Spatial Variations in the Surface Water Chemistry of Subtropical Peatlands (Central China) Linked to Anthropogenic Pressures
Abstract
:1. Introduction
2. Materials and Methods
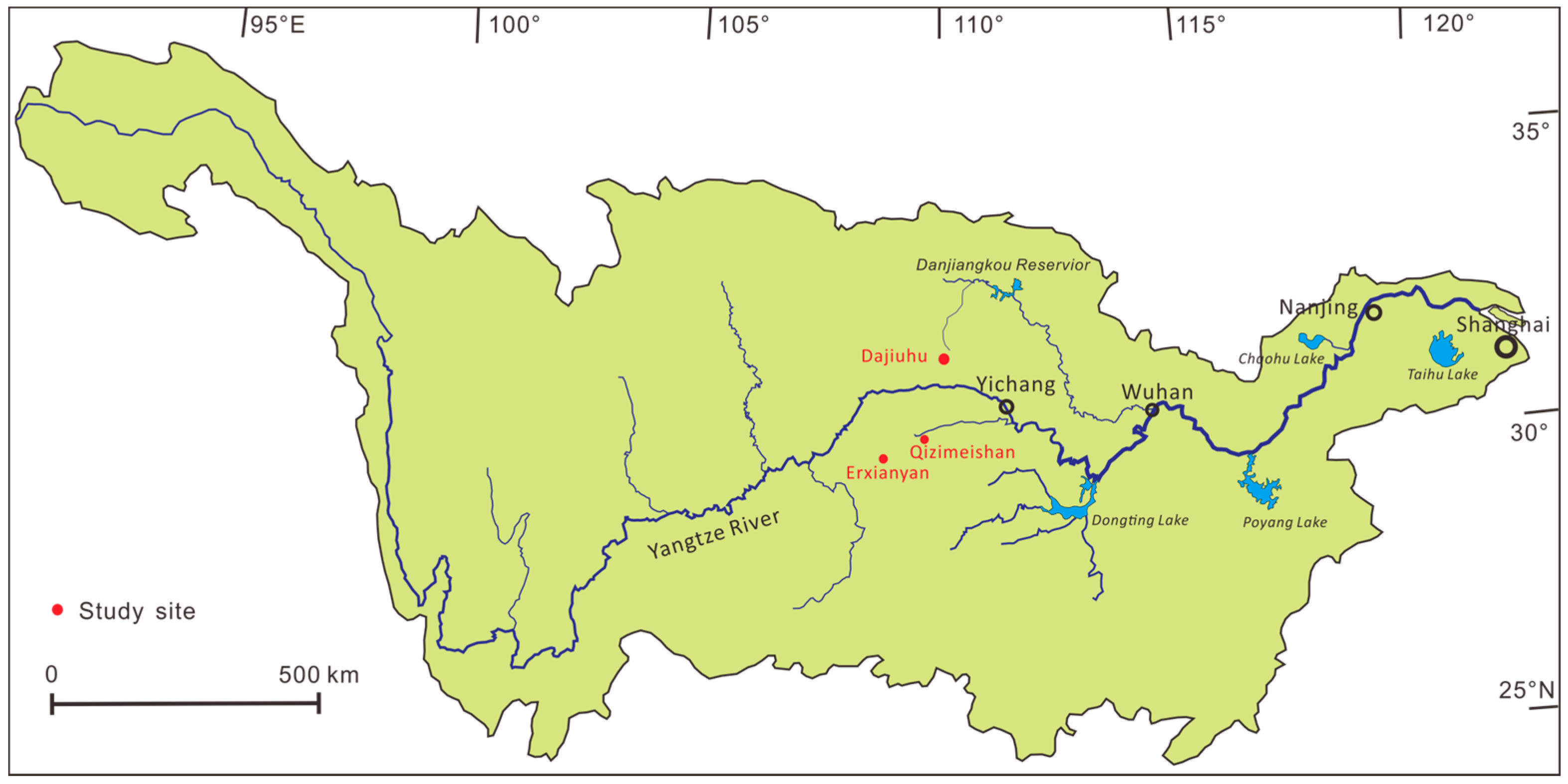
2.1. Site Description
2.2. Measurements and Sampling
2.3. Statistical Analysis
2.4. The Health Risk Analysis
2.5. Data Collection for Comparison
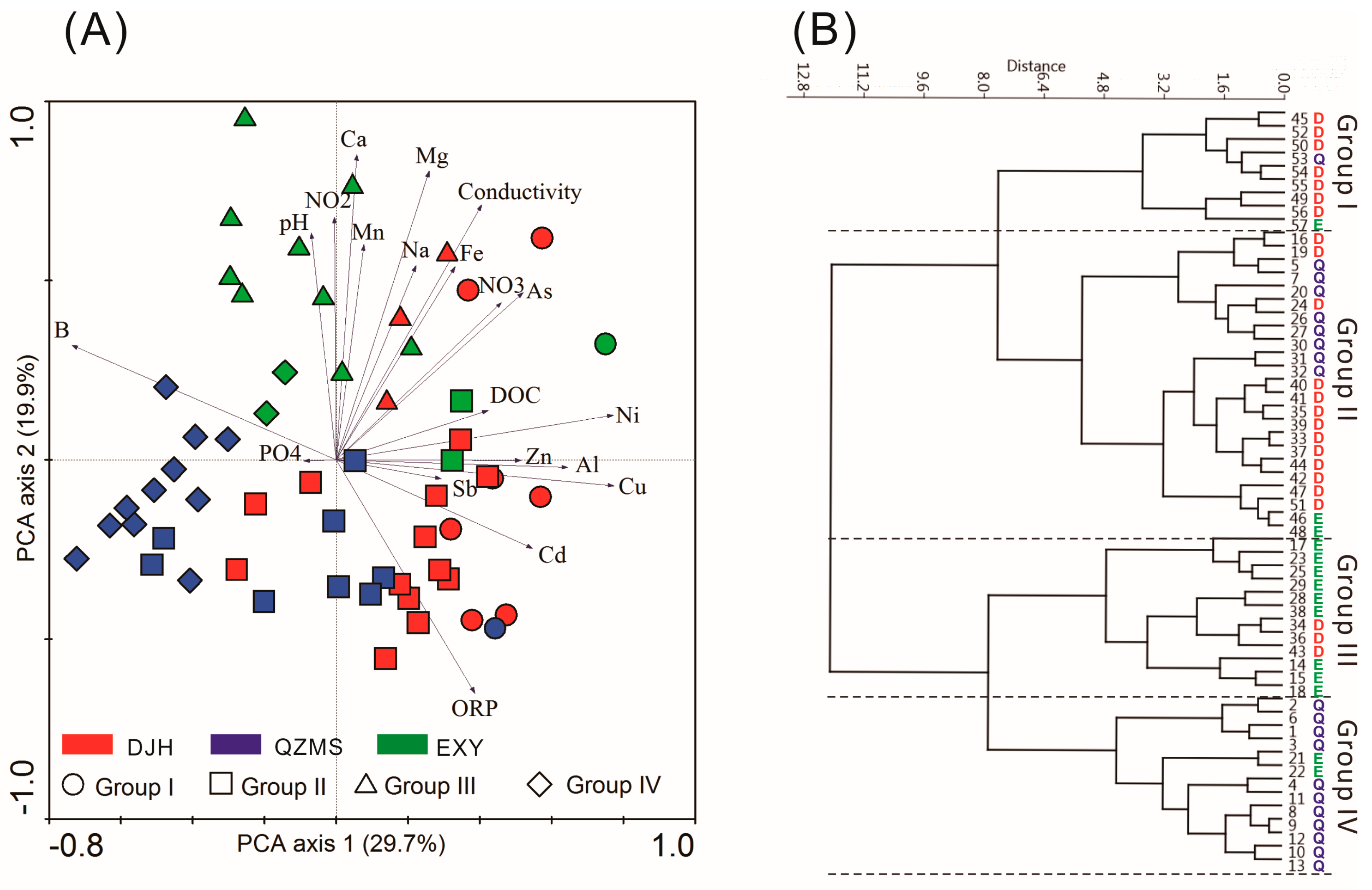
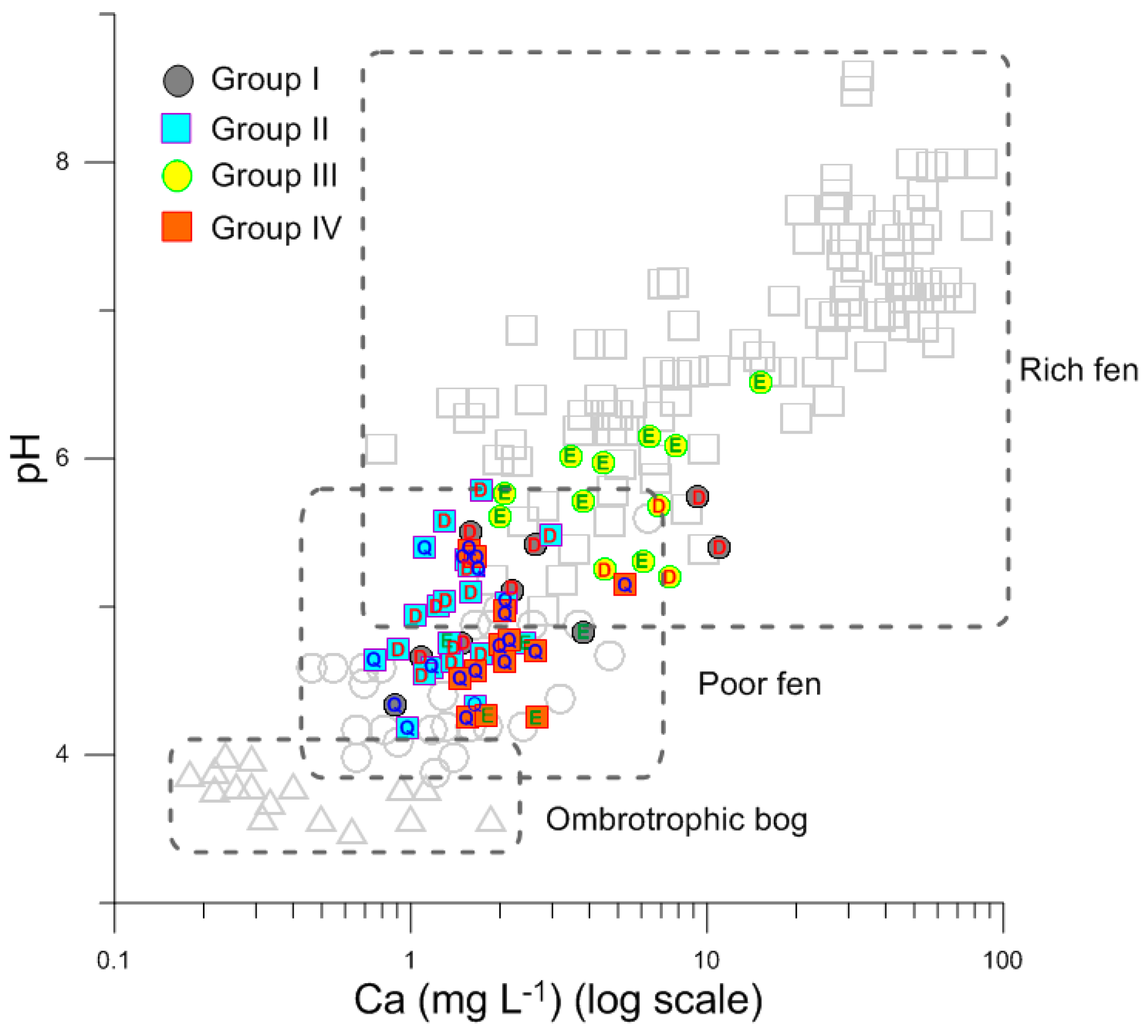
3. Results
4. Discussion
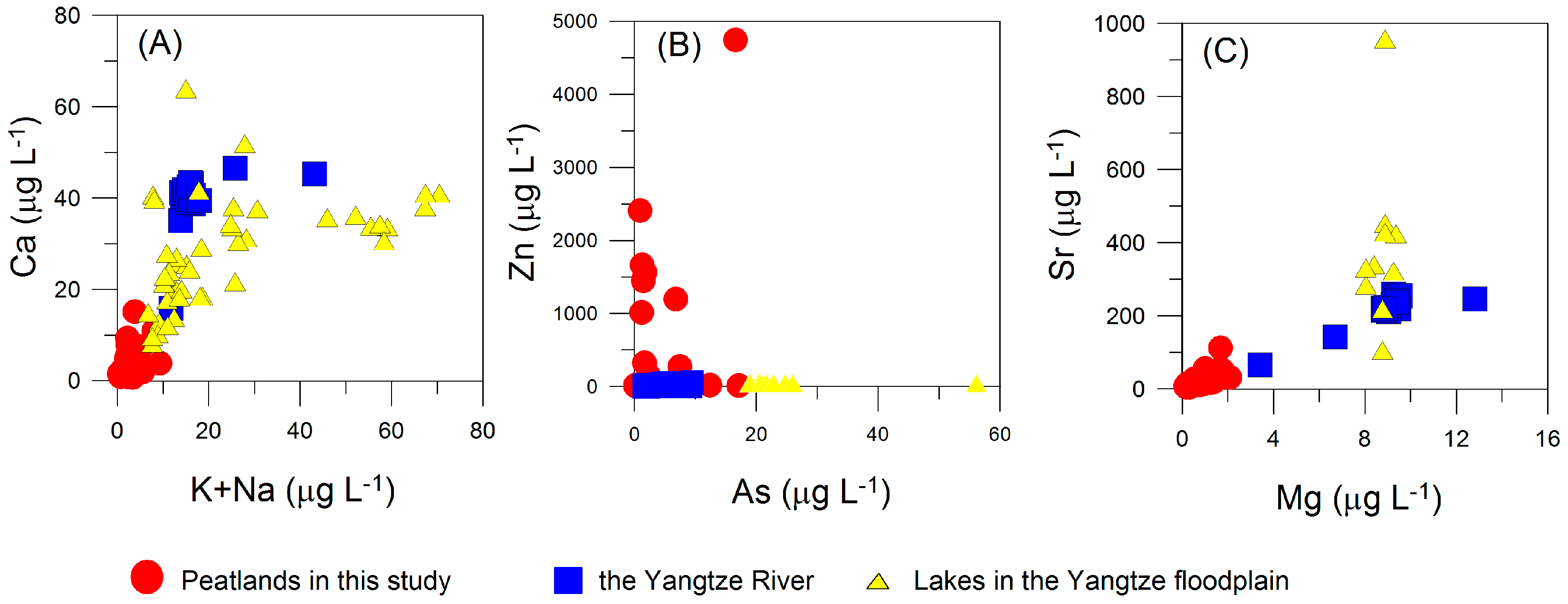
4.1. Generic Characteristics of Water Chemistry
4.2. Spatial Variations of Water Chemistry
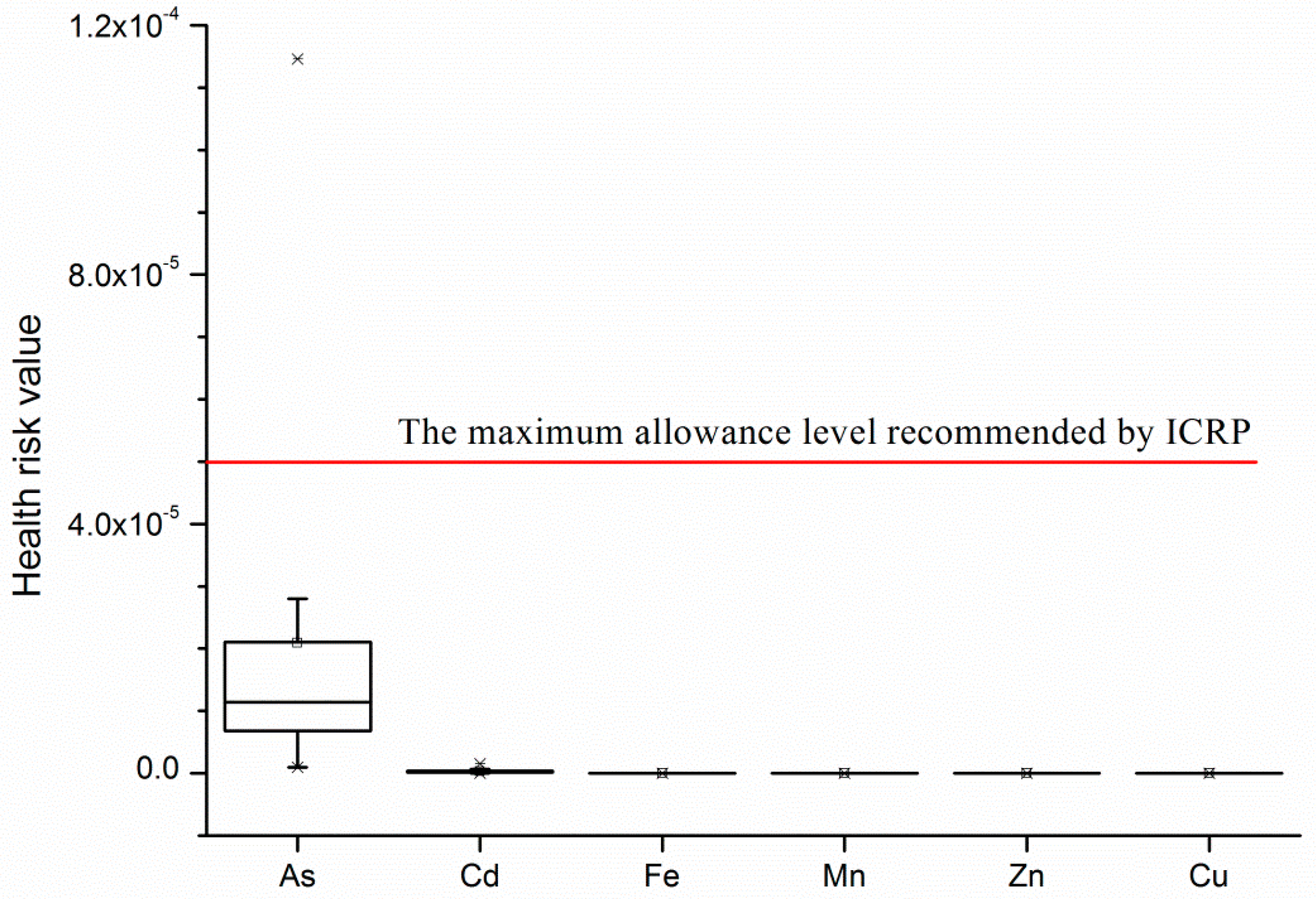
4.3. Health Risk
4.4. Conclusions and Implications for Peatland Conservation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martini, P.; Cortizas, A.M.; Chesworth, W. Peatlands: Evolution and Records of Environmental and Climate Changes (Vol. 9); Elsevier: Amsterdam, The Netherlands, 2007; pp. 17–51. [Google Scholar]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands; International Peat Society: Saarijärvi, Finland, 2002. [Google Scholar]
- Moore, P.D. The future of cool temperate Bogs. Environ. Conserv. 2002, 29, 3–20. [Google Scholar] [CrossRef]
- Sjörs, H.; Gunnarsson, U. Calcium and pH in north and central Swedish mire waters. J. Ecol. 2002, 90, 650–657. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Vitt, D.H.; Bayley, S.E.; Jin, T.L. Seasonal variation in water chemistry over a bog-rich fen gradient in Continental Western Canada. Can. J. Fish. Aquat. Sci. 1995, 52, 587–606. [Google Scholar] [CrossRef]
- Bourbonniere, R.A. Review of water chemistry research in natural and disturbed peatlands. Can. Water Resour. J. 2009, 34, 393–414. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, OH, USA, 2013. [Google Scholar]
- Chai, X. Peatland; Geological Publishing House: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Du, Y.; Cai, S.M.; Wang, X.L.; He, B.Y.; Xu, G.L.; Jiang, M.X.; Xue, H.P.; Xiao, F. Enivironmental background and ecological restoration of the Dajiuhu sub-alpine wetland in Mt. Shennongjia. Res. Environ. Yangtze Basin 2008, 17, 915–919. [Google Scholar]
- Zhao, S.; Li, E.; Cai, X.; Wang, X.; Jiang, L.; Yan, R. Research on the higher plant diversity of subalpine Sphagnum mire in western Hubei Province, China. Res. Environ. Yangtze Basin 2013, 22, 468–475. [Google Scholar]
- Pan, X.B.; He, Y.; Yan, M.; Ji, Z.H.; Li, T.; Yan, R.K.; Zhou, C.Z.; Wang, J.M. Analysis on current situation of hydrology and water resources and protection measures of Dajiu Lake in Shenlongjia. Hubei Agric. Sci. 2013, 52, 3033–3037. [Google Scholar] [CrossRef]
- Chen, X.; Qin, Y.; Stevenson, M.A.; McGowan, S. Diatom communities along pH and hydrological gradients in three montane mires, central China. Ecol. Indic. 2014, 45, 123–129. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Xiao, R.; Gao, H.; Liu, P. Spatial distribution of Fe, Cu, Mn in the surface water system and their effects on wetland vegetation in the Pearl River Estuary of China. Clean–Soil Air Water 2012, 40, 1085–1092. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, H. Heavy metal pollution in sediments from aquatic ecosystems in China. Clean-Soil Air Water 2013, 41, 878–882. [Google Scholar] [CrossRef]
- Shao, H.B.; Cui, B.S.; Bai, J.H. Wetland ecology in China. Clean-Soil Air Water 2012, 40, 1011–1014. [Google Scholar] [CrossRef]
- Cillero, C.; Díaz-Varela, R.A.; Rubinos, M.; Ramil-Rego, P. Assessment of anthropogenic pressures on South European Atlantic Bogs (NW Spain) based on hydrochemical data. Hydrobiologia 2016, 774, 137–154. [Google Scholar] [CrossRef]
- Wind-Mulder, H.L.; Vitt, D.H. Comparisons of water and peat chemistries of a post-harvested and undisturbed peatland with relevance to restoration. Wetlands 2000, 20, 616–628. [Google Scholar] [CrossRef]
- Santelmann, M.V.; Gorham, E. The influence of airborne road dust on the chemistry of Sphagnum mosses. J. Ecol. 1988, 76, 1219–1231. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The Global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv. Biol. 1998, 12, 502–515. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lei, Y.; Liu, S.X.; Fang, Y.P.; Man, J.S.; Peng, Z.L.; Zhang, L.; Ma, G.L. One subalpine sphagnum wetland being discovered in Qizimei Mountains Nature Reserve, Hubei. J. Cent. China Norm. Univ. (Nat. Sci.) 2005, 39, 387–388. [Google Scholar]
- Zhao, Y.; Yu, Z.; Tang, Y.; Li, H.; Yang, B.; Li, F.; Zhao, W.; Sun, J.; Chen, J.; Li, Q.; et al. Peatland initiation and carbon accumulation in China over the last 50,000 years. Earth-Sci. Rev. 2014, 128, 139–146. [Google Scholar] [CrossRef]
- Zeng, L.; Ning, D.; Xu, L.; Mao, X.; Chen, X. Sedimentary evidence of environmental degradation in Sanliqi Lake, Daye City (a typical mining city, central china). Bull. Environ. Contam. Toxicol. 2015, 95, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.E.; Billett, M.F.; Baird, A.J.; Chapman, P.J.; Dinsmore, K.J.; Holden, J. Regional variation in the biogeochemical and physical characteristics of natural peatland pools. Sci. Total Environ. 2016, 545, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Section on Permutation Methods; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Dean, J.F.; Billett, M.F.; Baxter, R.; Dinsmore, K.J.; Lessels, J.S.; Street, L.E.; Subke, J.-A.; Tetzlaff, D.; Washbourne, I.; Wookey, P.A. Biogeochemistry of “pristine” freshwater stream and lake systems in the western Canadian Arctic. Biogeochemistry 2016, 130, 191–213. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- USEPA. Quality Criteria for Water; US Department of Commerce, National Technical Information Service, United States Environmental Protection Agency (USEPA): Springfield, VA, USA, 1986.
- Müller, B.; Berg, M.; Yao, Z.P.; Zhang, X.F.; Wang, D.; Pfluger, A. How polluted is the Yangtze river? Water quality downstream from the Three Gorges Dam. Sci. Total Environ. 2008, 402, 232–247. [Google Scholar] [CrossRef]
- Liu, H.; Li, W. Dissolved trace elements and heavy metals from the shallow lakes in the middle and lower reaches of the Yangtze River region, China. Environ. Earth Sci. 2011, 62, 1503–1511. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Yu, H.; Ma, L.; Xu, L.; Qin, B. Water and sediment quality in lakes along the middle and lower reaches of the Yangtze River, China. Water Resour. Manag. 2012, 26, 3601–3618. [Google Scholar] [CrossRef]
- Mao, R.; Wang, Z.X.; Lei, Y.; Li, Z.Q.; Man, J.S.; Peng, Z.L. Profile characteristics and element vertical distribution in sphagnum wetland in Qizimei Mountains Nature Reserve, Hubei. Acta Pedol. Sin. 2009, 46, 159–163. [Google Scholar]
- Kalmykova, Y.; Strömvall, A.M.; Steenari, B.M. Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard. Mater. 2008, 152, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Shotyk, W. Review of the inorganic geochemistry of peats and peatland waters. Earth Sci. Rev. 1988, 25, 95–176. [Google Scholar] [CrossRef]
- Rothwell, J.J.; Taylor, K.G.; Ander, E.L.; Evans, M.G.; Daniels, S.M.; Allott, T.E.H. Arsenic retention and release in ombrotrophic peatlands. Sci. Total Environ. 2009, 407, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, B.; Wu, F. Spatial and temporal variations of Rb/Sr ratios of the bulk surface sediments in Lake Qinghai. Geochem. Trans. 2010, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, Y.; Tian, R.; Hu, H.; Chen, B.; Chen, L.K.; Xu, F. Modeling the purification effects of the constructed Sphagnum wetland on phosphorus and heavy metals in Dajiuhu Wetland Reserve, China. Ecol. Model. 2013, 252, 23–31. [Google Scholar] [CrossRef]
- Clymo, R.S.; Hayward, P.M. The Ecology of Sphagnum. In Bryophyte Ecology; Smith, A.J.E., Ed.; Springer: Berlin, Germany, 1982; pp. 229–289. [Google Scholar]
- Stone, E.L. Boron deficiency and excess in forest trees: A review. Forest Ecol. Manag. 1990, 37, 49–75. [Google Scholar] [CrossRef]
- Deng, F.; Li, R.D.; Wang, H.F. Dynamic changes of cultivated land in Hubei Province from 2000 to 2007. Resour. Environ. Yangtze Basin 2010, 19, 1171–1176. [Google Scholar]
| Variable/Unit | Group I | Group II | Group III | Group IV | All | p-Value |
|---|---|---|---|---|---|---|
| (n = 9) | (n = 23) | (n = 12) | (n = 13) | (n = 57) | ||
| pH | 5.08 ± 0.46 | 4.95 ± 0.41 | 5.77 ± 0.40 | 4.73 ± 0.39 | 5.09 ± 0.55 | <0.001 |
| Cond./μS cm−1 | 30.3 ± 12.9 | 21.2 ± 7.6 | 55.8 ± 24.1 | 16.3 ± 9.8 | 28.8 ± 19.9 | <0.001 |
| ORP/mV | 393.5 ± 40.5 | 390.4 ± 49.5 | 286.4 ± 70.8 | 347.0 ± 22.2 | 359.1 ± 63.6 | <0.001 |
| DOC/mg L−1 | 52.3 ± 39.7 | 41.5 ± 40.2 | 36.3 ± 21.6 | 29.0 ± 20.8 | 39.3 ± 33.2 | 0.45 |
| NH4+/mg L−1 | 0.95 ± 0.96 | 0.82 ± 0.69 | 1.71 ± 0.78 | 0.71 ± 0.54 | 1.00 ± 0.80 | <0.01 |
| NO2−/μg L−1 | 10.74 ± 9.53 | 6.54 ± 3.51 | 28.51 ± 46.22 | 7.45 ± 3.81 | 12.03 ± 22.72 | <0.01 |
| NO3−/μg L−1 | 1672 ± 933 | 1163 ± 769 | 1691 ± 834 | 1209 ± 1051 | 1365 ± 889 | 0.16 |
| PO43−/μg L−1 | 16.04 ± 3.35 | 18.09 ± 10.77 | 16.14 ± 3.45 | 18.22 ± 2.58 | 17.39 ± 7.21 | <0.05 |
| K/mg L−1 | 1.97 ± 2.02 | 1.18 ± 0.74 | 2.09 ± 0.98 | 1.18 ± 0.87 | 1.50 ± 1.15 | <0.05 |
| Ca/mg L−1 | 3.78 ± 3.74 | 1.48 ± 0.50 | 5.85 ± 3.52 | 2.20 ± 1.01 | 2.92 ± 2.77 | <0.001 |
| Na/mg L−1 | 1.43 ± 1.74 | 0.92 ± 0.59 | 1.65 ± 0.82 | 0.78 ± 0.31 | 1.12 ± 0.92 | <0.01 |
| Mg/mg L−1 | 0.87 ± 0.72 | 0.36 ± 0.14 | 1.09 ± 0.43 | 0.41 ± 0.14 | 0.61 ± 0.47 | <0.001 |
| Fe/mg L−1 | 2.34 ± 3.34 | 1.06 ± 1.26 | 3.91 ± 4.54 | 1.22 ± 1.46 | 1.90 ± 2.83 | 0.37 |
| Mn/mg L−1 | 0.26 ± 0.31 | 0.08 ± 0.09 | 0.90 ± 1.03 | 0.10 ± 0.10 | 0.29 ± 0.58 | 0.14 |
| Si/mg L−1 | 1.50 ± 0.74 | 0.91 ± 0.62 | 1.75 ± 1.06 | 1.04 ± 0.53 | 1.21 ± 0.79 | <0.05 |
| Zn/mg L−1 | 1.51 ± 1.43 | 0.02 ± 0.01 | 0.12 ± 0.34 | 0.02 ± 0.01 | 0.27 ± 0.78 | <0.001 |
| B/μg L−1 | 4.0 ± 3.8 | 4.3 ± 5.5 | 37.0 ± 37.9 | 168.3 ± 103.0 | 48.6 ± 84.0 | <0.001 |
| Al/μg L−1 | 237.8 ± 201.5 | 179.2 ± 156.5 | 136.3 ± 124.7 | 122.6 ± 158.5 | 166.5 ± 159.3 | 0.32 |
| V/μg L−1 | 0.68 ± 0.80 | 0.52 ± 0.59 | 0.64 ± 0.90 | 0.36 ± 0.61 | 0.53 ± 0.69 | 0.20 |
| Co/μg L−1 | 1.25 ± 1.11 | 0.82 ± 0.54 | 2.52 ± 2.43 | 0.71 ± 0.48 | 1.21 ± 1.41 | 0.23 |
| Ni/μg L−1 | 1.90 ± 0.91 | 1.06 ± 0.52 | 1.27 ± 0.90 | 0.64 ± 0.28 | 1.14 ± 0.75 | <0.001 |
| Cu/μg L−1 | 80.7 ± 70.3 | 2.2 ± 1.6 | 1.3 ± 0.8 | 0.6 ± 0.5 | 14.0 ± 39.4 | <0.001 |
| As/μg L−1 | 3.84 ± 5.18 | 2.54 ± 2.69 | 5.03 ± 5.22 | 1.82 ± 1.83 | 3.11 ± 3.76 | 0.19 |
| Sr/μg L−1 | 21.1 ± 18.9 | 8.9 ± 3.2 | 32.2 ± 28.2 | 12.3 ± 5.7 | 16.5 ± 17.4 | <0.001 |
| Cd/μg L−1 | 0.16 ± 0.10 | 0.14 ± 0.12 | 0.06 ± 0.02 | 0.08 ± 0.05 | 0.11 ± 0.10 | <0.01 |
| Sb/μg L−1 | 1.42 ± 0.27 | 1.49 ± 0.26 | 1.42 ± 0.42 | 1.24 ± 0.45 | 1.41 ± 0.35 | 0.36 |
| Ba/μg L−1 | 26.3 ± 30.5 | 18.5 ± 18.4 | 34.1 ± 35.4 | 12.3 ± 8.0 | 21.6 ± 24.2 | 0.16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.-M.; Chen, X.; Bu, Z.-J.; Zeng, L.-H. Spatial Variations in the Surface Water Chemistry of Subtropical Peatlands (Central China) Linked to Anthropogenic Pressures. Water 2017, 9, 505. https://doi.org/10.3390/w9070505
Cao Y-M, Chen X, Bu Z-J, Zeng L-H. Spatial Variations in the Surface Water Chemistry of Subtropical Peatlands (Central China) Linked to Anthropogenic Pressures. Water. 2017; 9(7):505. https://doi.org/10.3390/w9070505
Chicago/Turabian StyleCao, Yan-Min, Xu Chen, Zhao-Jun Bu, and Ling-Han Zeng. 2017. "Spatial Variations in the Surface Water Chemistry of Subtropical Peatlands (Central China) Linked to Anthropogenic Pressures" Water 9, no. 7: 505. https://doi.org/10.3390/w9070505





