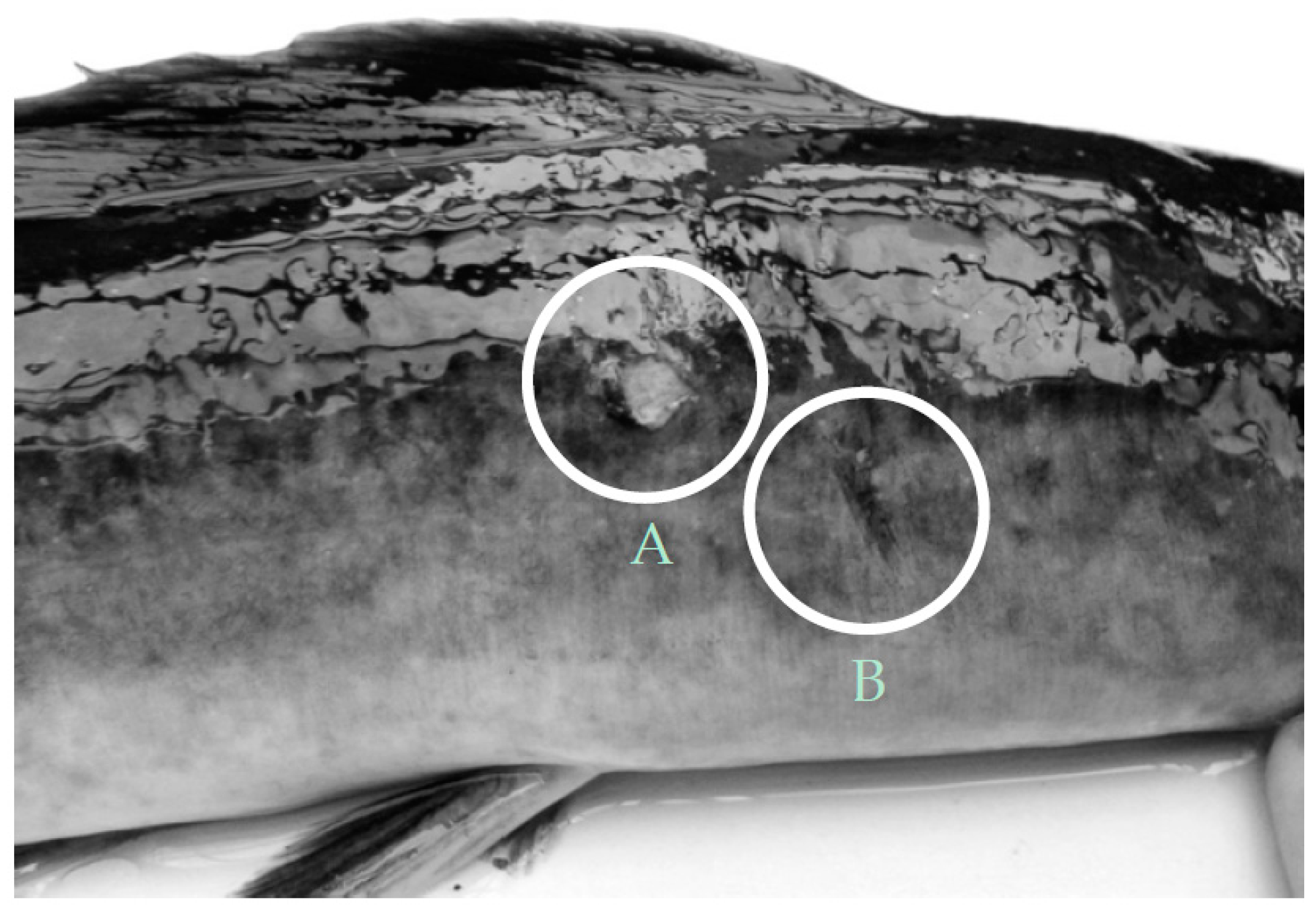
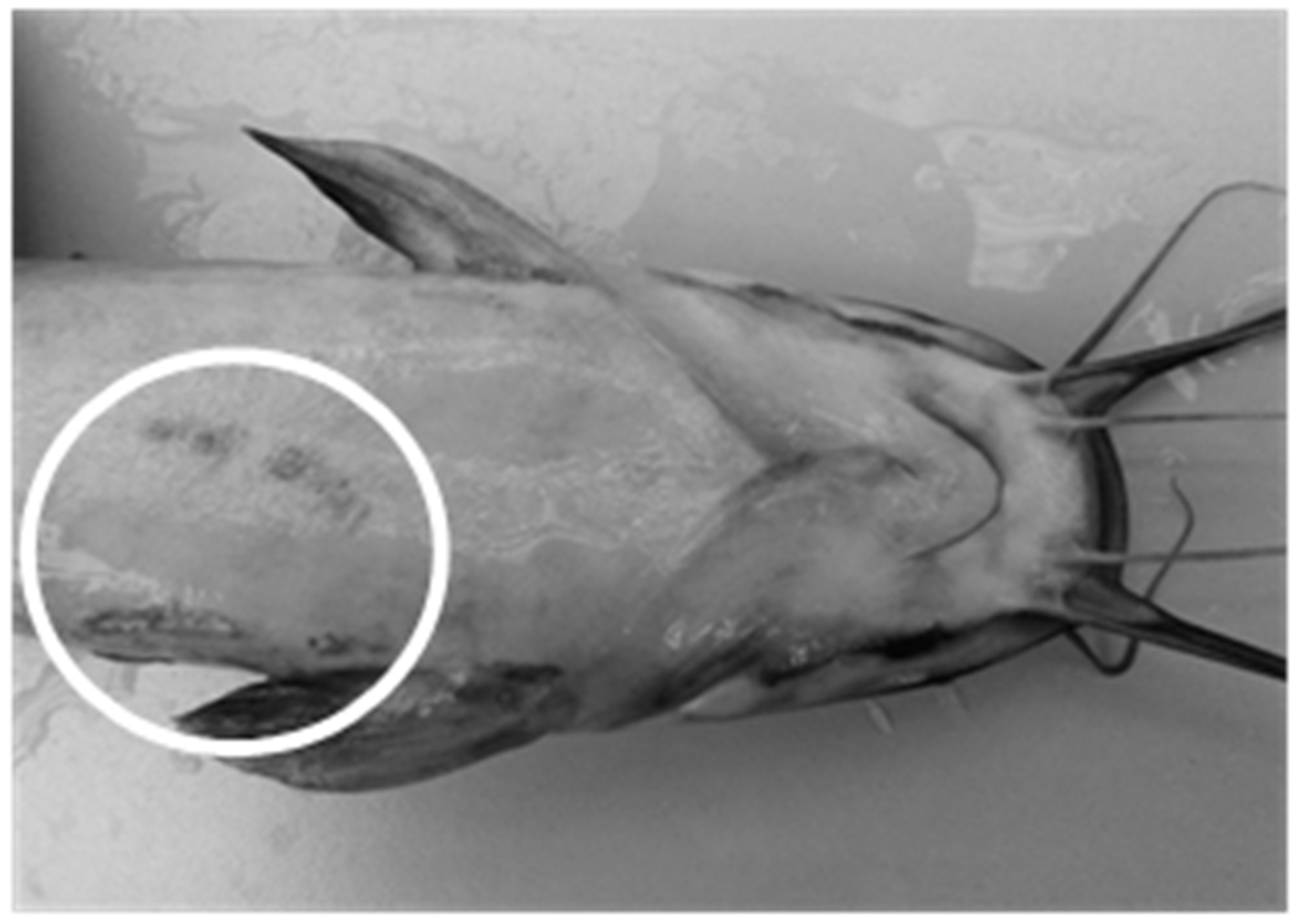
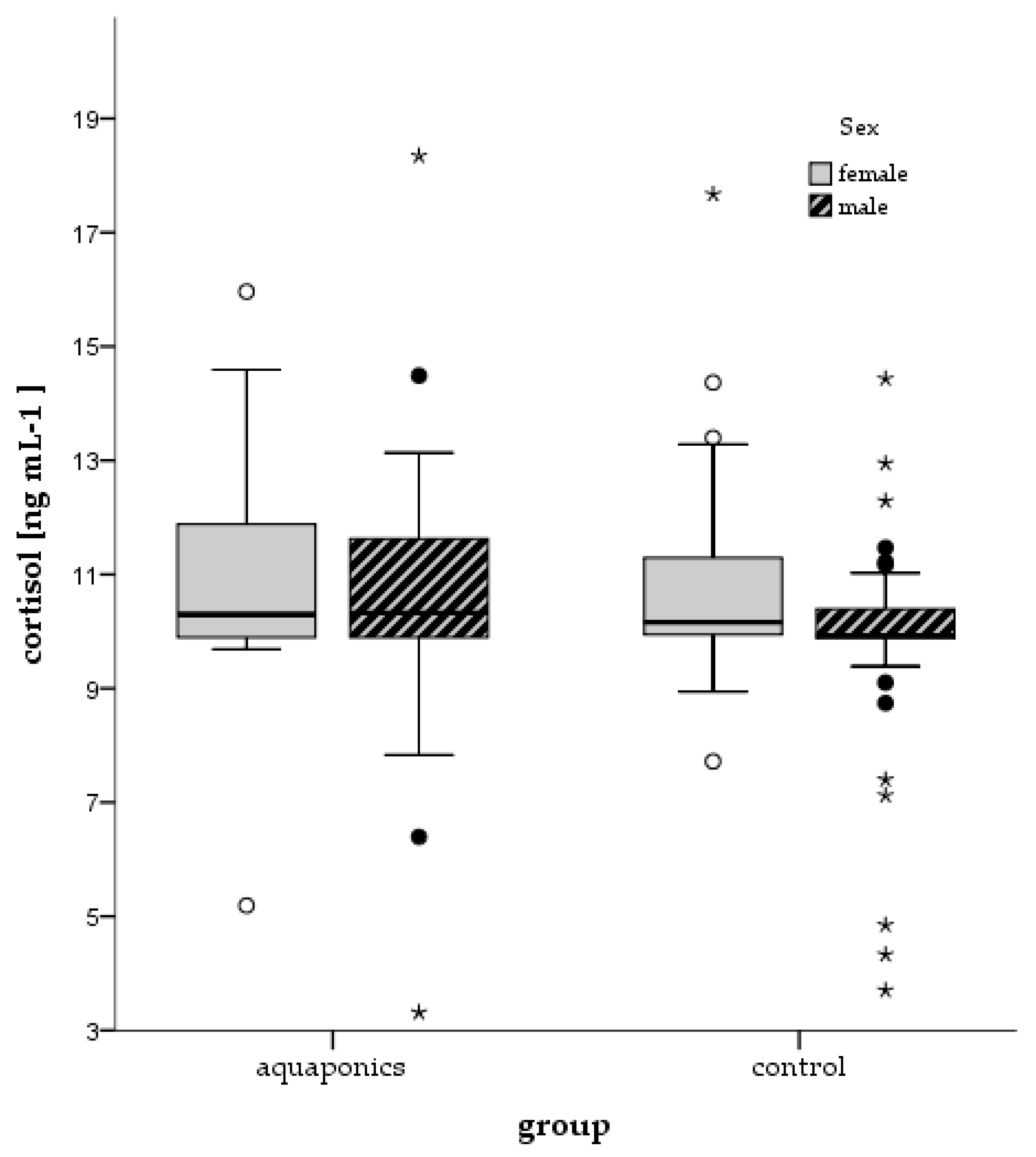
3.4. Stress Responses
4. Discussion
4.1. Water Quality
4.2. Plant Production
4.3. Fish Production
4.4. Stress and Welfare Aspects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Supplementary Data
| System | Parameter | Sampling Days 6–13 | Sampling Days 40–42 | Sampling Days 68–74 | Final Sampling on Day 87 |
|---|---|---|---|---|---|
| Control | Cortisol (ng mL−1) | 10.04 ± 0.05 | 12.17 ± 0.43 | 9.33 ± 0.30 | 14.77 ± 0.60 |
| Aquaponics | 10.17 ± 0.10 | 12.51 ± 0.31 | 10.12 ± 0.38 | 12.69 ± 0.42 | |
| Control | Glucose (mmol L−1) | 2.00 ± 0.14 | 2.51 ± 0.18 | 2.78 ± 0.09 | 3.16 ± 0.10 |
| Aquaponics | 2.27 ± 0.12 | 2.49 ± 0.13 | 2.75 ± 0.23 | 3.16 ± 0.14 | |
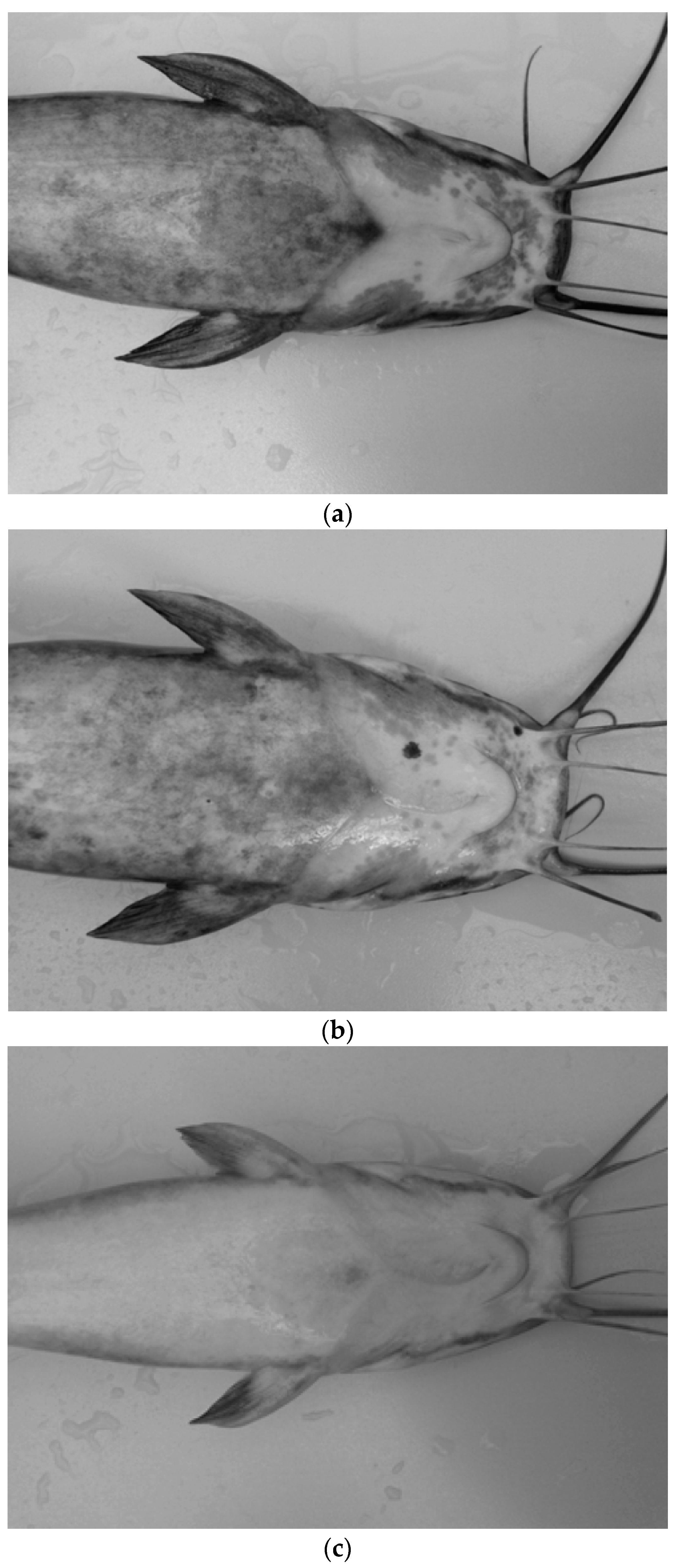
| Control | External injuries | 1.07 ± 0.32 | 2.35 ± 0.63 | 0.75 ± 0.17 | 0.98 ± 0.17 |
| Aquaponics | 1.53 ± 0.79 | 1.00 ± 0.23 | 0.36 ± 0.16 | 0.22 ± 0.06 |
References
- United Nations 2013. Department of Economic and Social Affairs, Population Division. Available online: http://www.unpopulation.org (assessed on 3 April 2017).
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalin. Water Treat. 2011, 32, 422–430. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; SRAC Publication: Stoneville, MS, USA, 2006; No. 454; pp. 1–16. [Google Scholar]
- FAO 2015. Food and Agriculture Organization of the United Nations, Cultured Aquatic Species Information Programme Clarias gariepinus. Available online: http://www.fao.org (assessed on 15 December 2015).
- FAO 2015. Food and Agriculture Organization of the United Nations, World Inventory of Fisheries, Impacts of Fishery Activities, Issues Fact Sheets. Available online: www.fao.org (assessed on 15 December 2015).
- Ip, Y.K.; Chew, S.F.; Wilson, J.M.; Randall, D.J. Defences against ammonia toxicity in tropical air-breathing fishes exposed to high concentrations of environmental ammonia: A review. J. Comp. Physiol. B 2004, 174, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.K.; Zubaidah, R.M.; Liew, P.C.; Loong, A.M.; Hiong, K.C.; Wong, W.P.; Chew, S.F. African sharptooth catfish Clarias gariepinus does not detoxify ammonia to urea or amino acids but actively excretes ammonia during exposure to environmental ammonia. Physiol. Biochem. Zool. 2004, 77, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Schram, E.; Roques, J.A.; Abbink, W.; Spanings, T.; De Vries, P.; Bierman, S.; van de Vis, H.; Flik, G. The impact of elevated water ammonia concentration on physiology, growth and feed intake of African catfish (Clarias gariepinus). Aquaculture 2010, 306, 108–115. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Chandroo, K.P.; Duncan, I.J.; Moccia, R.D. Can fish suffer?: Perspectives on sentience, pain, fear and stress. Appl. Anim. Behav. Sci. 2004, 86, 225–250. [Google Scholar] [CrossRef]
- Braithwaite, V.A.; Boulcott, P. Can Fish Suffer? Fish Welfare; Blackwell Scientific Publications: Oxford, UK, 2008; pp. 78–92. [Google Scholar]
- Braithwaite, V.A.; Huntingford, F.A. Fish and welfare: Do fish have the capacity for pain perception and suffering? Anim. Welf. 2004, 13, 87–92. [Google Scholar]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Barber, I. Parasites, Behaviour and welfare in fish. Appl. Anim. Behav. Sci. 2007, 104, 251–264. [Google Scholar] [CrossRef]
- Van de Nieuwegiessen, P.G.; Boerlage, A.S.; Verreth, J.A.; Schrama, J.W. Assessing the effects of a chronic stressor, stocking density, on welfare indicators of juvenile African catfish, Clarias gariepinus Burchell. Appl. Anim. Behav. Sci. 2008, 115, 233–243. [Google Scholar] [CrossRef]
- Van de Nieuwegiessen, P.G.; Olwo, J.; Khong, S.; Verreth, J.A.; Schrama, J.W. Effects of age and stocking density on the welfare of African catfish, Clarias gariepinus Burchell. Aquaculture 2009, 288, 69–75. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Pankhurst, N.W. The endocrinology of stress in fish: An environmental perspective. Gen. Comp. Endocr. 2011, 170, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Flik, G.; Klaren, P.H.; Van den Burg, E.H.; Metz, J.R.; Huising, M.O. CRF and stress in fish. Gen. Comp. Endocr. 2006, 146, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bonga, S.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar]
- Wu, H.; Aoki, A.; Arimoto, T.; Nakano, T.; Ohnuki, H.; Murata, M.; Ren, H.; Endo, H. Fish stress become visible: A new attempt to use biosensor for real-time monitoring fish stress. Biosens. Bioelectr. 2015, 67, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef]
- Migaud, H.; Cowan, M.; Taylor, J.; Ferguson, H.W. The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 2007, 270, 390–404. [Google Scholar] [CrossRef]
- Oppedal, F.; Juell, J.E.; Johansson, D. Thermo-and photoregulatory swimming behaviour of caged Atlantic salmon: Implications for photoperiod management and fish welfare. Aquaculture 2007, 265, 70–81. [Google Scholar] [CrossRef]
- Grewal, H.S.; Maheshwari, B.; Parks, S.E. Water and nutrient use efficiency of a low-cost hydroponic greenhouse for a cucumber crop: An Australian case study. Agric. Water Manag. 2011, 98, 841–846. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Oh, S.; Oh, M.M.; Son, J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic. 2007, 111, 330–334. [Google Scholar] [CrossRef]
- Hickey, G.M. Wound healing in fish larvae. J. Exp. Mar. Biol. Ecol. 1982, 57, 149–168. [Google Scholar] [CrossRef]
- Bovendeur, J.; Eding, E.H.; Henken, A.M. Design and performance of a water recirculation system for high-density culture of the African catfish, Clarias gariepinus (Burchell 1822). Aquaculture 1987, 63, 329–353. [Google Scholar] [CrossRef]
- Akinwole, A.O.; Faturoti, E.O. Biological performance of African Catfish (Clarias gariepinus) cultured in recirculating system in Ibadan. Aquac. Eng. 2007, 36, 18–23. [Google Scholar] [CrossRef]
- Eding, E.; Kamstra, A. Design and performance of recirculation systems for European eel Anguilla anguilla and African catfish Clarias gariepinus. In Proceedings of the AES Workshop, Orlando, FL, USA, 23 January 2001; pp. 18–28. [Google Scholar]
- Masser, M.P.; Rakocy, J.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Management of Recirculating Systems; SRAC Publication: Stoneville, MS, USA, 1999; p. 452. [Google Scholar]
- Losordo, T.M.; Masser, M.P.; Rakocy, J. Recirculating Aquaculture Tank Production Systems: Overview of Critical Considerations; SRAC Publication: Stoneville, MS, USA, 1998; p. 451. [Google Scholar]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Gislerød, H.R.; Kempton, R.J. The oxygen content of flowing nutrient solutions used for cucumber and tomato culture. Sci. Hortic. 1983, 20, 23–33. [Google Scholar] [CrossRef]
- Zeroni, M.; Gale, J.; Ben-Asher, J. Root aeration in a deep hydroponic system and its effect on growth and yield of tomato. Sci. Hortic. 1983, 19, 213–220. [Google Scholar] [CrossRef]
- Palm, H.W.; Bissa, K.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic systems. Part II: Fish and plant growth. AACL Bioflux 2014, 7, 162–175. [Google Scholar]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immun. 2008, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Shingles, A.; McKenzie, D.J.; Claireaux, G.; Domenici, P. Reflex Cardioventilatory Responses to Hypoxia in the Flathead Gray Mullet (Mugil cephalus) and Their Behavioral Modulation by Perceived Threat of Predation and Water Turbidity. Physiol. Biochem. Zool. 2005, 78, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Rueda, P.; Schrama, J.W.; Verreth, J.A. Behavioural responses under different feeding methods and light regimes of the African catfish (Clarias gariepinus) juveniles. Aquaculture 2004, 231, 347–359. [Google Scholar] [CrossRef]
- Fatollahi, M.; Kasumyan, A.O. The study of sensory bases of the feeding behavior of the African catfish Clarias gariepinus (Clariidae, Siluriformes). J. Ichthyol. 2006, 46, 161–172. [Google Scholar] [CrossRef]
- Clayton, R.D.; Stevenson, T.L.; Summerfelt, R.C. Fin erosion in intensively cultured walleyes and hybrid walleyes. Progress. Fish-Cult. 1998, 60, 114–118. [Google Scholar] [CrossRef]
- Pickering, A.D. Growth and stress in fish production. Aquaculture 1993, 111, 51–63. [Google Scholar] [CrossRef]
- Cericato, L.; Neto, J.G.M.; Fagundes, M.; Kreutz, L.C.; Quevedo, R.M.; Finco, J.; da Rosa, J.G.; Koakoski, G.; Centenaro, L.; Pottker, E.; et al. Cortisol response to acute stress in jundiá Rhamdia quelen acutely exposed to sub-lethal concentrations of agrichemicals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Hosoya, S.; Johnson, S.C.; Afonso, L.O. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short-and long-term stress. Fish Shellfish Immun. 2008, 24, 194–204. [Google Scholar] [CrossRef] [PubMed]

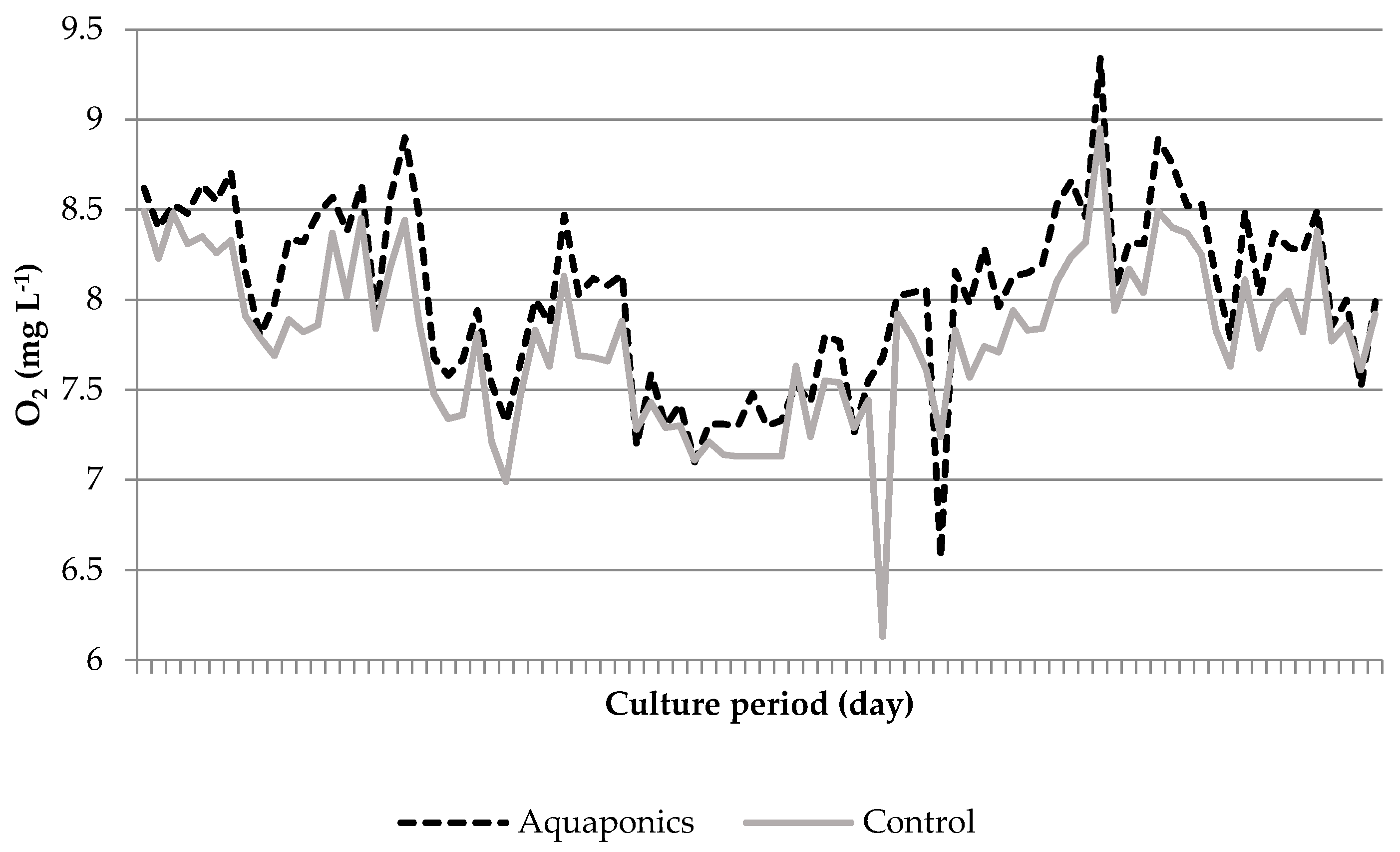
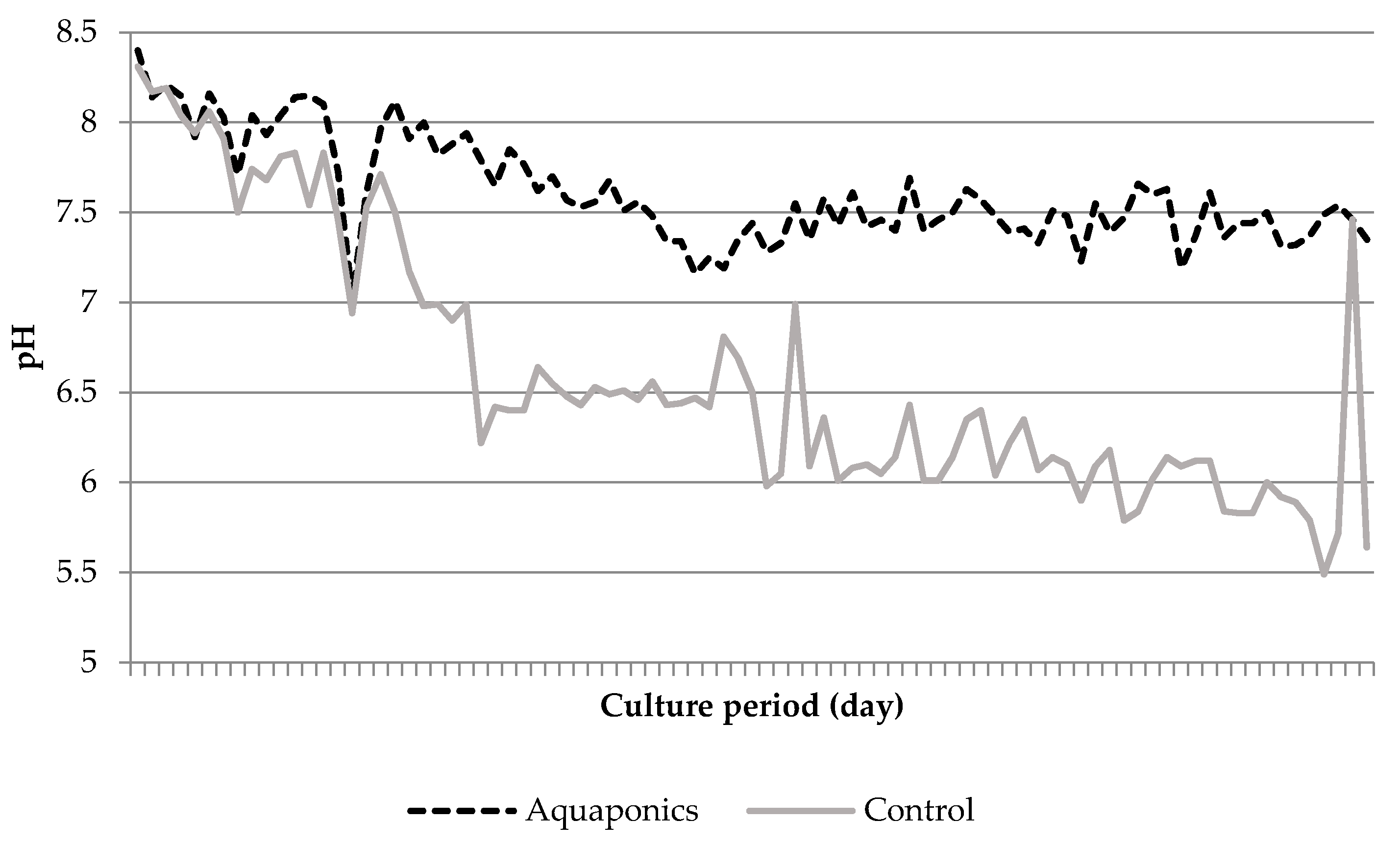
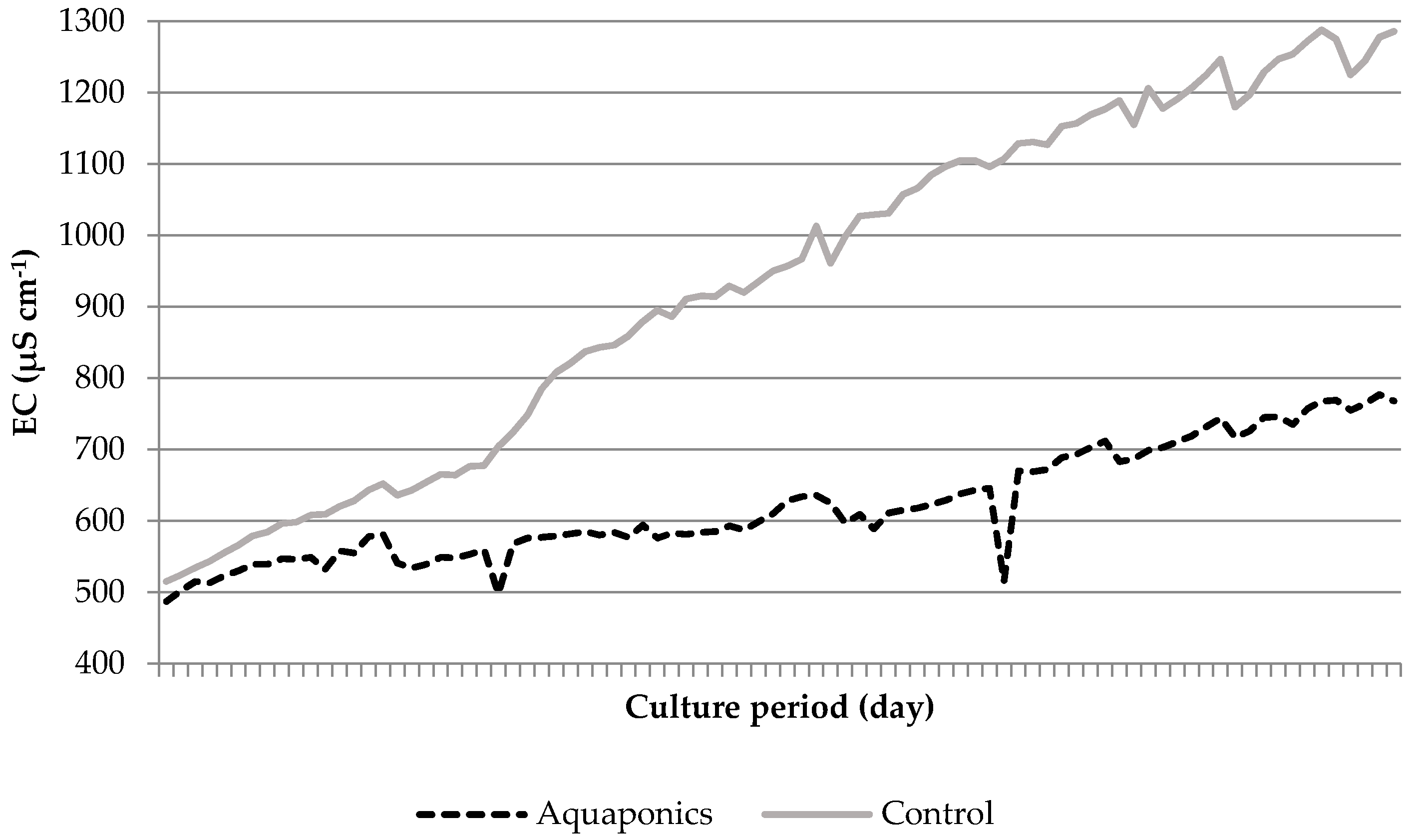


| System | DO (mg L−1) | O2 (%) | pH | Temperature (°C) | EC (μS cm−1) | TAN (mg L−1) | Nitrite-N (mg L−1) | Nitrate-N (mg L−1) |
|---|---|---|---|---|---|---|---|---|
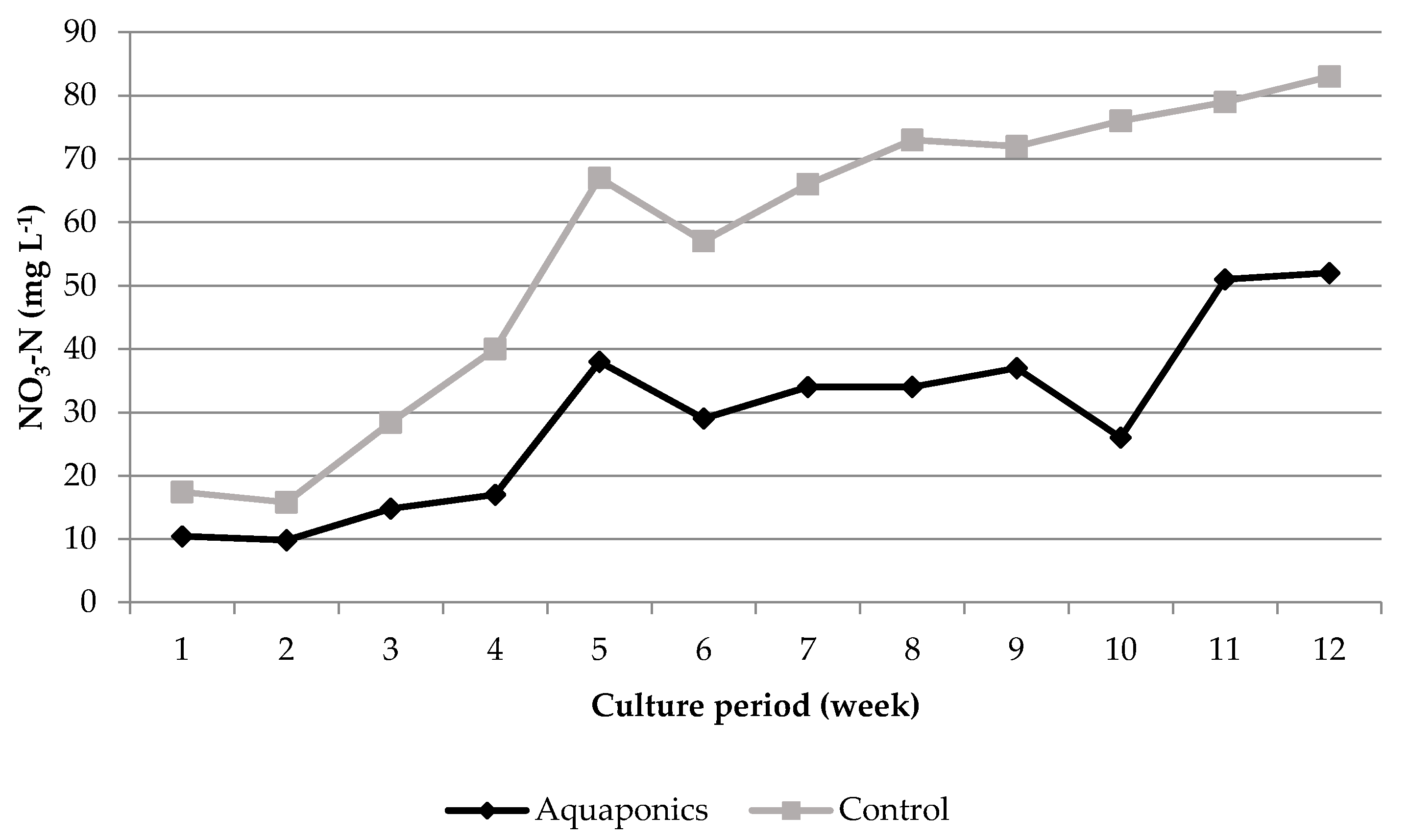
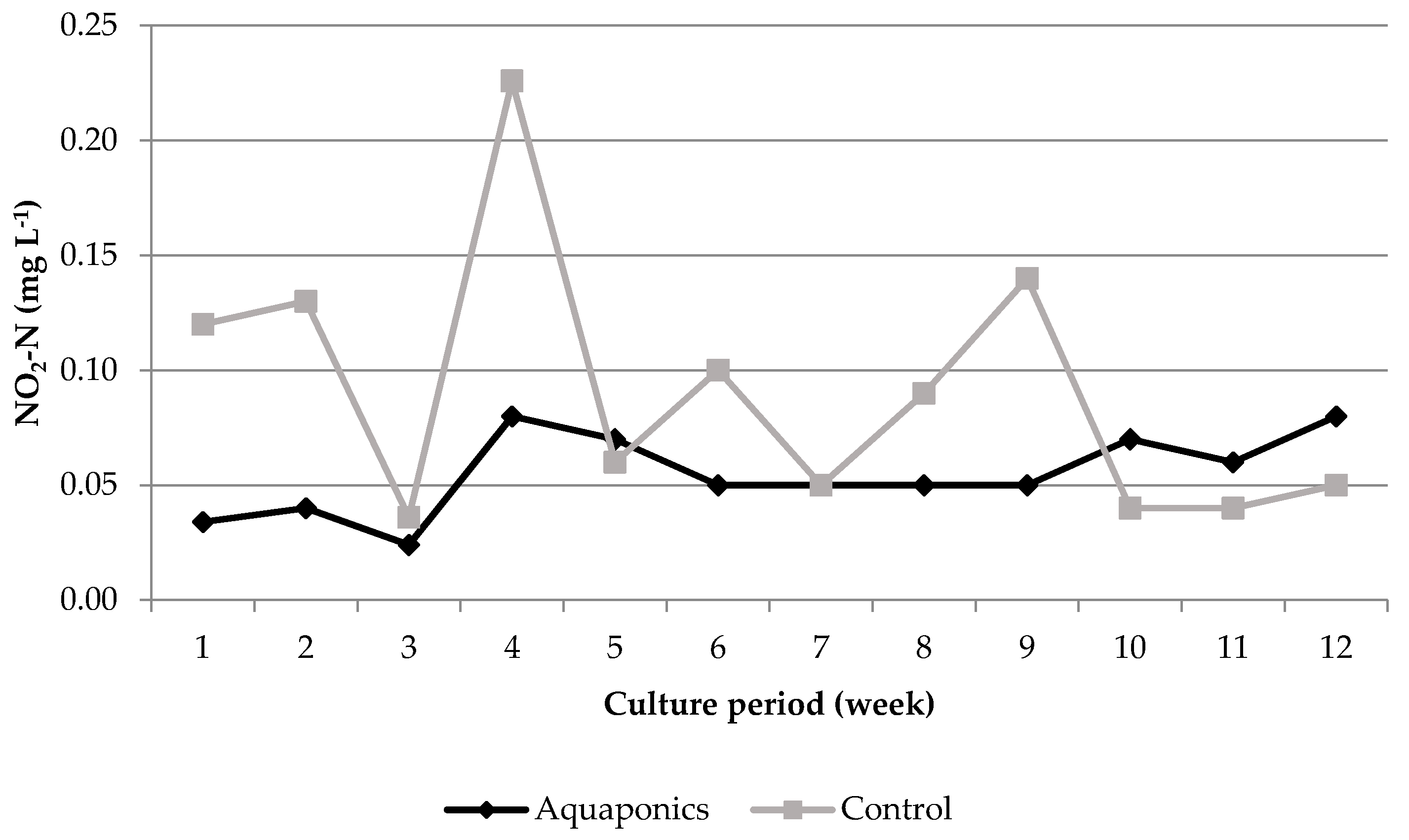
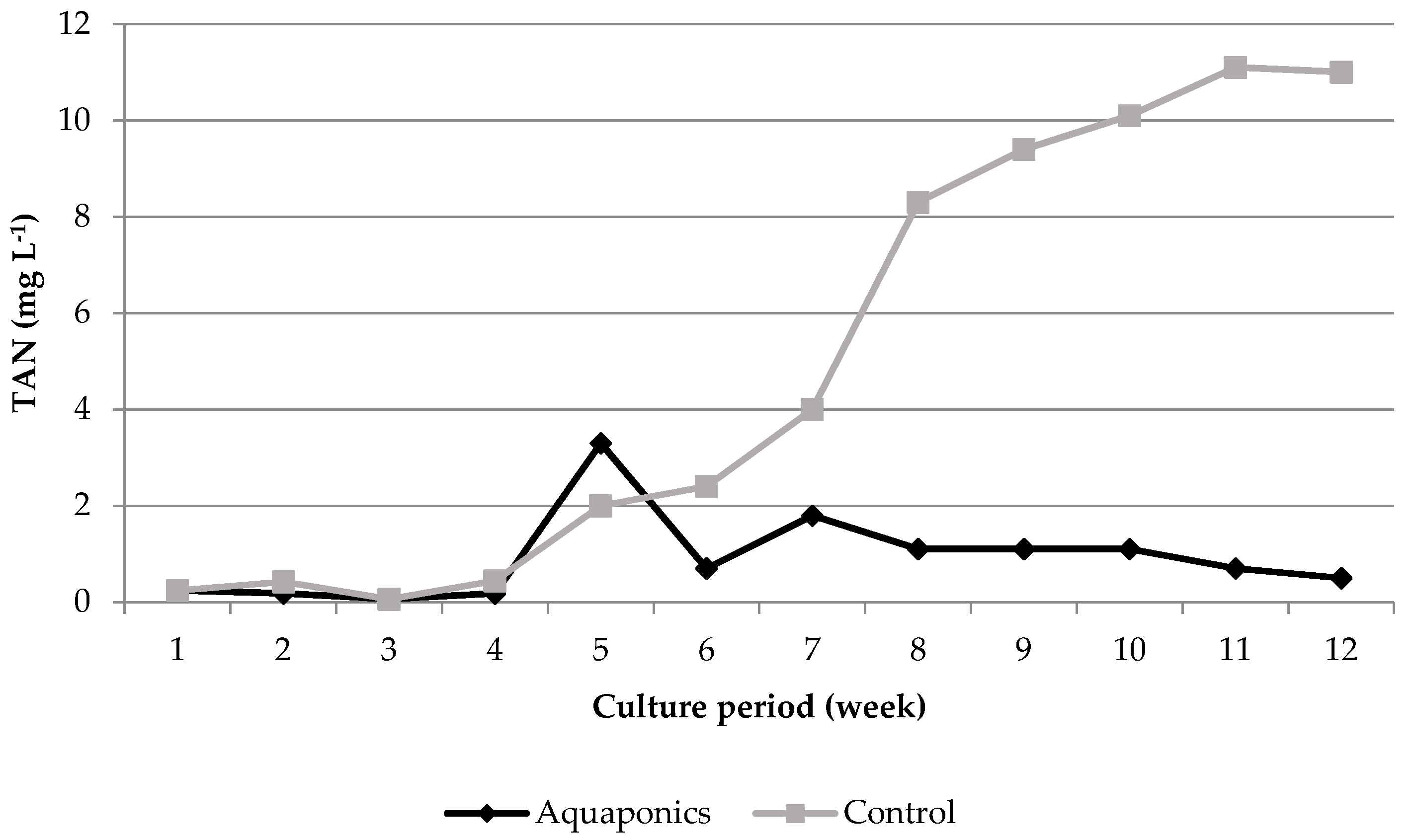
| Aquaponics | 8.04 * ± 0.05 | 95.87 * ± 0.25 | 7.61 * ± 0.03 | 24.21 ± 0.29 | 620.74 * ± 8.50 | 0.91 * ± 0.26 | 0.06 ± 0.01 | 29.42 * ± 4.15 |
| Control | 7.79 * ± 0.05 | 93.53 * ± 0.31 | 6.60 * ± 0.07 | 24.50 ± 0.51 | 934.84 * ± 26.31 | 4.96 * ± 1.33 | 0.09 ± 0.02 | 56.22 * ± 7.04 |
| System | 320 nm | 450 nm | 600 nm | 750 nm |
|---|---|---|---|---|
| Aquaponics | 0.313 * ± 0.007 | 0.064 * ± 0.003 | 0.025 * ± 0.002 | 0.015 * ± 0.002 |
| Control | 0.561 * ± 0.001 | 0.112 * ± 0.002 | 0.036 * ± 0.002 | 0.020 * ± 0.002 |
| System | Initial Weight (g/Fish) a | Final Weight (g/Fish) a | Initial Length (cm/Fish) a | Final Length (cm/Fish) a | FCR b | SGR c | DGR (g/Fish/Day) d |
|---|---|---|---|---|---|---|---|
| Aquaponics | 214.42 ± 5.95 | 333.33 ± 8.61 | 31.45 ± 1.39 | 35.17 ± 1.78 | 1.02 | 0.48 | 1.37 |
| Control | 327.58 ± 9.10 | 34.91 ± 1.71 | 1.09 | 0.41 | 1.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baßmann, B.; Brenner, M.; Palm, H.W. Stress and Welfare of African Catfish (Clarias gariepinus Burchell, 1822) in a Coupled Aquaponic System. Water 2017, 9, 504. https://doi.org/10.3390/w9070504
Baßmann B, Brenner M, Palm HW. Stress and Welfare of African Catfish (Clarias gariepinus Burchell, 1822) in a Coupled Aquaponic System. Water. 2017; 9(7):504. https://doi.org/10.3390/w9070504
Chicago/Turabian StyleBaßmann, Björn, Matthias Brenner, and Harry W. Palm. 2017. "Stress and Welfare of African Catfish (Clarias gariepinus Burchell, 1822) in a Coupled Aquaponic System" Water 9, no. 7: 504. https://doi.org/10.3390/w9070504






