Responses of Phragmites australis to Nitrogen Addition along Salinity Gradients in Coastal Saline–Alkali Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sampling and Analyses
2.4. Soil Properties
2.5. Data Analysis
3. Results
3.1. Soil pH and Water Contents in Different Growth Stages
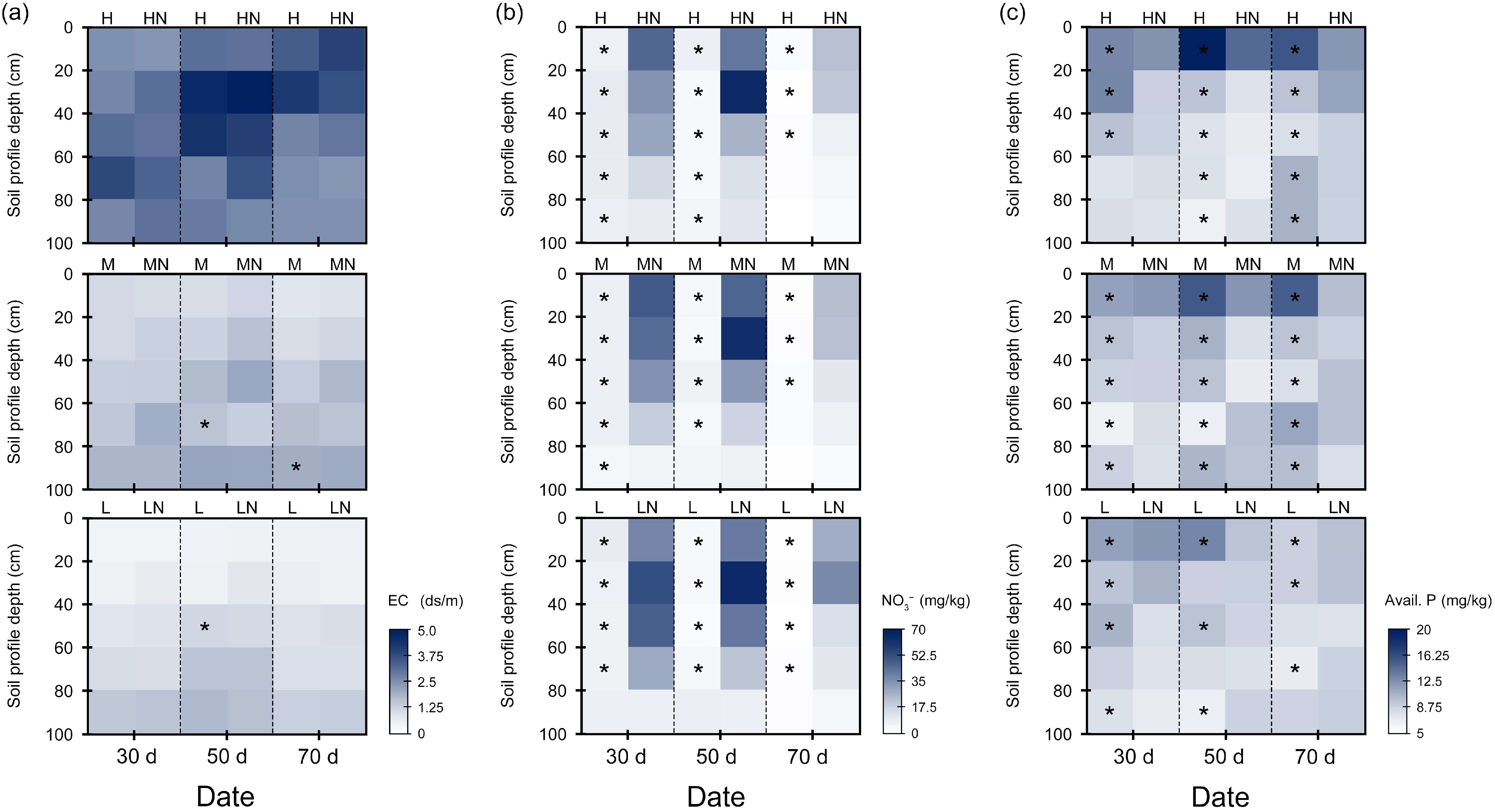
3.2. Soil Salt and Nutrient Characteristics in Different Growth Stages
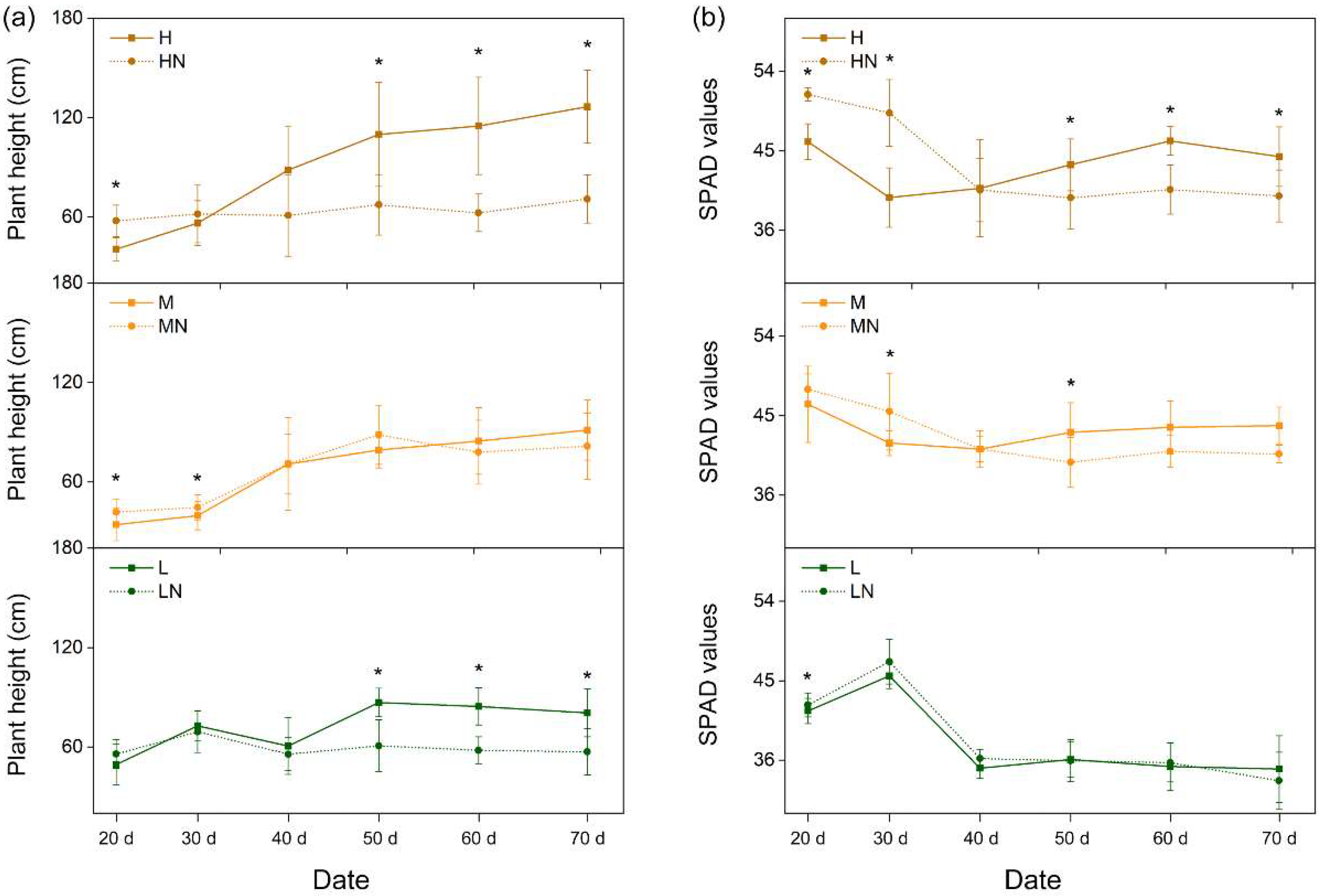
3.3. Growth Indices of P. australis
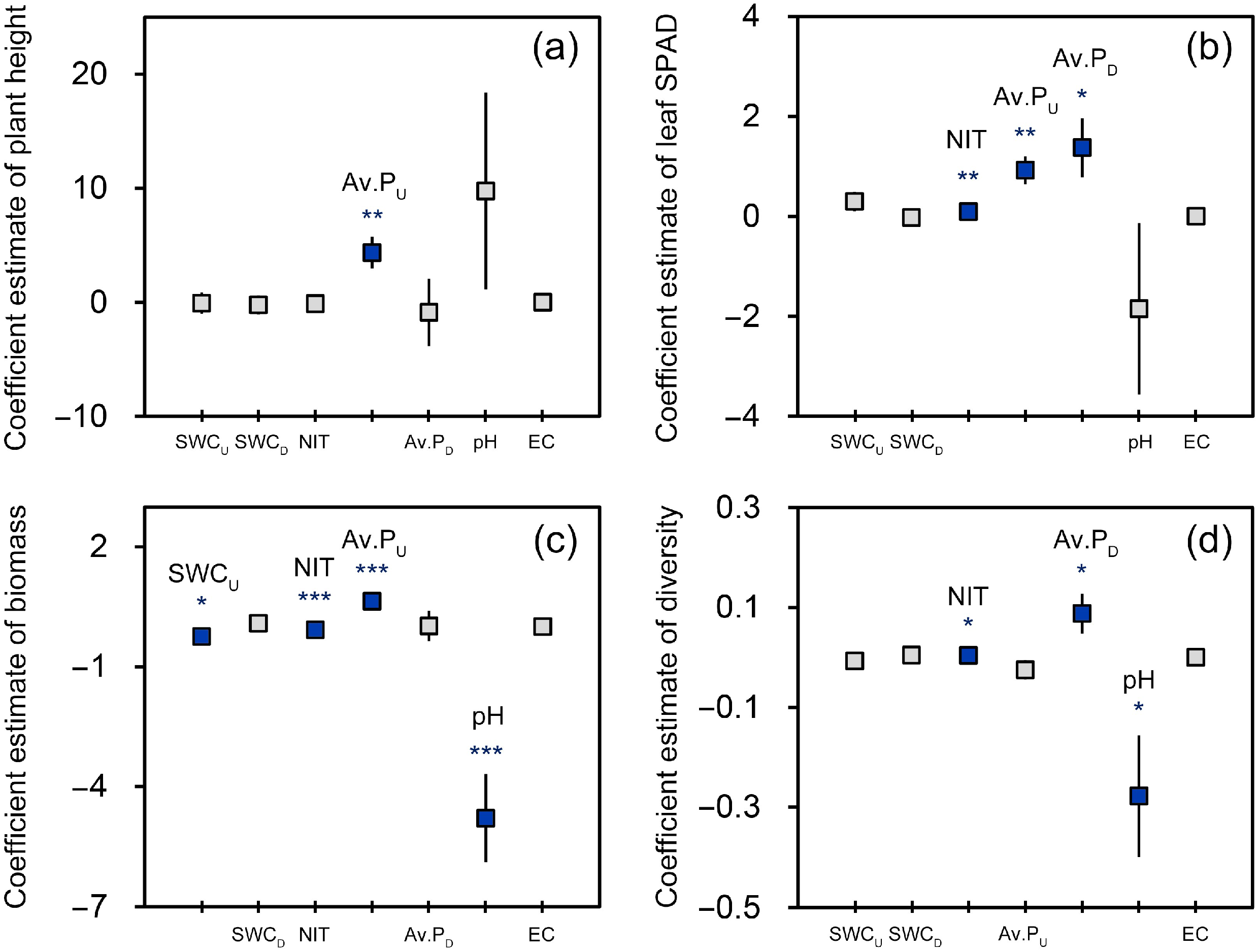
3.4. The Relationship between P. australis Growth and Soil Characteristics
4. Discussion
4.1. Responses of Soil Characteristics at Salinity Levels to N Addition
4.2. Effects of N Addition on Growth Indices of P. australis at Different Salinity Levels
4.3. Relationship between P. australis Growth and Soil Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zerbe, S.; Han, W.; Thevs, N.; Li, W.; He, P.; Schmitt, A.O.; Liu, Y.; Ji, C. Nitrogen and phosphorus stoichiometry of common reed (Phragmites australis) and its relationship to nutrient availability in northern China. Aquat. Bot. 2014, 112, 84–90. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.; Fedorenko, G.; Nevidomskaya, D.; Fedorenko, A.; Chaplygin, V.; Mandzhieva, S. Morphological and anatomical changes of Phragmites australis Cav. due to the uptake and accumulation of heavy metals from polluted soils. Sci. Total Environ. 2018, 636, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, X.; Fu, C.; Shi, T.; Yan, P. Effects of influent nitrogen loads on nitrogen and COD removal in horizontal subsurface flow constructed wetlands during different growth periods of Phragmites australis. Sci. Total Environ. 2018, 635, 1360–1366. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Fu, Z.; Qiao, H.; Liu, F. Assessing wetland nitrogen removal and reed (Phragmites australis) nutrient responses for the selection of optimal harvest time. J. Environ. Manag. 2021, 280, 111783. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, H.; Xia, L.; Yang, W.; Zhao, Y.; Jeelani, N.; An, S. Nitrogen cycling in plant and soil subsystems is driven by changes in soil salinity following coastal embankment in typical coastal saltmarsh ecosystems of Eastern China. Ecol. Eng. 2022, 174, 106467. [Google Scholar] [CrossRef]
- Hazelton, E.L.; Mozdzer, T.J.; Burdick, D.M.; Kettenring, K.M.; Whigham, D.F. Phragmites australis management in the United States: 40 years of methods and outcomes. AoB Plants 2014, 6, plu001. [Google Scholar] [CrossRef] [Green Version]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.J.; Stoffer, J.; Nestlerode, J.A. Wetlands as sinks for reactive nitrogen at continental and global scales: A meta-analysis. Ecosystems 2011, 14, 144–155. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, G.; Xing, Y.; Chen, F.; Zhang, X.; Wang, J.; Zhang, J.; Dai, G.; Zheng, X.; Sun, W. Nitrogen deposition and decreased precipitation does not change total nitrogen uptake in a temperate forest. Sci. Total Environ. 2019, 651, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Seghatoleslami, M.J.; Bradaran, R.; Ansarinia, E.; Mousavi, S.G. Effect of irrigation and nitrogen level on yield, yield components and some morphological traits of sunflower. Pak. J. Bot. 2012, 44, 1551–1555. [Google Scholar]
- Ravit, B.; Ehrenfeld, J.G.; Häggblom, M.M.; Bartels, M. The effects of drainage and nitrogen enrichment on Phragmites australis, Spartina alterniflora, and their root-associated microbial communities. Wetlands 2007, 27, 915–927. [Google Scholar] [CrossRef]
- Clevering, O.A. An investigation into the effects of nitrogen on growth and morphology of stable and die-back populations of Phragmites australis. Aquat. Bot. 1998, 60, 11–25. [Google Scholar] [CrossRef]
- Koppitz, H.; Dewender, M.; Ostendorp, W.; Schmieder, K. Amino acids as indicators of physiological stress in common reed Phragmites australis affected by an extreme flood. Aquat. Bot. 2004, 79, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.L. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. J. Appl. Ecol. 2003, 40, 344–353. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Y.; Chen, J.; Li, B. Ecophysiological characteristics of invasive Spartina alterniflora and native species in salt marshes of Yangtze River estuary, China. Estuar. Coast. Shelf Sci. 2009, 81, 74–82. [Google Scholar] [CrossRef]
- Qing, H.; Cai, Y.; Xiao, Y.; Yao, Y.; An, S. Nitrogen uptake and use efficiency of invasive Spartina alterniflora and native Phragmites australis: Effect of nitrogen supply. Clean Soil Air Water 2015, 43, 305–311. [Google Scholar] [CrossRef]
- Mao, W.; Kang, S.; Wan, Y.; Sun, Y.; Li, X.; Wang, Y. Yellow river sediment as a soil amendment for amelioration of saline land in the Yellow River Delta. Land Degrad. Dev. 2016, 27, 1595–1602. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zhang, W.; Song, Z.; Ding, Z.; Zhang, X. Comprehensive evaluation of the eco-environmental vulnerability in the Yellow River Delta wetland. Ecol. Indic. 2021, 125, 107514. [Google Scholar] [CrossRef]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Sun, X.-S.; Chen, Y.-H.; Zhuo, N.; Cui, Y.; Luo, F.-L.; Zhang, M.-X. Effects of salinity and concomitant species on growth of Phragmites australis populations at different levels of genetic diversity. Sci. Total Environ. 2021, 780, 146516. [Google Scholar] [CrossRef]
- Munzarova, E.; Lorenzen, B.; Brix, H.; Vojtiskova, L.; Votrubova, O. Effect of NH4+/NO3− availability on nitrate reductase activity and nitrogen accumulation in wetland helophytes Phragmites australis and Glyceria maxima. Environ. Exp. Bot. 2006, 55, 49–60. [Google Scholar] [CrossRef]
- Lu, G.; Xie, B.; Cagle, G.A.; Wang, X.; Han, G.; Wang, X.; Hou, A.; Guan, B. Effects of simulated nitrogen deposition on soil microbial community diversity in coastal wetland of the Yellow River Delta. Sci. Total Environ. 2021, 757, 143825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Bai, J.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X. Bacterial succession in salt marsh soils along a short-term invasion chronosequence of Spartina alterniflora in the Yellow River Estuary, China. Microb. Ecol. 2020, 79, 644–661. [Google Scholar] [CrossRef]
- Zhu, W.; Rezaei, E.E.; Nouri, H.; Yang, T.; Li, B.; Gong, H.; Lyu, Y.; Peng, J.; Sun, Z. Quick detection of field-scale soil comprehensive attributes via the integration of UAV and sentinel-2B remote sensing data. Remote Sens. 2021, 13, 4716. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Roncucci, N.; Bonari, E. Seasonal dynamics of aboveground and belowground biomass and nutrient accumulation and remobilization in giant reed (Arundo donax L.): A three-year study on marginal land. BioEnergy Res. 2013, 6, 725–736. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: New York, NY, USA, 2020; Volume 14. [Google Scholar]
- Luke, S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 2017, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, T.; Feng, X.; Zhu, W.; Zhang, L.; Pan, D.; Akram, N.A.; Ma, Q.; Zhong, Z.; Mahroof, S. Simulated grazing and nitrogen addition facilitate spatial expansion of Leymus chinensis clones into saline-alkali soil patches: Implications for Songnen grassland restoration in northeast China. Land Degrad. Dev. 2022, 33, 710–722. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, X.; Zou, Y.; Ding, S.; Zhang, J. Nitrogen Addition Effects on Wetland Soils Depend on Environmental Factors and Nitrogen Addition Methods: A Meta-Analysis. Water 2022, 14, 1748. [Google Scholar] [CrossRef]
- Grisso, R.D.; Alley, M.M.; Holshouser, D.L.; Thomason, W.E. Precision Farming Tools. Soil Electrical Conductivity; Virginia State University: Ettrick, VA, USA, 2005. [Google Scholar]
- Šimek, M.; Cooper, J. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Moseman-Valtierra, S.; Gonzalez, R.; Kroeger, K.D.; Tang, J.; Chao, W.C.; Crusius, J.; Bratton, J.; Green, A.; Shelton, J. Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O. Atmos. Environ. 2011, 45, 4390–4397. [Google Scholar] [CrossRef]
- Liu, C.; Yao, M.; Stegen, J.C.; Rui, J.; Li, J.; Li, X. Long-term nitrogen addition affects the phylogenetic turnover of soil microbial community responding to moisture pulse. Sci. Rep. 2017, 7, 17492. [Google Scholar] [CrossRef] [Green Version]
- Long, G.; Li, L.; Wang, D.; Zhao, P.; Tang, L.; Zhou, Y.; Yin, X. Nitrogen levels regulate intercropping-related mitigation of potential nitrate leaching. Agric. Ecosyst. Environ. 2021, 319, 107540. [Google Scholar] [CrossRef]
- Bostic, E.; White, J. Soil phosphorus and vegetation influence on wetland phosphorus release after simulated drought. Soil Sci. Soc. Am. J. 2007, 71, 238–244. [Google Scholar] [CrossRef]
- Whigham, D.F.; Verhoeven, J.T.; Samarkin, V.; Megonigal, P.J. Responses of Avicennia germinans (black mangrove) and the soil microbial community to nitrogen addition in a hypersaline wetland. Estuaries Coasts 2009, 32, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Change Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Change Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Lissner, J.; Schierup, H.-H. Effects of salinity on the growth of Phragmites australis. Aquat. Bot. 1997, 55, 247–260. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]
- Hassan, M.S.; Khair, A.; Haque, M.M.; Azad, A.K.; Hamid, A. Genotypic variation in traditional rice varieties for chlorophyll content, SPAD value and nitrogen use efficiency. Bangladesh J. Agric. Res. 2009, 34, 505–515. [Google Scholar] [CrossRef]
- Zhao, D.; Raja Reddy, K.; Kakani, V.; Read, J.; Carter, G. Corn (Zea mays L.) growth, leaf pigment concentration, photosynthesis and leaf hyperspectral reflectance properties as affected by nitrogen supply. Plant Soil 2003, 257, 205–218. [Google Scholar] [CrossRef]
- Weese, D.J.; Heath, K.D.; Dentinger, B.T.; Lau, J.A. Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 2015, 69, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Rickey, M.A.; Anderson, R.C. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. J. Appl. Ecol. 2004, 41, 888–896. [Google Scholar] [CrossRef]
- Hirtreiter, J.; Potts, D. Canopy structure, photosynthetic capacity and nitrogen distribution in adjacent mixed and monospecific stands of Phragmites australis and Typha latifolia. Plant Ecol. 2012, 213, 821–829. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, J.; Chen, Q.; Huang, W.; Hu, L.; Zhang, Y.; Fan, C. An investigation of the effects of capping on internal phosphorus release from sediments under rooted macrophytes (Phragmites australis) revegetation. Environ. Sci. Pollut. Res. 2018, 25, 24682–24694. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.-P.; Yang, Y.; Yu, M.; Wang, C.; Yan, J. Interactive effects of nitrogen and phosphorus additions on plant growth vary with ecosystem type. Plant Soil 2019, 440, 523–537. [Google Scholar] [CrossRef]


| Salinity Level | Salt Contents (g kg−1) | EC (ds m−1) | pH | Organic Matter (g kg−1) | Total N (g kg−1) | Avail. P (mg kg−1) | Avail. N (mg kg−1) |
|---|---|---|---|---|---|---|---|
| High | |||||||
| Range | 13.06–8.54 | 2.23–3.39 | 8.28–7.97 | 8–4.19 | 0.53–0.25 | 13.4–12.3 | 43.9–18.9 |
| Mean | 11.03 | 2.87 | 8.07 | 5.92 | 0.39 | 12.7 | 26.6 |
| S.E.s | 1.71 | 0.47 | 0.11 | 1.30 | 0.11 | 0.4 | 9.2 |
| Medium | |||||||
| Range | 6.94–4.19 | 1.11–1.82 | 8.49–8.13 | 12.16–2.8 | 0.86–0.47 | 12.3–11.2 | 49.1–26.7 |
| Mean | 5.43 | 1.42 | 8.29 | 6.97 | 0.55 | 11.7 | 40.0 |
| S.E.s | 0.95 | 0.27 | 0.12 | 3.66 | 0.14 | 0.4 | 9.0 |
| Low | |||||||
| Range | 3.97–2.02 | 0.55–1.05 | 8.65–8.24 | 11.5–5.72 | 0.76–0.43 | 12.4–11.1 | 82.7–20.7 |
| Mean | 3.20 | 0.85 | 8.49 | 7.92 | 0.60 | 11.7 | 49.1 |
| S.E.s | 0.75 | 0.22 | 0.15 | 1.90 | 0.10 | 0.5 | 21.9 |
| Soil Properties | Salinity (S) | N Addition (N) | Growth Stage (G) | Soil Depth (D) | S × N |
|---|---|---|---|---|---|
| pH | *** | *** | *** | * | *** |
| SWC | * | ** | *** | *** | ** |
| Soil Properties | Salinity (S) | N Addition (N) | Growth Stage (G) | Soil Depth (D) | S × N |
|---|---|---|---|---|---|
| EC | *** | * | *** | *** | – |
| NO3− | * | *** | *** | *** | *** |
| Avail. P | *** | *** | – | *** | *** |
| Growth Indices | Salinity (S) | N Addition (N) | Growth Stage (G) | S × N | S × N × G |
|---|---|---|---|---|---|
| Plant height | *** | *** | *** | *** | *** |
| SPAD | *** | – | *** | – | * |
| Treatments | Biomass (t ha−1 F.W.) | Dry Matter (t ha−1 D.W.) | Plant Moisture (%) | Vegetation Diversity |
|---|---|---|---|---|
| H | 33.5 ± 1.2 a | 18.1 ± 0.8 a | 53.8 ± 0.6 a | 0.50 ± 0.01 c |
| HN | 21.7 ± 0.5 c | 11.5 ± 0.3 c | 53.2 ± 0.3 ab | 0.75 ± 0.01 b |
| M | 28.5 ± 1.1 b | 14.7 ± 0.6 b | 51.5 ± 0.6 b | 0.82 ± 0.02 b |
| MN | 18 ± 0.4 d | 8.8 ± 0.2 d | 48.6 ± 0.3 c | 1.36 ± 0.02 a |
| L | 12.5 ± 0.5 e | 5.9 ± 0.3 e | 47 ± 0.5 c | 0.44 ± 0.01 c |
| LN | 7.6 ± 1.2 f | 3.6 ± 0.5 f | 46.9 ± 1 c | 0.74 ± 0.06 b |
| S | *** | *** | *** | *** |
| N | *** | *** | * | *** |
| S × N | ** | ** | – | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Han, Y.; Li, J.; Liu, Z.; Hou, R.; Zhang, Y.; Dou, W.; Wang, B.; Ouyang, Z. Responses of Phragmites australis to Nitrogen Addition along Salinity Gradients in Coastal Saline–Alkali Soil. Land 2022, 11, 2320. https://doi.org/10.3390/land11122320
Gong H, Han Y, Li J, Liu Z, Hou R, Zhang Y, Dou W, Wang B, Ouyang Z. Responses of Phragmites australis to Nitrogen Addition along Salinity Gradients in Coastal Saline–Alkali Soil. Land. 2022; 11(12):2320. https://doi.org/10.3390/land11122320
Chicago/Turabian StyleGong, Huarui, Yanyun Han, Jing Li, Zhen Liu, Ruixing Hou, Yitao Zhang, Wenjun Dou, Bing Wang, and Zhu Ouyang. 2022. "Responses of Phragmites australis to Nitrogen Addition along Salinity Gradients in Coastal Saline–Alkali Soil" Land 11, no. 12: 2320. https://doi.org/10.3390/land11122320






