Comparing the Spectroscopic and Molecular Characteristics of Different Dissolved Organic Matter Fractions Isolated by Hydrophobic and Anionic Exchange Resins Using Fluorescence Spectroscopy and FT-ICR-MS
Abstract
1. Introduction
2. Material and Methods
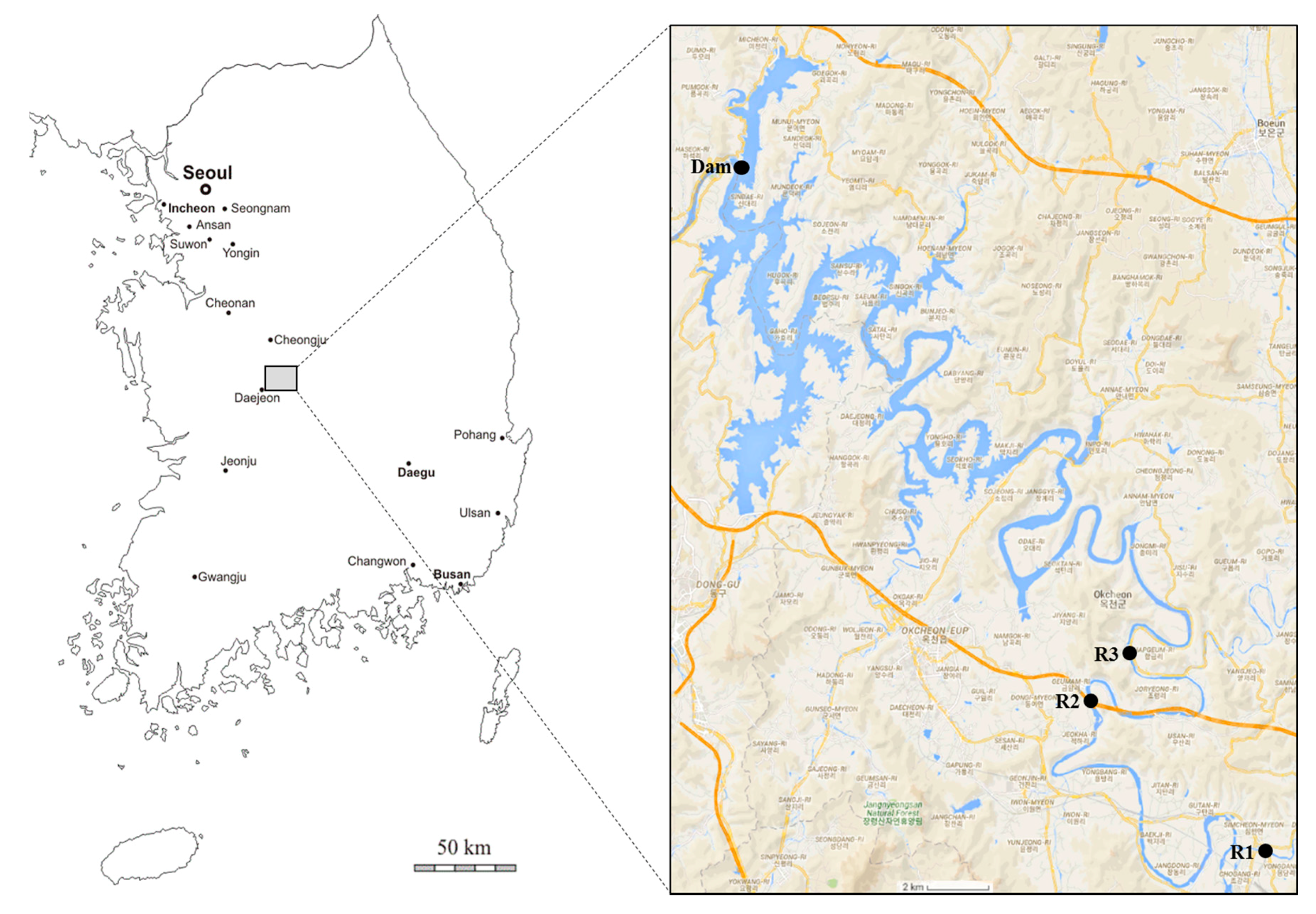
2.1. Study Area and Sampling
2.2. Resin Fractionation
2.3. Measurements of Dissolved Organic Carbon, Absorption, and Fluorescence Spectra
2.4. FT-ICR MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
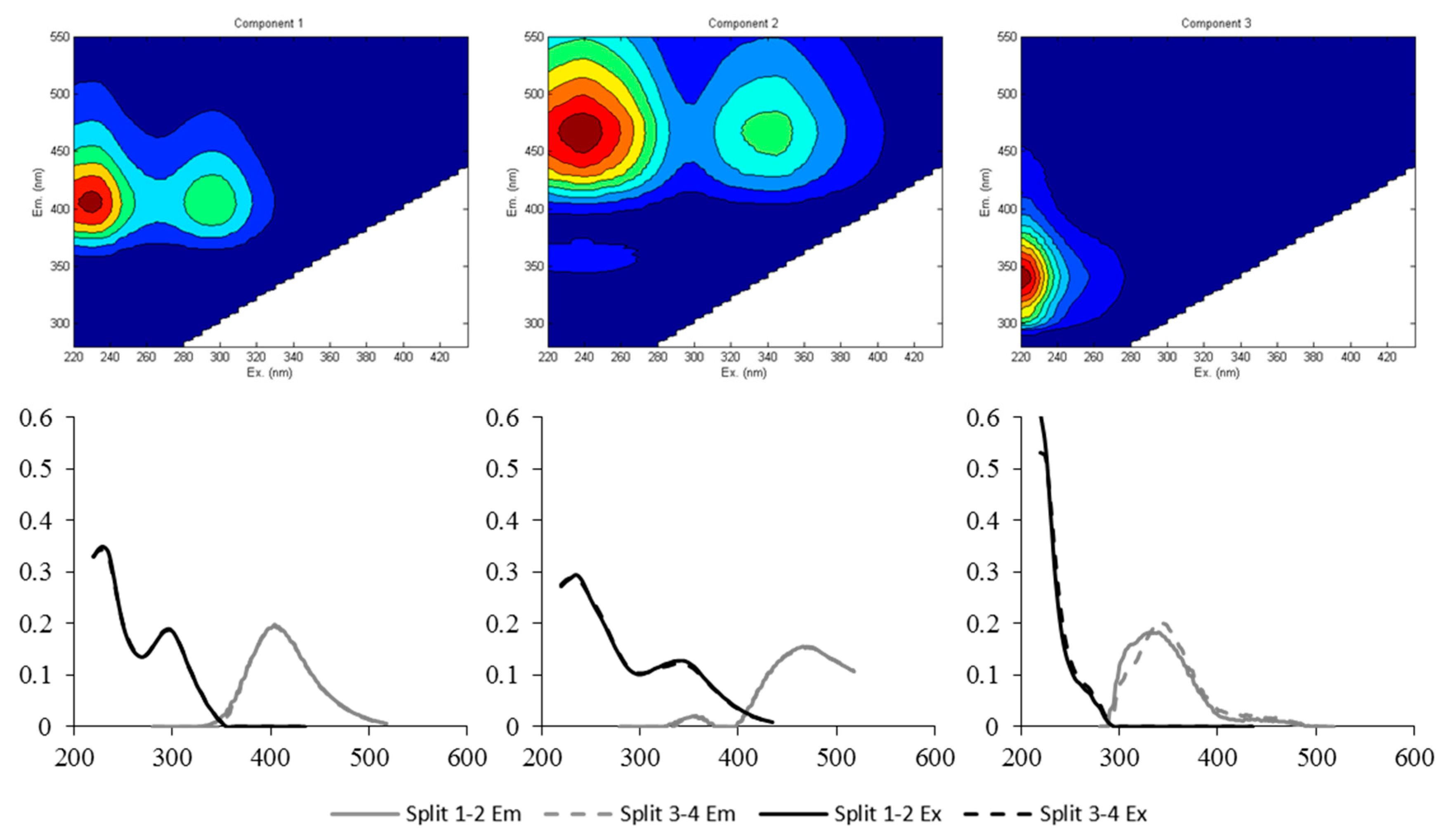
3.1. Identification of Fluorescent Components from EEM-PARAFAC
3.2. Fluorescence Properties of Three Different Chemical Fractions
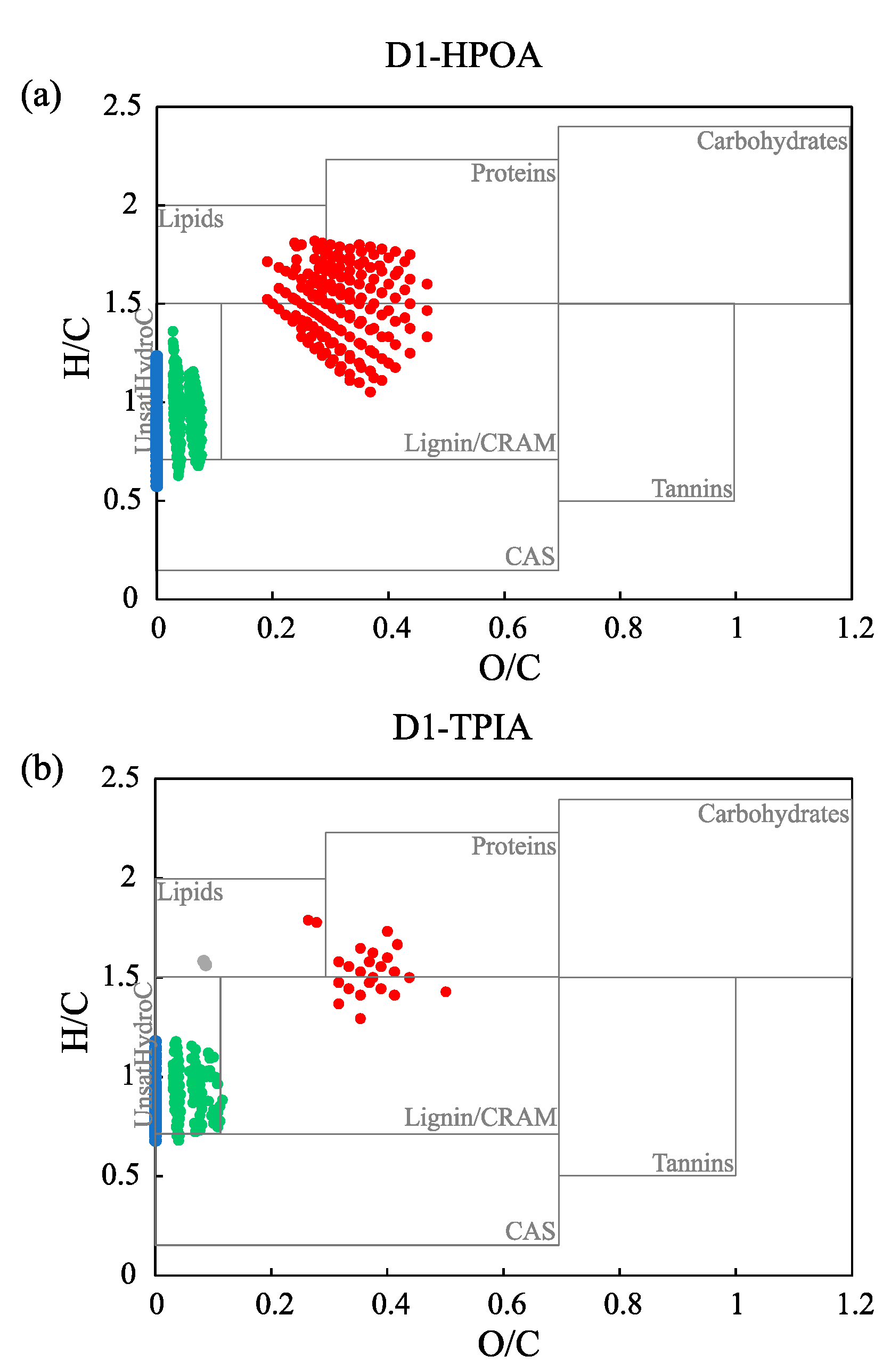
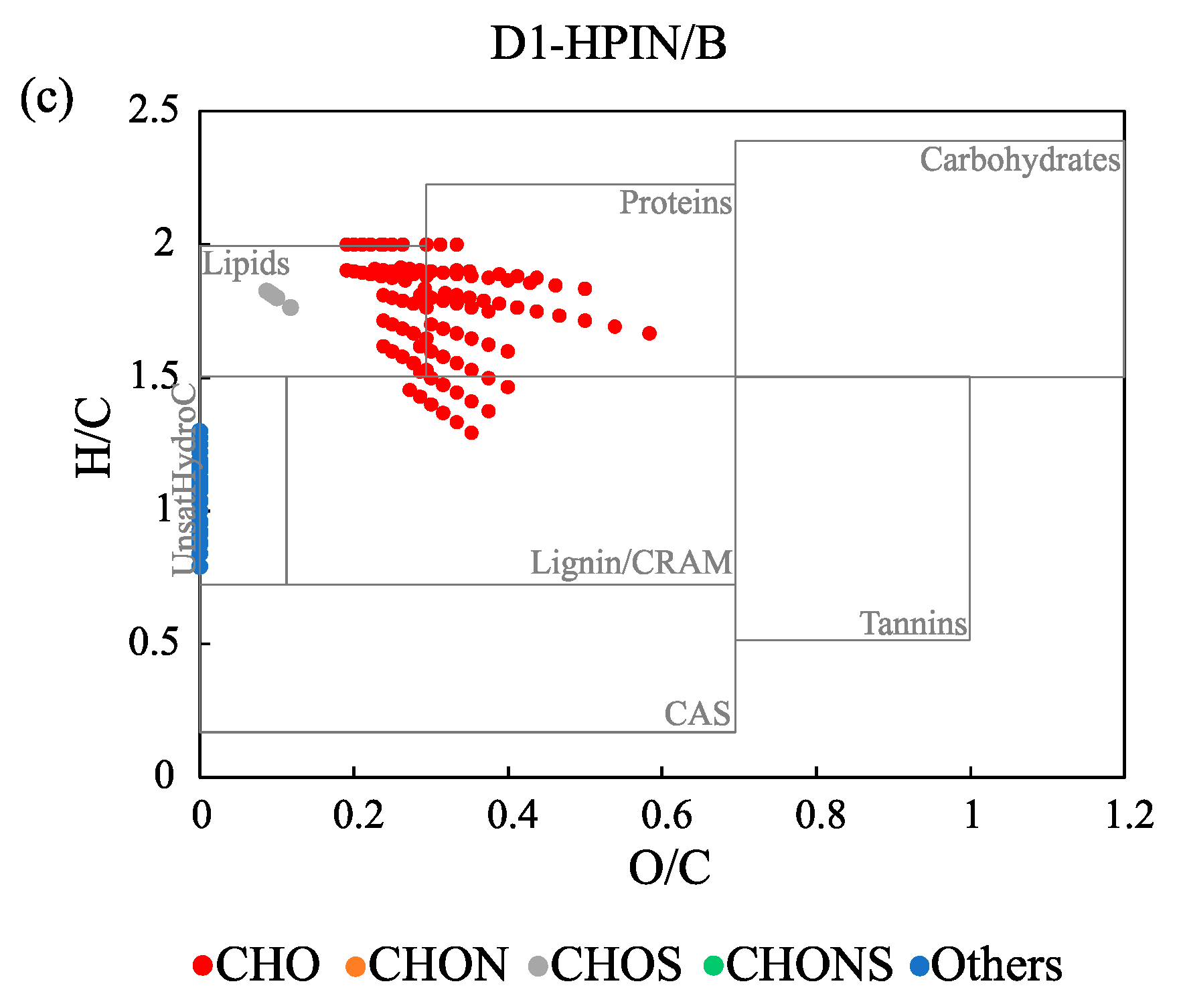
3.3. Comparison of Molecular Composition of the Three Chemical Fractions
3.4. Comparison Between the Different Chemical Fractions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, J.A.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Melendez-Perez, J.J.; Martinez-Mejia, M.J.; Taj Awan, A.; Fadini, P.S.; Mozeto, A.A.; Ngueira Eberlin, M. Characterization and comparison of riverine, lacustrine, marine and estuarine dissolved organic matter by ultra-high resolution and accuracy Fourier transform mass spectrometry. Org. Geochem. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- He, W.; Hur, J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter. Water Res. 2015, 83, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Mesfioui, R.; Dotson, A.; Westerhoff, P.; Hatcher, P. Comparison of hydrophobic and amphiphilic fractions of dissolved organic matter from a water reservoir by Fourier transform ion cyclotron resonance mass spectrometry. J. Soils Sediments 2016. [Google Scholar] [CrossRef]
- Fang, Z.; He, C.; Li, Y.; Chung, K.H.; Xu, C.; Shi, Q. Fractionation and characterization of dissolved organic matter (DOM) in refinery wastewater by revised phase retention and ion-exchange adsorption solid phase extraction followed by ESI FT-ICR MS. Talanta 2017, 162, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Leenheer, J. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters. Environ. Sci. Technol. 1981, 15, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Fukushima, T.; Matsushige, K.; Kim, Y.H. Fractionation and characterization of dissolved organic matter in a shallow eutrophic lake, its inflowing rivers, and other organic sources. Water Res. 2001, 35, 4019–4028. [Google Scholar] [CrossRef]
- Kim, H.-C.; Dempsey, B.A. Comparison of two fractionation strategies for characterization of wastewater effluent organic matter and diagnosis of membrane fouling. Water Res. 2012, 46, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruis, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Singh, S.; D’Sa, E.J.; Swenson, E.M. Chromophoric dissolved organic matter (CDOM) variability in Barataria Basin using excitation-emission matrix (EEM) fluorescence and parallel factor analysis (PARAFAC). Sci. Total Environ. 2010, 408, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hur, L. Critical evaluation of spectroscopic indices for organic matter source tracing via end member mixing analysis based on two contrasting sources. Water Res. 2014, 59, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Williams, M.A.; Schlautman, M.A. Evaluating spectroscopic and chromatographic techniques to resolve dissolved organic matter via end member mixing analysis. Chemosphere 2006, 63, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Singh, S.; Dutta, S.; Levia, D.; Mitchell, M.; Scott, D.; Bais, H.; McHale, P. Fluorescence characteristics and sources of dissolved organic matter for stream water during storm events in a forested mid-Atlantic watershed. J. Geophys. Res. 2011, 116G03043. [Google Scholar] [CrossRef]
- Fichot, C.G.; Kaiser, K.; Hooker, S.B.; Amon, R.M.W.; Babin, M.; Bélanger, S.; Walker, S.A.; Benner, R. Pan-Arctic distributions of continental runoff in the Arctic Ocean. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, M.; Park, J.E.; Hur, J. Molecular diversity of riverine alkaline-extractable sediment organic matter and its linkages with spectral indicators and molecular size distributions. Water Res. 2016, 100, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Yang, L.; Hur, J. Lipid biomarkers and spectroscopic indices for identifying organic matter sources in aquatic environments: A review. Water Res. 2017, 112, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Hendrickson, C.L. Fourier transform ion cyclotron resonance detection: Principles and experimental configurations. Int. J. Mass Spectrom. 2002, 215, 59–75. [Google Scholar] [CrossRef]
- Koch, B.P.; Ludwichowski, K.U.; Kattner, G.; Dittmar, T.; Witt, M. Advanced characterization of marine dissolved organic matter by combining reversed-phase liquid chromatography and FT-ICR-MS. Mar. Chem. 2008, 111, 233–241. [Google Scholar] [CrossRef]
- Sleighter, R.L.; Hatcher, P.G. Fourier transform mass spectrometry for the molecular level characterization of natural organic matter: Instrument capabilities, applications, and limitations. In Fourier Transforms-Approach to Scientific Principles; Nikolic, G., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Li, Y.; Xu, C.; Chung, K.H.; Shi, Q. Molecular characterization of dissolved organic matter and its subfractions in refinery process water by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2015, 29, 2923–2930. [Google Scholar]
- Leenheer, J.A.; Croue, J.P. Peer reviewed: Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18–26. [Google Scholar] [CrossRef]
- Wei, L.-L.; Zhoa, Q.-L.; Xue, S.; Jia, T. Removal and transformation of dissolved organic matter in secondary effluent during granular activated carbon treatment. J. Zhejiang Uni. SCIENCE A 2008, 9, 994–1003. [Google Scholar] [CrossRef]
- Chung, S.W.; Hipsey, M.R.; Imberger, J. Modelling the propagation of turbid density inflows into a stratified lake: Daecheong Reservoir, Korea. Environ. Model. Softw. 2009, 24, 1467–1482. [Google Scholar] [CrossRef]
- Yu, S.J.; Lee, J.Y.; Ha, S.R. Effect of the seasonal diffuse pollution migration on natural organic matter behavior in a stratified dam reservoir. J. Environ. Sci. 2010, 22, 908–914. [Google Scholar] [CrossRef]
- Murphy, K.R.; Butler, K.D.; Spencer, R.G.M.; Stedmon, C.A.; Boehme, J.R.; Aiken, G.R. Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison. Environ. Sci. Technol. 2010, 44, 9405–9412. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Hwang, S.J.; Shin, J.K. Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water Air Soil Pollut. 2008, 191, 231–243. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, G.H. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the Van Krevelen Diagram. Anal. Chem. 2003, 72, 5336–5344. [Google Scholar] [CrossRef]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef]
- Lapierre, J.F.; del Giorgio, P.A. Partial coupling and differential regulation of biologically and photochemically labile dissolved organic carbon across boreal aquatic networks. Biogeosciences 2014, 11, 5969–5985. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterization of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Meth. 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Lee, B.-M.; Deo, Y.-S.; Hur, J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence Eemparafac. Water Res. 2015, 73, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Martager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using Parafac analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Lu, Y.; Bauer, J.E.; Canuel, E.A.; Yamashita, Y.; Chambers, R.M.; Jaffe, R. Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use. J. Geophys. Res. Biogeosci. 2013, 118, 566–580. [Google Scholar] [CrossRef]
- Singh, S.; Inamdar, S.; Scott, D. Comparison of two PARAFAC models of dissolved organic matter fluorescence for a mid-atlantic forested watershed in the USA. J. Ecosyst. 2013, 2013, 532424. [Google Scholar] [CrossRef]
- Mai, N.; Scully, N.M.; Pisani, O.; Jaffe, R. Composition of a protein-like fluorophore of dissolved organic matter in coastal wetland and estuarine ecosystems. Water Res. 2007, 41, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hernes, P.J.; Bergamaschi, B.A.; Eckard, R.S.; Spencer, R.G.M. Fluorescence-based proxies for lignin in freshwater dissolved organic matter. J. Geophys. Lett. 2009, 114, G00F03. [Google Scholar] [CrossRef]
- Reemtsma, T. Determination of molecular formulas of natural organic matter molecules by (ultra-) high-resolution mass spectrometry Status and needs. J. Chromatogr. A 2009, 1216, 3687–3701. [Google Scholar] [CrossRef] [PubMed]
- Novotny, N.R.; Capley, E.N.; Stenson, A.C. Fact or artifact: The representativeness of ESI-MS for complex natural organic mixtures. J. Mass Spectrom. 2014, 49, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.L.; Rice, J.A. Effect of experimental parameters on the ESI FT-ICR Mass Spectrum of fulvic acid. Anal. Chem. 2000, 72, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.L.; Muddiman, D.C. Leveling response factors in the electrospray ionization process using a heated capillary interface. J. Am. Soc. Mass. Spectrom. 2005, 16, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Wilm, M. Principles of Electrospray Ionization. Mol. Cell. Prot. 2011, 10, M111.009407. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mazumdar, S. Electrospray ionization mass spectrometry: A technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012, 2012, 282574. [Google Scholar] [CrossRef] [PubMed]
- Bagag, A.; Jault, J.-M.; Sidahmed-Adrar, N.; Refregiers, M.; Giuliani, A.; Le Naour, F. Characterization of Hydrophobic Peptides in the Presence of Detergent by Photoionization Mass Spectrometry. PLoS ONE 2013, 8, e79033. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Takayama, M. Influence of hydrophobicity on positive- and negative-ion yields of peptides in electrospray ionization mass spectrometry. Rapid Commun. Mass Spctrom. 2014, 28, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. A molecular-level approach for characterizing water-insoluble components of ambient organic aerosol particulates using ultrahigh-resolution mass spectrometry. Atmos. Chem. Phys. 2014, 14, 10299–10314. [Google Scholar] [CrossRef]
- Derrien, M.; Lee, Y.K.; Park, J.-E.; Li, P.; Chen, M.; Lee, S.H.; Lee, S.H.; Lee, J.-B.; Hur, J. Spectroscopic and molecular characterization of humic substances (HS) from soils and sediments in a watershed: Comparative study of HS chemical fractions and the origins. Environ. Sci. Pollut. Res. 2017, 24, 16933–16945. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kim, S.; Park, J.-E.; Jung, H.-J.; Hur, H. Structural and compositional changes of dissolved organic matter upon solid-phase extraction tracked by multiple analytical tools. Anal. Bioanal. Chem. 2016, 23, 6249–6258. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, S.; Luo, L.; Cao, D. Solid-phase extraction-stepwise elution (SPE-SE) procedure for isolation of dissolved organic matter prior to ESI-FT-ICR-MS analysis. Anal. Chim. Acta 2016, 948, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gireeshkumar, T.R.; Deepulal, P.M.; Chandramohanakumar, N. Distribution and sources of aliphatic hydrocarbons and fatty acids in surface sediments of a tropical estuary south west coast of India (Cochin estuary). Environ. Monit. Assess. 2015, 187, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
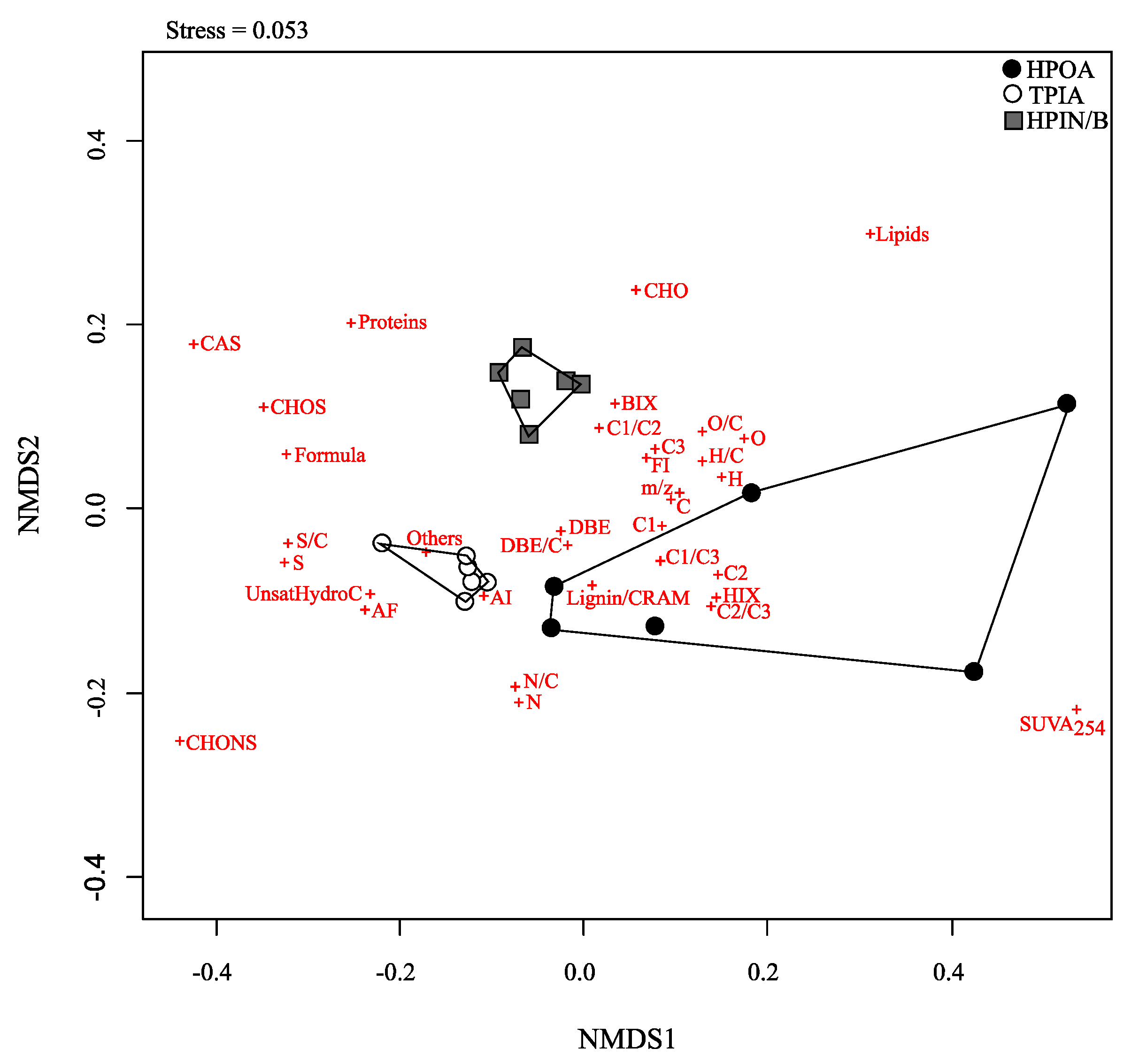
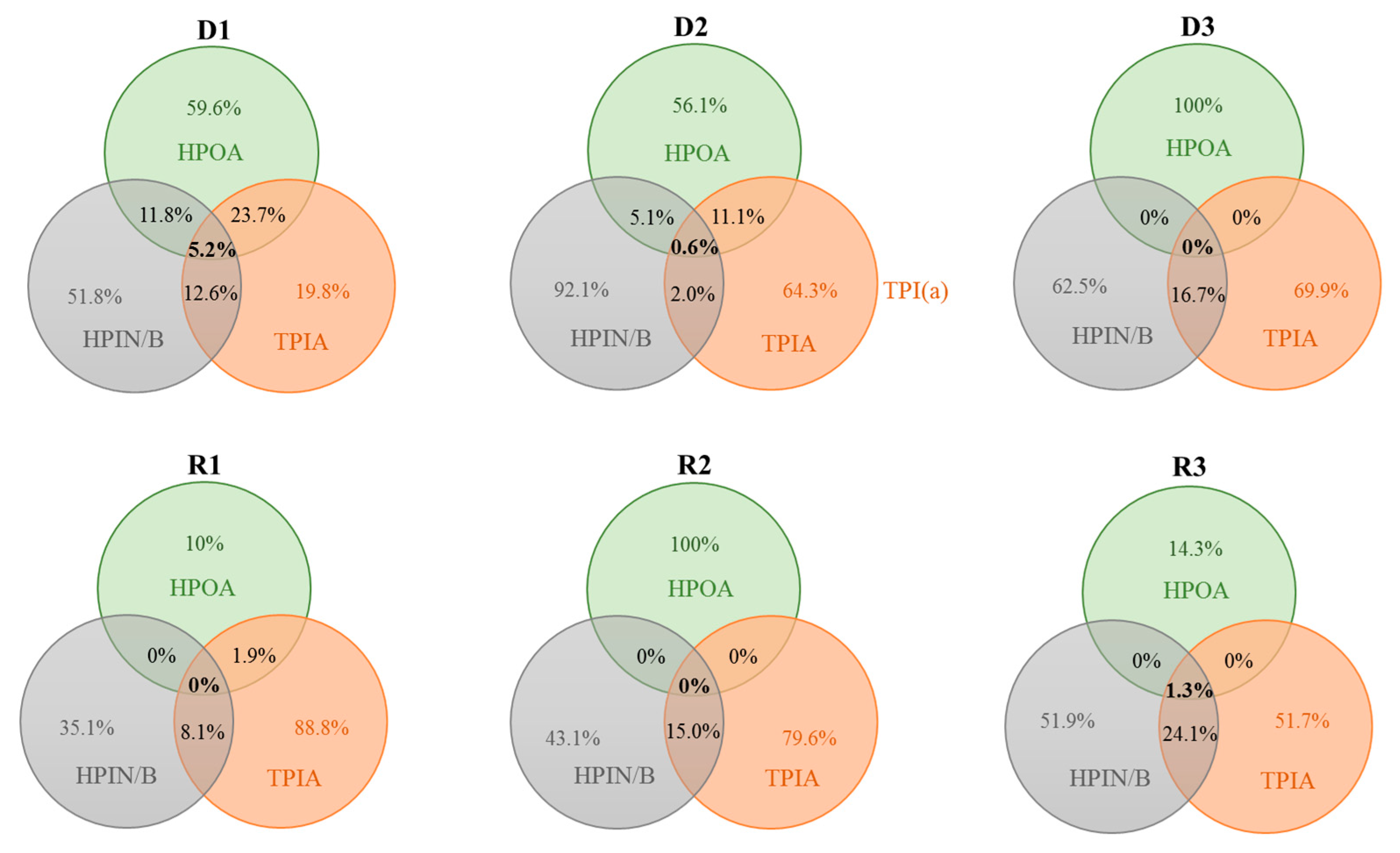
| Sample | HPOA | TPIA | HPIN/B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Min | Max | Range | Average | Min | Max | Range | Average | Min | Max | Range | |
| SUVA254 | 3.8 ± 1.9 | 1.9 | 5.93 | 4.1 | 0.4 ± 0.1 | 0.2 | 0.6 | 0.40 | 0.2 ± 0.2 | 0.1 | 0.6 | 0.5 |
| %C1 | 30.1 ± 3.8 | 24.1 | 35.3 | 11.2 | 30.4 ± 7.3 | 14.4 | 35.3 | 20.9 | 21.7 ± 1.7 | 19.6 | 23.9 | 4.4 |
| %C2 | 16.8 ± 3.3 | 12.2 | 21.5 | 9.3 | 12.7 ± 4.1 | 3.6 | 15.3 | 11.7 | 6.3 ± 1.0 | 4.7 | 7.6 | 2.9 |
| %C3 | 53.0 ± 6.8 | 43.2 | 61.7 | 18.5 | 57.0 ± 11.3 | 50.6 | 82.0 | 31.4 | 72.0 ± 2.0 | 68.5 | 74.6 | 6.1 |
| FI | 1.2 ± 0.0 | 1.1 | 1.2 | 0.1 | 1.2 ± 0.1 | 1.2 | 1.3 | 0.2 | 1.6 ± 0.1 | 1.5 | 1.7 | 0.2 |
| HIX | 2.5 ± 0.6 | 1.7 | 3.4 | 1.6 | 1.7 ± 0.6 | 0.5 | 2.1 | 1.6 | 0.8 ± 0.2 | 0.4 | 1.0 | 0.6 |
| BIX | 0.8 ± 0.1 | 0.7 | 0.9 | 0.2 | 1.0 ± 0.2 | 0.9 | 1.5 | 0.6 | 1.9 ± 0.7 | 1.4 | 3.5 | 2.1 |
| C1/C2 | 1.8 ± 0.2 | 1.5 | 2.2 | 0.7 | 2.6 ± 0.6 | 2.1 | 4.0 | 1.9 | 3.6 ± 0.7 | 3.1 | 5.0 | 1.9 |
| C1/C3 | 0.6 ± 0.1 | 0.4 | 0.8 | 0.4 | 0.6 ± 0.2 | 0.2 | 0.7 | 0.5 | 0.3 ± 0.1 | 0.3 | 0.3 | 0.1 |
| C2/C3 | 0.3 ± 0.1 | 0.2 | 0.5 | 0.3 | 0.2 ±0.1 | 0.0 | 0.3 | 0.2 | 0.1 ± 0.0 | 0.1 | 0.1 | 0.0 |
| Number of formulas | 82 ± 145 | 1 | 403 | 402 | 378 ± 295 | 42 | 939 | 897 | 203 ± 53 | 134 | 267 | 133 |
| m/zwa | 392.47 ± 38.22 | 346.37 | 441.29 | 94.92 | 383.21 ± 14.17 | 354.68 | 398.22 | 43.54 | 356.07 ± 10.75 | 346.42 | 376.65 | 30.23 |
| Cwa | 22.97 ± 2.49 | 19.02 | 27.00 | 7.98 | 23.61 ± 0.83 | 21.92 | 24.47 | 2.55 | 20.76 ± 0.85 | 19.66 | 21.89 | 2.22 |
| Hwa | 33.79 ± 4.93 | 29.50 | 42.00 | 12.50 | 25.23 ± 0.95 | 23.19 | 25.95 | 2.76 | 30.05 ± 2.32 | 26.93 | 33.43 | 6.50 |
| Owa | 4.39 ± 1.40 | 2.66 | 7.00 | 4.34 | 2.92 ± 0.39 | 2.47 | 3.53 | 1.05 | 4.17 ± 0.54 | 3.34 | 5.18 | 1.83 |
| Nwa | 0.37 ± 0.33 | 0.00 | 1.00 | 1.00 | 0.61 ± 0.05 | 0.54 | 0.68 | 0.14 | 0.15 ± 0.04 | 0.09 | 0.21 | 0.12 |
| Swa | 0.25 ± 0.26 | 0.00 | 0.70 | 0.70 | 0.61 ± 0.05 | 0.52 | 0.69 | 0.17 | 0.27 ± 0.09 | 0.11 | 0.38 | 0.27 |
| H/Cwa | 1.51 ± 0.17 | 1.31 | 1.75 | 0.44 | 1.13 ± 0.02 | 1.11 | 1.16 | 0.05 | 1.53 ± 0.14 | 1.35 | 1.73 | 0.38 |
| O/Cwa | 0.21 ± 0.06 | 0.14 | 0.29 | 0.15 | 0.16 ± 0.02 | 0.14 | 0.18 | 0.04 | 0.23 ± 0.03 | 0.18 | 0.28 | 0.10 |
| N/Cwa | 0.01 ± 0.01 | 0.00 | 0.04 | 0.04 | 0.02 ± 0.00 | 0.02 | 0.03 | 0.00 | 0.01 ± 0.00 | 0.01 | 0.01 | 0.00 |
| S/Cwa | 0.01 ± 0.01 | 0.00 | 0.03 | 0.03 | 0.02 ± 0.00 | 0.02 | 0.03 | 0.01 | 0.01 ± 0.00 | 0.01 | 0.02 | 0.01 |
| DBEwa | 7.3 ± 2.6 | 3.7 | 10.0 | 6.2 | 12.3 ± 0.5 | 11.6 | 12.9 | 1.3 | 6.8 ± 1.9 | 4.0 | 9.4 | 5.4 |
| DBEwa/Cwa | 0.3 ± 0.1 | 0.2 | 0.4 | 0.3 | 0.5 ± 0.0 | 0.5 | 0.5 | 0.0 | 0.3 ± 0.1 | 0.2 | 0.4 | 0.2 |
| AImod,wa | 0.2 ± 0.1 | 0.0 | 0.3 | 0.3 | 0.4 ± 0.0 | 0.4 | 0.4 | 0.0 | 0.2 ± 0.1 | 0.1 | 0.3 | 0.2 |
| CHO (%) | 37.6 ± 32.1 | 0.0 | 100.0 | 100.0 | 29.5 ± 8.3 | 14.0 | 38.6 | 24.6 | 74.7 ± 6.3 | 66.0 | 86.2 | 20.2 |
| CHON (%) | 16.7 ± 37.3 | 0.0 | 100.0 | 100.0 | 1.9 ± 4.2 | 0.0 | 11.2 | 11.2 | 0.7 ± 1.0 | 0.0 | 2.4 | 2.4 |
| CHOS (%) | 2.9 ± 6.5 | 0.0 | 17.5 | 17.5 | 1.7 ± 1.7 | 0.0 | 4.8 | 4.8 | 3.1 ± 2.0 | 0.0 | 6.7 | 6.7 |
| CHONS (%) | 9.4 ± 14.1 | 0.0 | 36.2 | 36.2 | 41.5 ± 13.6 | 14.3 | 55.2 | 40.9 | 3.8 ± 6.4 | 0.0 | 17.8 | 17.8 |
| Others (%) | 33.4 ± 27.9 | 0.0 | 70.0 | 70.0 | 25.5 ± 14.5 | 11.1 | 54.8 | 43.7 | 17.6 ± 8.0 | 7.5 | 28.1 | 20.7 |
| AF (%) | 32.5 ± 25.3 | 0.0 | 70.0 | 70.0 | 46.4 ± 10.0 | 28.3 | 61.6 | 33.3 | 14.5 ± 5.5 | 6.0 | 21.8 | 15.9 |
| CAS (%) | 0.5 ± 1.2 | 0.0 | 3.2 | 3.2 | 10.5 ± 9.0 | 0.9 | 20.8 | 19.9 | 10.4 ± 10.9 | 0.0 | 28.0 | 28.0 |
| Lignin/CRAM (%) | 22.4 ± 35.7 | 0.0 | 100.0 | 100.0 | 20.9 ± 9.2 | 7.1 | 35.6 | 28.4 | 17.6 ± 4.6 | 9.5 | 24.6 | 15.1 |
| Protein (%) | 6.8 ± 6.8 | 0.0 | 14.3 | 14.3 | 10.7 ± 2.2 | 7.6 | 14.8 | 7.2 | 25.7 ± 7.5 | 19.1 | 39.6 | 20.5 |
| Carbohydrate (%) | 0.0 ± 0.0 | 0.0 | 0.0 | 0.0 | 0.0 ± 0.0 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.4 | 0.0 | 1.0 | 1.0 |
| Lipid (%) | 28.5 ± 33.2 | 0.0 | 100.0 | 100.0 | 3.3 ± 1.3 | 1.6 | 5.2 | 3.6 | 29.4 ± 9.7 | 9.7 | 39.4 | 29.7 |
| UnsatHydroC (%) | 43.1 ± 32.8 | 0.0 | 90.0 | 90.0 | 58.1 ± 14.9 | 32.3 | 79.1 | 46.8 | 21.2 ± 7.3 | 10.6 | 27.9 | 17.3 |
| Tannin (%) | 0.0 ± 0.0 | 0.0 | 0.0 | 0.0 | 0.0 ± 0.0 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.6 | 0.0 | 1.6 | 1.6 |
| Fluorescence and Molecular Parameters | p-Value | ||
|---|---|---|---|
| HPOA vs. TPIA | TPIA vs. HPIN/B | HPOA vs. HPIN/B | |
| SUVA254 | 0.0006 | 0.1315 | 0.0004 |
| %C1 | 0.9454 | 0.0436 | 0.0027 |
| %C2 | 0.1046 | 0.0125 | 0.0002 |
| %C3 | 0.5195 | 0.0257 | 0.0004 |
| FI | 0.1041 | 0.0000 | 0.0000 |
| HIX | 0.0644 | 0.0047 | 0.0001 |
| BIX | 0.0811 | 0.0318 | 0.0089 |
| C1/C2 | 0.0264 | 0.0601 | 0.0007 |
| C1/C3 | 0.8659 | 0.0163 | 0.0041 |
| C2/C3 | 0.1672 | 0.0070 | 0.0013 |
| Number of formulas | 0.0720 | 0.2235 | 0.1080 |
| m/zwa | 0.6224 | 0.0066 | 0.0675 |
| Cwa | 0.5994 | 0.0003 | 0.0896 |
| Hwa | 0.0034 | 0.0016 | 0.1542 |
| Owa | 0.0469 | 0.0019 | 0.7518 |
| Nwa | 0.1429 | 0.0000 | 0.1674 |
| Swa | 0.0116 | 0.0000 | 0.8574 |
| H/Cwa | 0.0007 | 0.0001 | 0.8665 |
| O/Cwa | 0.0879 | 0.0005 | 0.4691 |
| N/Cwa | 0.1248 | 0.0000 | 0.2131 |
| S/Cwa | 0.0142 | 0.0005 | 0.5865 |
| DBEwa | 0.0015 | 0.0001 | 0.7634 |
| DBEwa/Cwa | 0.0009 | 0.0003 | 0.8337 |
| AImod,wa | 0.0015 | 0.0000 | 0.6707 |
| CHO (%) | 0.5936 | 0.0000 | 0.0298 |
| CHON (%) | 0.3981 | 0.5689 | 0.3618 |
| CHOS (%) | 0.6893 | 0.2468 | 0.9512 |
| CHONS (%) | 0.0042 | 0.0002 | 0.4418 |
| Others (%) | 0.5848 | 0.3142 | 0.2524 |
| AF (%) | 0.2787 | 0.0001 | 0.1509 |
| CAS (%) | 0.0335 | 0.9870 | 0.0717 |
| Lignin/CRAM (%) | 0.9263 | 0.4942 | 0.7702 |
| Protein (%) | 0.2527 | 0.0016 | 0.0020 |
| Carbohydrate (%) | / | 0.1492 | 0.1492 |
| Lipid (%) | 0.1220 | 0.0001 | 0.9522 |
| UnsatHydroC (%) | 0.3759 | 0.0006 | 0.1740 |
| Tannin (%) | / | 0.3409 | 0.3409 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derrien, M.; Lee, Y.K.; Hur, J. Comparing the Spectroscopic and Molecular Characteristics of Different Dissolved Organic Matter Fractions Isolated by Hydrophobic and Anionic Exchange Resins Using Fluorescence Spectroscopy and FT-ICR-MS. Water 2017, 9, 555. https://doi.org/10.3390/w9080555
Derrien M, Lee YK, Hur J. Comparing the Spectroscopic and Molecular Characteristics of Different Dissolved Organic Matter Fractions Isolated by Hydrophobic and Anionic Exchange Resins Using Fluorescence Spectroscopy and FT-ICR-MS. Water. 2017; 9(8):555. https://doi.org/10.3390/w9080555
Chicago/Turabian StyleDerrien, Morgane, Yun Kyung Lee, and Jin Hur. 2017. "Comparing the Spectroscopic and Molecular Characteristics of Different Dissolved Organic Matter Fractions Isolated by Hydrophobic and Anionic Exchange Resins Using Fluorescence Spectroscopy and FT-ICR-MS" Water 9, no. 8: 555. https://doi.org/10.3390/w9080555
APA StyleDerrien, M., Lee, Y. K., & Hur, J. (2017). Comparing the Spectroscopic and Molecular Characteristics of Different Dissolved Organic Matter Fractions Isolated by Hydrophobic and Anionic Exchange Resins Using Fluorescence Spectroscopy and FT-ICR-MS. Water, 9(8), 555. https://doi.org/10.3390/w9080555





