Mineralogical Characterization of Dolomitic Aggregate Concrete: The Camarasa Dam (Catalonia, Spain)
Abstract
:1. Introduction
dolomite portlandite calcite brucite
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
3. Results
3.1. Raw Dolostones
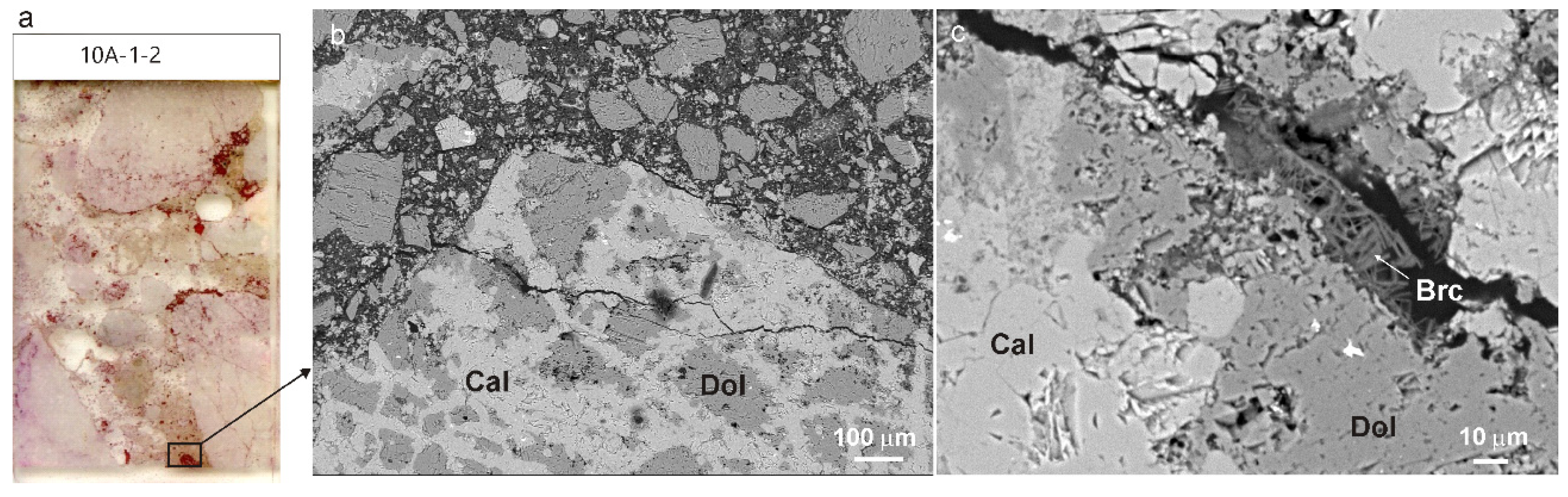
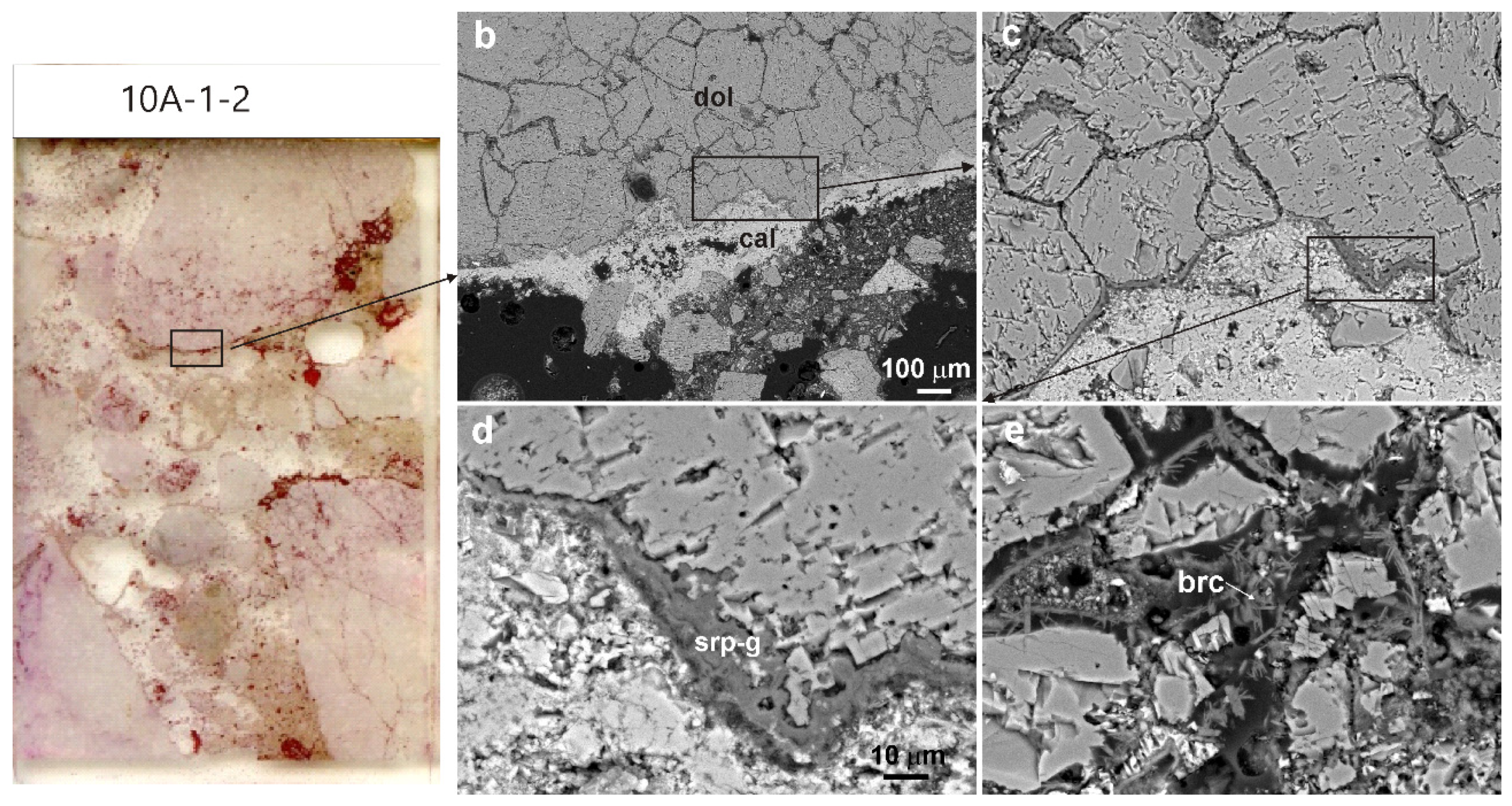
3.2. Concrete Petrography and Mineralogy
3.2.1. Type A Concrete
3.2.2. Type B Concrete
4. Discussion
CSH brucite serpentine group mineral portlandite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swenson, E.G. A Reactive Aggregate Undetected by ASTM Tests. ASTM Bull. 1957, 226, 48–51. [Google Scholar]
- Swenson, E.G.; Gillott, J.E. Characteristics of Kingston carbonate rock reaction. Highw. Res. Board Bull. 1960, 275, 18–31. [Google Scholar]
- Hadley, D.W. Alkali Reactivity of Carbonate Rocks-Expansion and Dedolomitization. Highw. Res. Board Proc. 1961, 40, 462–474. [Google Scholar]
- Deng, M.; Tang, M.S. Mechanism of dedolomitization and expansion of dolomitic rocks. Cem. Concr. Res. 1993, 23, 1397–1408. [Google Scholar]
- Tong, L.; Deng, M.; Lan, X.H.; Tang, M.S. A case study of two airport runways affected by alkali-carbonate reaction. Part one: Evidence of deterioration and evaluation of aggregates. Cem. Concr. Res. 1997, 27, 321–328. [Google Scholar] [CrossRef]
- Gao, P.; Lu, X.; Geng, F.; Li, X.; Hou, J.; Lin, H.; Shi, N. Production of MgO-type expansive agent in dam concrete by use of industrial by-products. Build. Environ. 2008, 43, 453–457. [Google Scholar] [CrossRef]
- Prinčič, T.; Štukovnik, P.; Pejovnik, S.; De Schutter, G.; Bosiljkov, V.B. Observations on dedolomitization of carbonate concrete aggregates, implications for ACR and expansion. Cem. Concr. Res. 2013, 54, 151–160. [Google Scholar] [CrossRef]
- Tong, L.; Tang, M. Correlation between reaction and expansion of alkali-carbonate reaction. Cem. Concr. Res. 1995, 25, 470–476. [Google Scholar] [CrossRef]
- Swenson, E.G.; Gillott, J.E. Alkali reactivity of dolomitic limestone aggregate. Mag. Concr. Res. 1967, 19, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Katayama, T. A critical review of carbonate rock reactions—Is their reactivity useful or harmful? In Proceedings of the 9th Int. Conf. on Alkali-Aggregate Reaction in Concrete (ICAAR), London, UK, 27–31 July 1992; pp. 508–517. [Google Scholar]
- Charlwood, R.; Sims, I.A. Review of the Effectiveness of Strategies to Manage Expansive Chemical Reactions in Dams and Hydro Projects. In Proceedings of the Dam Swelling Concrete DSC, London, UK, 15 June 2017; p. 3. [Google Scholar]
- Newell, V.A.; Wagner, C.D. Modifications to Hiwassee Dam and planned modification to Fontana and Chickamauga Dams by the Tennessee Valley Authority to manage alkali-aggregate reaction. In Proceedings of the 2nd Int. Conf. on Alkali-Aggregate Reaction in Hydroelectric Plants and Dams. USCOLD, Chattanooga, TN, USA, 22–27 October 1995; pp. 83–100. [Google Scholar]
- Galí, S.; Ayora, C.; Alfonso, P.; Tauler, E.; Labrador, M. Kinetics of dolomite-portlandite reaction, Application to Portland cement concrete. Cem. Concr. Res. 2001, 31, 933–939. [Google Scholar] [CrossRef]
- García, E.; Alfonso, P.; Labrador, M.; Galí, S. Dedolomitization in different alkaline media: Application to Portland cement paste. Cem. Concr. Res. 2003, 33, 1443–1448. [Google Scholar] [CrossRef]
- García, E.; Alfonso, P.; Tauler, E.; Galí, S. Surface alteration of dolomite in dedolomitization reaction in alkaline media. Cem. Concr. Res. 2003, 33, 1449–1456. [Google Scholar] [CrossRef]
- Wang, H.; Gillott, J.E. Alkali–carbonate reaction: significance of chemical and mineral admixtures. Mag. Concr. Res. 1995, 47, 69–75. [Google Scholar] [CrossRef]
- Katayama. The so-called alkali-carbonate reaction (ACR)—Its mineralogical and geochemical details, with special reference to ASR. Cem. Concr. Res. 2010, 40, 643–675. [Google Scholar] [CrossRef]
- Sant John, D.A.; Poole, A.W.; Sims, I. Concrete Petrography; Edward Arnold: London, UK, 1998; 474p. [Google Scholar]
- Qian, G.; Deng, M.; Lan, X.; Xu, Z.; Tang, M. Alkali carbonate reaction expansion of dolomitic limestone aggregates with porphyrotopic texture. Eng. Geol. 2002, 63, 17–29. [Google Scholar] [CrossRef]
- López-Buendía, A.M.; Climent, V.; Verdú, P. Lithological influence of aggregate in the alkali-carbonate reaction. Cem. Concr. Res. 2006, 36, 1490–1500. [Google Scholar] [CrossRef]
- Girard, J.P.; Sanjuan, B.; Czernichowski-Lauriol, I.; Fouillac, C. Diagenesis of the Oseberg Sandstone Reservoir (North Sea): An example of integration of core, formation fluid and geochemical modelling studies. AAPG Bull. 1996, 5. (CONF-960527). [Google Scholar]
- Lanza, V.; Alaejos, P. Optimized Gel Pat Test for Detection of Alkali-Reactive Aggregates. ACI Mater. J. 2012, 109, 403. [Google Scholar]
- Lindgård, J.; Nixon, P.J.; Borchers, I.; Schouenborg, B.; Wigum, B.J.; Haugen, M.; Åkesson, U. The EU “PARTNER” Project—European standard tests to prevent alkali reactions in aggregates: Final results and recommendations. Cem. Concr. Res. 2010, 40, 611–635. [Google Scholar] [CrossRef] [Green Version]
- Díez-Cascón, J.; Bueno, F. Ingeniería de Presas: Presas de fábrica; Univ. de Cantabria Publ. Santander: Cantabria, Spain, 2001; p. 474. [Google Scholar]
- Solana, J. Sand-cement. Rev. de Obras Públicas 1916, 64, 85–88. [Google Scholar]
- Martínez-Roig, J.M. Instalación de la confluencia. Construcción de la presa de Camarasa, Col. Tècnico-Històrica de FECSA; FECSA: Barcelona, Spain, 1995; 84p. [Google Scholar]
- Pocoví, A. Estudio geológico de las Sierras Marginales Catalanas (Prepirineo de Lérida). Acta Geol. Hisp. 1998, XIII, 73–79. [Google Scholar]
- Rodríguez-Carvajal, J. An introduction to the program FullProf 2000, Laboratoire Leon Brillouin (CEA-CNRS), Gif-sur-Yvette, France. 2001. Available online: https://www.psi.ch/sites/default/files/import/sinq/dmc/ManualsEN/fullprof.pdf (accessed on 20 December 2019).
- Blanco, A.; Segura, I.; Cavalaro, S.H.P.; Chinchón-Payá, S.; Aguado, A. Sand-cement concrete in the century-old Camarasa dam. J. Perform. Constr. Facil. 2015, 30. [Google Scholar] [CrossRef] [Green Version]
- Hewlett, P.C.; Liska, M. Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Katayama, T.; Jensen, V.; Rogers, C.A. The enigma of the ‘so-called’alkali–carbonate reaction. Proc. Inst. Civ. Eng. -Constr. Mater. 2016, 169, 223–232. [Google Scholar] [CrossRef]
- Jin, F.; Wang, F.; Al-Tabbaa, A. Three-year performance of in-situ solidified/stabilised soil using novel MgO-bearing binders. Chemosphere 2016, 144, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Machner, A.; Zajac, M.; Haha, M.B.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Stability of the hydrate phase assemblage in Portland composite cements containing dolomite and metakaolin after leaching, carbonation, and chloride exposure. Cem. Concr. Res. 2018, 89, 89–106. [Google Scholar] [CrossRef]
- Cabrera Vélez, P.J. La Evolución de los Conglomerantes Hidráulicos en Presas. Master’s Thesis, Universitat Politécnica de Catalunya, Barcelona, Spain, 2013. [Google Scholar]
- Lee, H.; Cody, R.D.; Cody, A.M.; Spry, P.G. Observations on brucite formation and the role of brucite in Iowa highway concrete deterioration. Environ. Eng. Geosci. 2002, 8, 137–145. [Google Scholar] [CrossRef]
- Katayama, T. How to identify carbonate rock reactions in concrete. Mater. Charact. 2004, 53, 85–104. [Google Scholar] [CrossRef]
- Beyene, M.; Snyder, A.; Lee, R.J.; Blaszkiewicz, M. Alkali Silica Reaction (ASR) as a root cause of distress in a concrete made from Alkali Carbonate Reaction (ACR) potentially susceptible aggregates. Cem. Concr. Res. 2013, 51, 85–95. [Google Scholar] [CrossRef]
- Locati, F.; Falcone, D.; Marfil, S. Dedolomitization and alkali-silica reactions in low-expansive marbles from the province of Córdoba, Argentina. A microstructural and chemical study. Constr. Build. Mater. 2014, 58, 171–181. [Google Scholar] [CrossRef]
- Štukovnik, P.; Prinčič, T.; Pejovnik, R.S.; Bokan, B.V. Alkali-carbonate reaction in concrete and its implications for a high rate of long-term compressive strength increase. Const. Building Mater. 2014, 50, 699–709. [Google Scholar] [CrossRef]








| Component | Particle Size (mm) | Type A (wt%) | Type B (wt%) |
|---|---|---|---|
| Dolomitic aggregate | 10–150 | - | 66 |
| Dolomitic aggregate | 10–70 | 62 | - |
| Dolomitic aggregate | 1–10 | 13 | 12 |
| Dolomitic aggregate | 0.1–1 | 12.2 | 10.8 |
| Dolomitic aggregate | <0.1 | 3.8 | 6 |
| Portland cement | 9.0 | 5.2 |
| Type | Sample | Calcite | Dolomite | Quartz | Brucite | Microcline |
|---|---|---|---|---|---|---|
| A | 5bi-1 | 35 | 52 | - | 13 | - |
| A | 5bi-2 | 27 | 66 | - | 7 | - |
| A | P5bc | 56 | 40 | 2 | 2 | - |
| B | 4a | 16 | 66 | 11 | 1 | 6 |
| B | 13a | 18 | 59 | 15 | 1 | 7 |
| B | P4a | 18 | 67 | 11 | <1 | 4 |
| B | P6a | 19 | 65 | 11 | <1 | 4 |
| B | P10a1 | 18 | 65 | 12 | <1 | 5 |
| B | P10a2 | 26 | 55 | 13 | 1 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, E.; Alfonso, P.; Tauler, E. Mineralogical Characterization of Dolomitic Aggregate Concrete: The Camarasa Dam (Catalonia, Spain). Minerals 2020, 10, 117. https://doi.org/10.3390/min10020117
Garcia E, Alfonso P, Tauler E. Mineralogical Characterization of Dolomitic Aggregate Concrete: The Camarasa Dam (Catalonia, Spain). Minerals. 2020; 10(2):117. https://doi.org/10.3390/min10020117
Chicago/Turabian StyleGarcia, Encarnación, Pura Alfonso, and Esperança Tauler. 2020. "Mineralogical Characterization of Dolomitic Aggregate Concrete: The Camarasa Dam (Catalonia, Spain)" Minerals 10, no. 2: 117. https://doi.org/10.3390/min10020117






