Review of Filters for Air Sampling and Chemical Analysis in Mining Workplaces
Abstract
:1. Introduction
2. Ambient and Workplace Aerosol Sampling and Analysis
3. Filter Characteristics
3.1. Filter Material and Structure
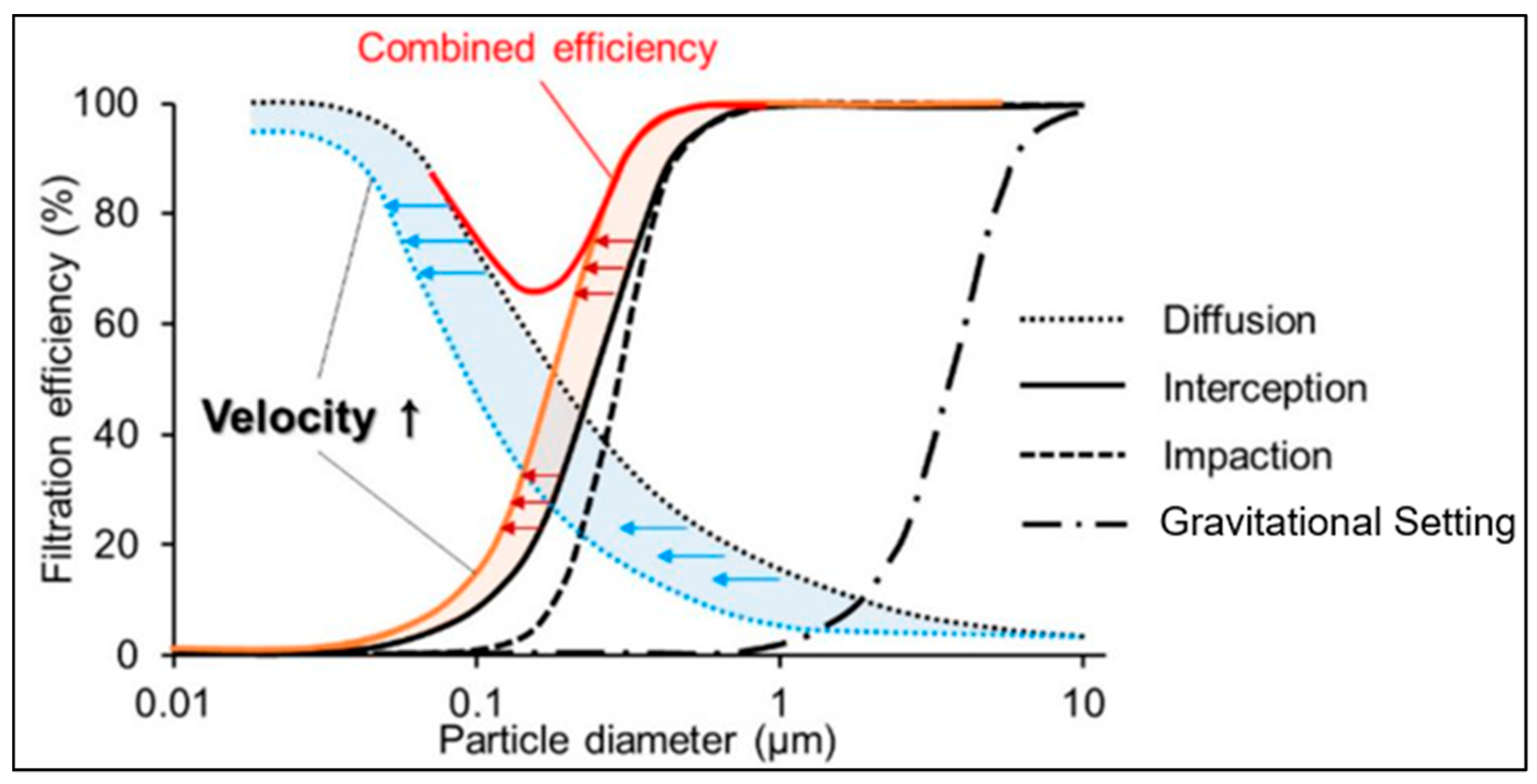
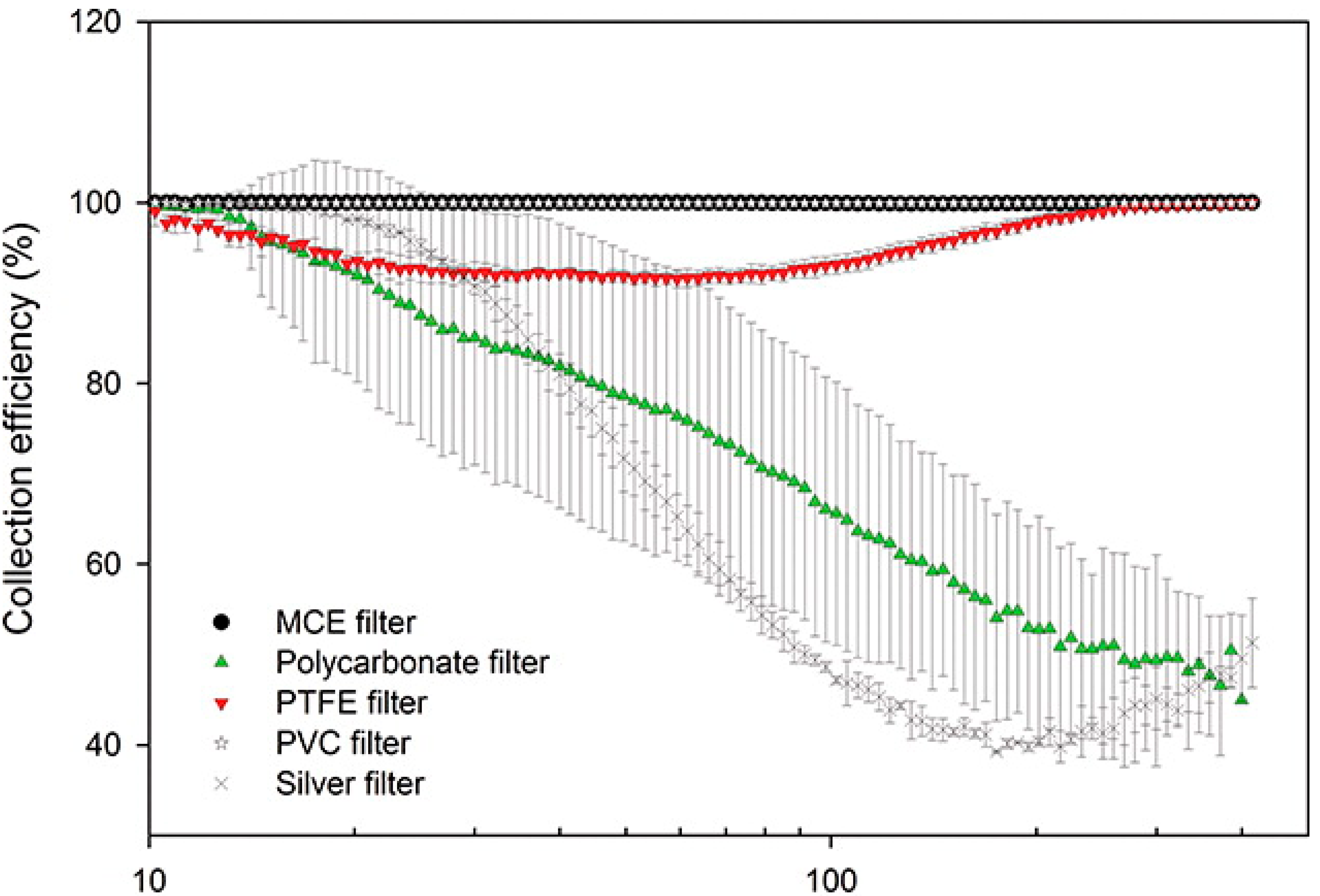
3.2. Filter Collection Efficiency
3.3. Potential Environmental Artifacts
3.4. Inhomogeneous Sample Deposits and Filter Cassette Assembly
3.5. Cost and Availability
4. Membrane Filters for Respirable Crystalline Silica (RCS) Quantification
4.1. Spectroscopic Analysis Methods for Coal Mine Dust
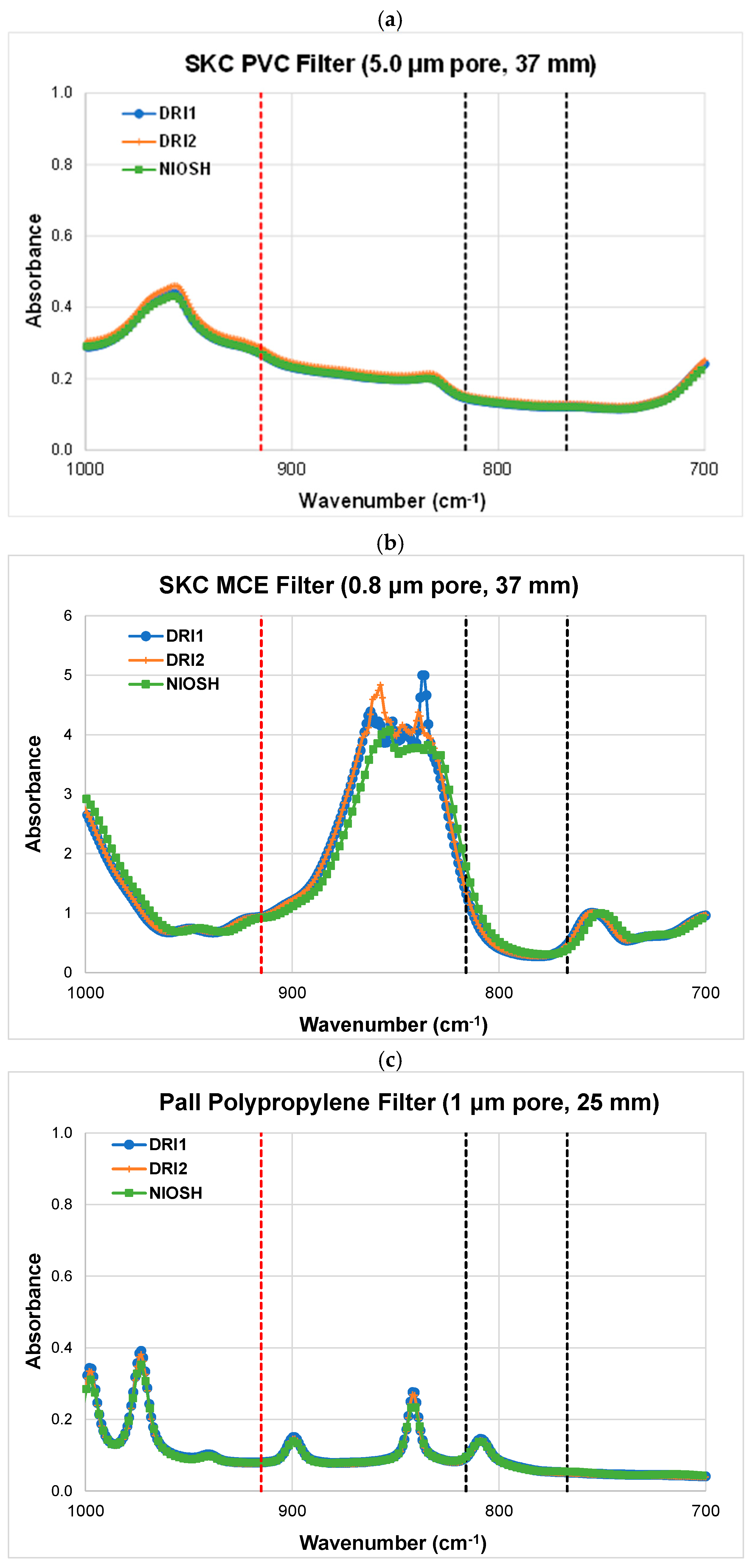
4.1.1. FTIR Spectrometry
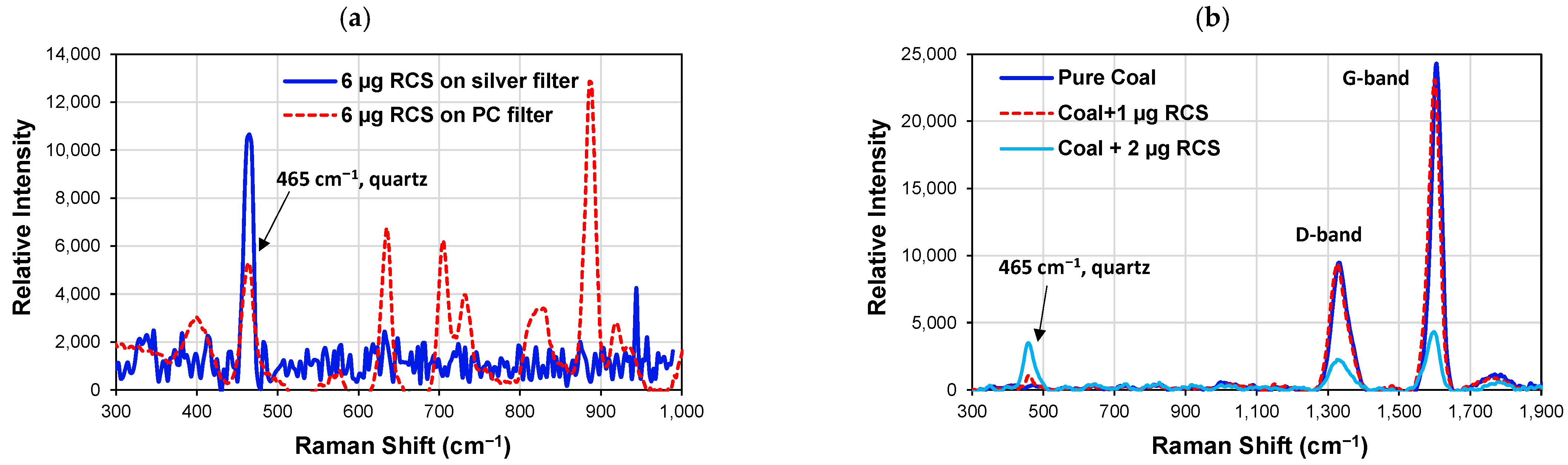
4.1.2. Raman Spectroscopy
4.2. Detection Limits
5. Major Findings and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Spurny, K.R. The history of dust and aerosol filtration. In Advances in Aerosol Filtration; Spurny, K.R., Ed.; CRC Press LLC: Boca Raton, FL, USA, 1998; pp. 3–12. [Google Scholar]
- Davies, C.N.; Aylward, M. Photoelectric measurement of coal dust stains of filter paper. Br. J. Ind. Med. 1949, 6, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldhaus, G.M. Schutzmasken in vergangegen Jahrhunderten. Gasmaske 1929, 1, 104–114. [Google Scholar]
- Fieldner, A.C.; Oberfell, G.G.; Teague, M.C.; Lawrence, J.N. Methods of testing gas masks and absorbents. J. Ind. Eng. Chem. 1919, 11, 519–540. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, N.A. The Mechanics of Aerosols; Pergamon Press Ltd.: New York, NY, USA, 1964. [Google Scholar]
- Spurný, K. Membrane filters in aerosology. II. Filtration mechanisms in membrane filters. Zent. Biol. Aerosolforsch. 1965, 12, 530–545. [Google Scholar]
- Davies, C.N. Air Filtration; Academic Press: London, UK, 1973. [Google Scholar]
- Davies, C.N. Filtration of aerosols. J. Aerosol Sci. 1983, 14, 147–161. [Google Scholar] [CrossRef]
- Langmuir, I. Report on Smokes and Filters, Section I; U.S. Office of Scientific Research and Development: Washington, DC, USA, 1942.
- Langmuir, I. The Collected Works of Irving Langmuir; Pergamon Press: London, UK, 1962. [Google Scholar]
- Kaufman, A. Die Faserstoffe für Atemschutzfilter−Wirkungsweise und Verbesserungsmöglichkeiten. VDI 1936, 80, 593–599. [Google Scholar]
- Jung, S.; Kim, J. Advanced Design of Fiber-Based Particulate Filters: Materials, Morphology, and Construction of Fibrous Assembly. Polymers 2020, 12, 1714. [Google Scholar] [CrossRef]
- Barrett, L.W.; Rousseau, A.D. Aerosol loading performance of electret filter media. Am. Ind. Hyg. Assoc. J. 1998, 59, 532–539. [Google Scholar] [CrossRef]
- Lee, H.; Segets, D.; Sub, S.; Peukert, W.; Chen, S.C.; Pui, D.Y.H. Liquid filtration of nanoparticles through track-etched membrane filters under favorable and different ionic strength conditions: Experiments and modeling. J. Membr. Sci. 2017, 524, 682–690. [Google Scholar] [CrossRef]
- Cai, H.; Chen, M.; Chen, Q.; Du, F.; Liu, J.; Shi, H. Microplastic quantification affected by structure and pore size of filters. Chemosphere 2020, 257, 127198. [Google Scholar] [CrossRef]
- Wright, S.L.; Levermore, J.M.; Kelly, F.J. Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ. Sci. Technol. 2019, 53, 8947–8956. [Google Scholar] [CrossRef]
- Mainelis, G. Bioaerosol sampling: Classical approaches, advances, and perspectives. Aerosol Sci. Technol. 2020, 54, 496–519. [Google Scholar] [CrossRef]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol sampling: Sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Wigginton, R.K.; Vikesland, P.J. Gold-coated polycarbonate membrane filter for pathogen concentration and SERS-based detection. Analyst 2010, 135, 1320–1326. [Google Scholar] [CrossRef]
- Kildeso, J.; Nielsen, B.H. Exposure assessment of airborne microorganisms by fluorescence microscopy and image processing. Ann. Occup. Hyg. 1997, 41, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2016, 302, 1600353. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Pui, D.Y.H.; Rubow, K.L. Characteristics of air sampling filter media. In Aerosols in the Mining and Industrial Work Environments; Ann Arbor Science: Ann Arbor, MI, USA, 1983; pp. 989–1037. [Google Scholar]
- Li, X.W.; Hoff, S.J.; Bundy, D.S.; Harmon, J.; Xin, H.; Zhu, J. Biofilter—A malodor control technology for livestock industry. J. Environ. Sci. Health-Part A-Toxics/Haz. Subst. Env. Eng. 1996, 31, 2275–2285. [Google Scholar] [CrossRef]
- Spurny, K.R. (Ed.) Advances in Aerosol Filtration; CRC Press LLC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Lippmann, M. Filters and filter holders. In Air Sampling Instruments for Evaluation of Atmospheric Contaminants, 9th ed.; Cohen, B.S., McCammon, C.S., Jr., Eds.; American Conference of Governmental Industrial Hygienists (ACGIH): Cincinnati, OH, USA, 2001; pp. 281–314. [Google Scholar]
- Raynor, P.C.; Leith, D.; Lee, K.W.; Mukund, R. Sampling and analysis using filters. In Aerosol Measurement: Principles, Techniques and Applications, 3rd ed.; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 107–128. [Google Scholar]
- Chow, J.C. Critical review: Measurement methods to determine compliance with ambient air quality standards for suspended particles. J. Air Waste Manag. Assoc. 1995, 45, 320–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuchman, D.P. Research toward direct analysis of quartz dust on filters using FTIR spectroscopy. Bur. Mines Inf. Circ. 1992, 9309, 1–17. [Google Scholar]
- Tuchman, D.P.; Volkwein, J.C.; Vinson, R.P. Implementing infrared determination of quartz particulates on novel filters for a prototype dust monitor. J. Environ. Monit. 2008, 10, 671–678. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C. Ambient aerosol sampling. In Aerosol Measurement: Principles, Techniques and Applications, 3rd ed.; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 591–614. [Google Scholar]
- Watson, J.G.; Tropp, R.J.; Kohl, S.D.; Wang, X.L.; Chow, J.C. Filter processing and gravimetric analysis for suspended particulate matter samples. Aerosol Sci. Eng. 2017, 1, 193–205. [Google Scholar] [CrossRef]
- Lilienfeld, P.; Dulchinos, J. Portable instantaneous mass monitoring for coal mine dust. Am. Ind. Hyg. Assoc. J. 1972, 33, 136–145. [Google Scholar] [CrossRef]
- Patashnick, H. On-line, real-time instrumentation for diesel particulate testing. Diesel Prog. N. Am. 1987, 53, 43–44. [Google Scholar]
- Sorensen, C.M.; Gebhart, J.; O’Hern, T.J.; Rader, D.J. Optical measurement techniques: Fundamentals and applications. In Aerosol Measurement: Principles, Techniques and Applications, 3rd ed.; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 269–312. [Google Scholar]
- Chow, J.C.; Watson, J.G.; Park, K.; Lowenthal, D.H.; Robinson, N.F.; Magliano, K.L. Comparisn of particle light scattering and PM2.5 mass in central California. J. Air Waste Manag. Assoc. 2006, 56, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Chancellor, G.; Evenstad, J.; Farnsworth, J.E.; Hase, A.; Olson, G.M.; Sreenath, A.; Agarwal, J.K. A novel optical instrument for estimating size segregated aerosol mass concentration in real time. Aerosol Sci. Technol. 2009, 43, 939–950. [Google Scholar] [CrossRef]
- Kleinman, M.T.; Head, S.J.; Morris, R.E.; Stevenson, E.D.; Altshuler, S.L.; Chow, J.C.; Watson, J.G.; Hidy, G.M.; Mueller, P.K. Air quality measurements: From rubber bands to tapping the rainbow: Critical review discussion. J. Air Waste Manag. Assoc. 2017, 67, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidy, G.M.; Mueller, P.K.; Altshuler, S.L.; Chow, J.C.; Watson, J.G. Critical review: Air quality measurements—From rubber bands to tapping the rainbow. J. Air Waste Manag. Assoc. 2017, 67, 637–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mine Safety and Health Administration (MSHA). 30 CFR Part 74: Coal mine dust sampling devices. Fed. Regist. 2010, 70, 17523–17529. [Google Scholar] [CrossRef]
- Page, S.J.; Volkwein, J.C. A revised conversion factor relating respirable dust concentrations measured by 10 mm Dorr-Oliver nylon cyclones operated at 1.7 and 2.0 L min−1. J. Environ. Monit. 2009, 11, 684–689. [Google Scholar] [CrossRef]
- Patashnick, H.; Hemenway, C.L. Oscillating fiber microbalance. Rev. Sci. Instrum. 1969, 40, 1008–1011. [Google Scholar] [CrossRef]
- Patashnick, H.; Rupprecht, E.G. Continuous PM10 measurements using the tapered element oscillating microbalance. J. Air Waste Manag. Assoc. 1991, 41, 1079–1083. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Tuchman, D.P.; Vinson, R.P. Performance of a prototype personal dust monitor for coalmine use. In Mine Ventilation; AA Balkema: Lisse, The Netherlands, 2002. [Google Scholar]
- Volkwein, J.C.; Vinson, R.P.; McWilliams, L.J.; Tuchman, D.P.; Mischler, S.E. Performance of a New Personal Respirable Dust Monitor for Mine Use; National Institute of Occupational Safety and Health: Pittsburgh, PA, USA, 2004.
- Volkwein, J.C.; Vonson, R.P.; Page, S.J.; McWilliams, L.J.; Joy, G.J.; Mischler, S.E.; Tuchman, D.P. Laboratory and Field Performance of a Continuously Measuring Personal Respirable Dust Monitor; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Washington, DC, USA, 2006.
- Kohler, J. NIOSH analysis of comments questioning the use of the CPDM. In MSHA Public Hearings on the Proposed Rule to Limit Miners’ Exposure to Coalmine Dust; AB64-COMM-93; Mine Safety and Health Administration: Arlington, VA, USA, 2010. [Google Scholar]
- Page, S.J.; Volkwein, J.C.; Vinson, R.P.; Joy, G.J.; Mischler, S.E.; Tuchman, D.P.; McWilliams, L.J. Equivalency of a personal dust monitor to the current United States coal mine respirable dust sampler. J. Environ. Monit. 2008, 10, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Belle, B. Pairwise evaluation of PDM3700 and traditional gravimetric sampler for personal dust exposure assessment. In Proceedings, The Australian Mine Ventilation Conference; The Australasian Institute of Mining and Metallurgy: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Lidén, G.; Gudmundsson, A. Optimization of a cyclone to the 1993 international sampling convention for respirable dust. Appl. Occup. Environ. Hyg. 1996, 11, 1398–1408. [Google Scholar] [CrossRef]
- Verpaele, S.; Jouret, J. A comparison of the performance of samplers for respirable dust in workplaces and laboratory analysis for respirable quartz. Ann. Occup. Hyg. 2013, 57, 54–62. [Google Scholar]
- Watson, J.G.; Chow, J.C.; Shah, J.J.; Pace, T.G. The effect of sampling inlets on the PM10 and PM15 to TSP concentration ratios. J. Air Pollut. Control Assoc. 1983, 33, 114–119. [Google Scholar] [CrossRef]
- Farcas, D.; Lee, T.; Chisholm, W.P.; Soo, J.C.; Harper, M. Replacement of filters for respirable quartz measurement in coal mine dust by infrared spectroscopy. J. Occup. Environ. Hyg. 2016, 13, D16–D22. [Google Scholar] [CrossRef]
- Mine Safety and Health Administration (MSHA). Infrared Determination of Quartz in Respirable Coal Mine Dust-Method No. MSHA P7; Mine Safety and Health Administration: Pittsburgh, PA, USA, 2013.
- The National Institute of Occupational Safety and Health (NIOSH). Quartz in Coal Mine Dust, by IR (redeposition)—NIOSH Method 7603; The National Institute for Occupational Safety and Health (NIOSH): Washington, DC, USA, 2003.
- Lorberau, C.D.; Carsey, T.P.; Fischbach, T.J.; Mulligan, K.J. Evaluation of direct-on-filter methods for the determination of respirable α-quartz. Appl. Occup. Environ. Hyg. 1990, 5, 27–35. [Google Scholar] [CrossRef]
- Miller, A.L.; Drake, P.L.; Murphy, N.C.; Cauda, E.G.; Lebouf, R.F.; Markevicius, G. Deposition uniformity of coal dust on filters and its effect on the accuracy of FTIR analyses for silica. Aerosol Sci. Technol. 2013, 47, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.L.; Cauda, E.; Chubb, L.G.; Tuchman, D.P.; Rubinstein, E.N. Performance comparison of four portable FTIR instruments for direct-on-filter measurement of respirable crystalline silica. Ann. Work. Expo. Health 2020, 64, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Cauda, E.; Miller, A.; Drake, P. Promoting early exposure monitoring for respirable crystalline silica: Taking the laboratory to the mine site. J. Occup. Environ. Hyg. 2016, 13, D39–D45. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.F.; Autenrieth, D.A.; Cauda, E.; Chubb, L.; Spear, T.M.; Wock, S.; Rosenthal, S. A comparison of respirable crystalline silica concentration measurements using a direct-on-filter Fourier transform infrared (FT-IR) transmission method vs. a traditional laboratory X-ray diffraction method. J. Occup. Environ. Hyg. 2018, 15, 743–754. [Google Scholar] [CrossRef]
- Miller, A.L.; Drake, P.L.; Murphy, N.C.; Noll, J.D.; Volkwein, J.C. Evaluating portable infrared spectrometers for measuring the silica content of coal dust. J. Environ. Monit. 2012, 14, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.L.; Weakley, A.T.; Griffiths, P.R.; Cauda, E.G.; Bayman, S. Direct-on-filter α-quartz estimation in respirable coal mine dust using transmission Fourier Transform Infrared Spectrometry and partial least squares regression. Appl. Spectrosc. 2017, 71, 1014–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stach, R.; Barone, T.; Cauda, E.; Krebs, P.; Pejcic, B.; Daboss, S.; Mizaikoff, B. Direct infrared spectroscopy for the size-independent identification and quantification of respirable particles relative mass in mine dusts. Anal. Bioanal. Chem. 2020, 412, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Pampena, J.D.; Cauda, E.G.; Chubb, G.; Meadows, J.J. Use of the field-based silica monitoring technique in a coal mine: A case study. Min. Metall. Explor. 2020, 37, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Murphy, N.C.; Bayman, S.J.; Briggs, Z.P.; Kilpatrick, A.D.; Quinn, C.A.; Wadas, M.R.; Cauda, E.G.; Griffiths, P.R. Evaluation of diffuse reflection infrared spectrometry for end-of-shift measurement of a-quartz in coal dust samples. J. Occup. Environ. Hyg. 2015, 12, 421–430. [Google Scholar] [CrossRef]
- Chow, J.C.; Lowenthal, D.H.; Chen, L.-W.A.; Wang, X.L.; Watson, J.G. Mass reconstruction methods for PM2.5: A review. Air Qual. Atmos. Health 2015, 8, 243–263. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, M. Sampling aerosols by filtration. In Air Sampling Instruments for Evaluation of Atmospheric Contaminants, 7th ed.; Hering, S.V., Ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1989; pp. 305–336. [Google Scholar]
- Millette, J.R.; Brown, R.S. Chapter 13—Environmental forensic microscopy. In Introduction to Environmental Forensics, 3rd ed.; Murphy, B.L., Morrison, R.D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 487–511. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA). National ambient air quality standards for particulate matter: Final rule. Fed. Regist. 1997, 62, 38651–38760. [Google Scholar]
- Rattigan, O.V.; Carpenter, A.C.; Felton, H.D.; Civerolo, K.L. Optical carbon analysis on Teflon filters from the FRM network in New York. Atmos. Pollut. Res. 2021, 12, 101163. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Frazier, C.A. X-ray fluorescence analysis of ambient air samples. In Elemental Analysis of Airborne Particles; Landsberger, S., Creatchman, M., Eds.; Advances in Environmental, Industrial and Process Control Technologies; Gordon and Breach Science: Amsterdam, The Netherlands, 1999; Volume 1, pp. 67–96. [Google Scholar]
- Chow, J.C.; Watson, J.G. Enhanced ion chromatographic speciation of water-soluble PM2.5 to improve aerosol source apportionment. Aerosol Sci. Eng. 2017, 1, 7–24. [Google Scholar] [CrossRef]
- Solomon, P.A.; Crumpler, D.; Flanagan, J.B.; Jayanty, R.K.M.; Rickman, E.E.; McDade, C.E. US National PM2.5 Chemical Speciation Monitoring Networks-CSN and IMPROVE: Description of networks. J. Air Waste Manag. Assoc. 2014, 64, 1410–1438. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Magliano, K.L. Loss of PM2.5 nitrate from filter samples in central California. J. Air Waste Manag. Assoc. 2005, 55, 1158–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Chang, M.-C.O.; Robinson, N.F.; Trimble, D.L.; Kohl, S.D. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manag. Assoc. 2007, 57, 1014–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.C.; Watson, J.G.; Robles, J.; Wang, X.L.; Chen, L.-W.A.; Trimble, D.L.; Kohl, S.D.; Tropp, R.J.; Fung, K.K. Quality assurance and quality control for thermal/optical analysis of aerosol samples for organic and elemental carbon. Anal. Bioanal. Chem. 2011, 401, 3141–3152. [Google Scholar] [CrossRef]
- Chow, J.C.; Wang, X.L.; Sumlin, B.J.; Gronstal, S.B.; Chen, L.-W.A.; Trimble, D.L.; Kohl, S.D.; Mayorga, S.R.; Riggio, G.M.; Hurbain, P.R.; et al. Optical calibration and equivalence of a multiwavelength thermal/optical carbon analyzer. Aerosol Air Qual. Res. 2015, 15, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Cahill, T.A.; Ashbaugh, L.L.; Barone, J.B.; Eldred, R.A.; Feeney, P.J.; Flocchini, R.G.; Goodart, C.; Shadoan, D.J.; Wolfe, G. Analysis of respirable fractions in atmospheric particulates via sequential filtration. J. Air Pollut. Control Assoc. 1977, 27, 675–678. [Google Scholar] [CrossRef] [Green Version]
- Wittmaack, K. Combustion characteristics of water-insoluble elemental and organic carbon in size selected ambient aerosol particles. Atmos. Chem. Phys. 2005, 5, 1905–1913. [Google Scholar] [CrossRef]
- Engelbrecht, D.R.; Cahill, T.A.; Feeney, P.J. Electrostatic effects on gravimetric analysis of membrane filters. J. Air Pollut. Control Assoc. 1980, 30, 391–392. [Google Scholar] [CrossRef]
- Kröger, C.R.; Morales, J.R. Charge effect in nuclepore filter. Phys. Scr. 1988, 37, 270–273. [Google Scholar] [CrossRef]
- Romo-Kröger, C.M. Measurable electrostatic effects in Nuclepore filters. J. Air Pollut. Control Assoc. 1989, 39, 1465–1466. [Google Scholar] [CrossRef]
- Okuda, T.; Fujimori, E.; Hatoya, K.; Takada, H.; Kumata, H.; Nakajima, F.; Hatakeyama, S.; Uchida, M.; Tanaka, S.; He, K.B.; et al. Rapid and simple determination of multi-elements in aerosol samples collected on quartz fiber filters by using EDXRF coupled with fundamental parameter quantification technique. Aerosol Air Qual. Res. 2013, 13, 1864–1876. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.G.; Chow, J.C.; Chen, L.; Wang, X.L.; Merrifield, T.M.; Fine, P.M.; Barker, K. Measurement system evaluation for upwind/downwind sampling of fugitive dust emissions. Aerosol Air Qual. Res. 2011, 11, 331–350. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G. Ion chromatography in elemental analysis of airborne particles. In Elemental Analysis of Airborne Particles; Landsberger, S., Creatchman, M., Eds.; Advances in Environmental, Industrial and Process Control Technologies; Gordon and Breach Science: Amsterdam, The Netherlands, 1999; Volume 1, pp. 97–137. [Google Scholar]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The DRI Thermal/Optical Reflectance carbon analysis system: Description, evaluation and applications in U.S. air quality studies. Atmos. Environ. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Crow, D.; Lowenthal, D.H.; Merrifield, T.M. Comparison of IMPROVE and NIOSH carbon measurements. Aerosol Sci. Technol. 2001, 34, 23–34. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Arnott, W.P.; Moosmüller, H.; Fung, K.K. Equivalence of elemental carbon by Thermal/Optical Reflectance and Transmittance with different temperature protocols. Environ. Sci. Technol. 2004, 38, 4414–4422. [Google Scholar] [CrossRef]
- Chow, J.C.; Yu, J.Z.; Watson, J.G.; Ho, S.S.H.; Bohannan, T.L.; Hays, M.D.; Fung, K.K. The application of thermal methods for determining chemical composition of carbonaceous aerosols: A review. J. Environ. Sci. Health-Part A 2007, 42, 1521–1541. [Google Scholar] [CrossRef]
- Raja, S.; Chandrasekaran, S.R.; Lin, L.; Xia, X.Y.; Hopke, P.K.; Valsaraj, K.T. Analysis of beta attenuation monitor filter rolls for particulate matter speciation. Aerosol Air Qual. Res. 2017, 17, 14–23. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Chen, L.-W.A.; Frank, N.H. Methods to assess carbonaceous aerosol sampling artifacts for IMPROVE and other long-term networks. J. Air Waste Manag. Assoc. 2009, 59, 898–911. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Rice, J.; Frank, N.H. Quantification of PM2.5 organic carbon sampling artifacts in US networks. Atmos. Chem. Phys. 2010, 10, 5223–5239. [Google Scholar] [CrossRef] [Green Version]
- Lindeken, C.L.; Morgin, R.L.; Petrock, K.F. Collection efficiency of Whatman 41 filter paper for submicron aerosols. Health Phys. 1963, 9, 305–308. [Google Scholar] [CrossRef]
- Demuynck, M. Determination of irreversible absorption of water by cellulose filters. Atmos. Environ. 1975, 9, 523–528. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Bowen, J.L.; Frazier, C.A.; Gertler, A.W.; Fung, K.K.; Landis, D.; Ashbaugh, L.L. A sampling system for reactive species in the western United States. In Sampling and Analysis of Airborne Pollutants; Winegar, E.D., Keith, L.H., Eds.; Lewis Publishers: Ann Arbor, MI, USA, 1993; pp. 209–228. [Google Scholar]
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Hackney, R.; Magliano, K.L.; Lehrman, D.E.; Smith, T.B. Temporal variations of PM2.5, PM10, and gaseous precursors during the 1995 Integrated Monitoring Study in Central California. In Proceedings, PM2.5: A Fine Particle Standard; Chow, J.C., Koutrakis, P., Eds.; Air & Waste Management Association: Pittsburgh, PA, USA, 1998; pp. 59–77. [Google Scholar]
- Chow, J.C.; Watson, J.G. Chemical analyses of particle filter deposits. In Aerosols Handbook: Measurement, Dosimetry, and Health Effects, 2nd ed.; Ruzer, L., Harley, N.H., Eds.; CRC Press; Taylor & Francis: New York, NY, USA, 2013; pp. 179–204. [Google Scholar]
- ASTM F316-03; Standard Test Methods for Pore Size Characteristics of Membrane Filters by Bubble Point and Mean Flow Pore Test. American Society for Testing Materials International: West Conshohocken, PA, USA, 2011.
- Lindsley, W.G. Filter pore size and aerosol sample collection. In NIOSH Manual of Analytical Methods (NMAM), 5th ed.; National Institute for Occupational Safety and Health (NIOSH): Cincinnati, OH, USA, 2016. [Google Scholar]
- Sherwood, R.; Greenhalgh, D. A personal air sampler. Ann. Occup. Hyg. 1960, 2, 127–132. [Google Scholar] [PubMed]
- Sherwood, R. Historical perspectives: Realization, development, and first applications of the personal air sampler. Appl. Occup. Environ. Hyg. 1997, 12, 229–234. [Google Scholar] [CrossRef]
- First, M.W.; Silverman, L. Air sampling with membrane filters. In A.M.A. Archives of Industrial Hygiene and Occupational Medicine; American Medical Association (AMA): Chicago, IL, USA, 1953; Volume 7, pp. 1–11. [Google Scholar]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Lee, K.W.; Liu, B.Y.H. On the minimum efficiency and the most penetrating particle size for fibrous filters. J. Air Pollut. Control Assoc. 1980, 30, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, M.S.S.; Harley, N.H. Experimental investigation of sintered porous metal filters. J. Aerosol Sci. 2000, 31, 721–738. [Google Scholar] [CrossRef]
- Kim, S.C.; Harrington, M.S.; Pui, D.Y.H. Experimental study of nanoparticles penetration through commercial filter media. J. Nanopart. Res. 2006, 9, 117–125. [Google Scholar] [CrossRef]
- Zíkova, N.; Ondráček, J.; Ždímal, V. Size-resolved penetration through high-efficiency filter media typically used for aerosol sampling. Aerosol Sci. Technol. 2015, 49, 239–249. [Google Scholar] [CrossRef]
- Soo, J.C.; Monaghan, K.; Lee, T.; Kashon, M.; Harper, M. Air sampling filtration media: Collection efficiency for respirable size-selective sampling. Aerosol Sci. Technol. 2016, 50, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Breuer, D. Analytical performance issues: Flow resistance of samplers for personal monitoring in work areas and requirements for sampling pump performance. J. Occup. Environ. Hyg. 2012, 9, D25–D32. [Google Scholar] [CrossRef]
- Yamamoto, N.; Fujii, M.; Kumagai, K.; Yanagisawa, Y. Time course shift in particle penetration characteristics through capillary pore membrane filters. J. Aerosol Sci. 2004, 35, 731–741. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, X.L.; Chen, D.R. Effect of dust loading rate on the loading characteristics of high efficiency filter media. Powder Technol. 2016, 287, 20–28. [Google Scholar] [CrossRef]
- Appel, B.R.; Tokiwa, Y.; Haik, M.; Kothny, E.L. Artifact particulate sulfate and nitrate formation on filter media. Atmos. Environ. 1984, 18, 409–416. [Google Scholar] [CrossRef]
- Batterman, S.A.; Osak, I.; Gelman, C. SO2 sorption characteristics of air sampling filter media using a new laboratory test. Atmos. Environ. 1997, 31, 1041–1047. [Google Scholar] [CrossRef]
- Hsu, Y.M.; Kollett, J.; Wysocki, K.; Wu, C.Y.; Lundgren, D.A.; Birky, B.K. Positive artifact sulfate formation from SO2 adsorption in the silica gel sampler used in NIOSH method 7903. Environ. Sci. Technol. 2007, 41, 6205–6209. [Google Scholar] [CrossRef]
- Yamashita, T.; Sasaki, T.; Fujimura, M.; Hashimoto, Y. Adsorption of sulfur dioxide and nitrogen oxides on glass fiber filters for aerosol collection. Bunseki Kagaku 1978, 27, T1–T5. [Google Scholar] [CrossRef]
- Byers, R.L.; Davis, J.W. Sulfur dioxide adsorption and desorption on various filter media. J. Air Pollut. Control Assoc. 1970, 20, 236–238. [Google Scholar] [CrossRef]
- Gilbert, J.; Sartim, R.; Suringar, M.E.; Richards, J. Positive bias in particulate matter emissions data due to sulfur dioxide adsorption and oxidation on glass fiber filters. J. Air Waste Manag. Assoc. 2021, 71, 1076–1084. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Lurmann, F.W.; Musarra, S. Ammonium nitrate, nitric acid, and ammonia equilibrium in wintertime Phoenix, Arizona. J. Air Waste Manag. Assoc. 1994, 44, 405–412. [Google Scholar] [CrossRef]
- Hering, S.V.; Cass, G.R. The magnitude of bias in the measurement of PM2.5 arising from volatilization of particulate nitrate from Teflon filters. J. Air Waste Manag. Assoc. 1999, 49, 725–733. [Google Scholar] [CrossRef]
- Harrison, R.M.; Kitto, A.M.N. Field intercomparison of filter pack and denuder sampling methods for reactive gaseous and particulate pollutants. Atmos. Environ. 1990, 24, 2633–2640. [Google Scholar] [CrossRef]
- Febo, A.; Perrino, C.; Allegrini, I. Field intercomparison exercise on nitric acid and nitrate measurement (Rome, 1988)—A critical approach to the evaluation of the results. Sci. Total Environ. 1993, 133, 39–71. [Google Scholar] [CrossRef]
- Keck, L.; Wittmaack, K. Laboratory studies on the retention of nitric acid, hydrochloric acid and ammonia on aerosol filters. Atmos. Environ. 2005, 39, 2157–2162. [Google Scholar] [CrossRef]
- Keck, L.; Wittmaack, K. Effect of filter type and temperature on volatilisation losses from ammonium salts in aerosol matter. Atmos. Environ. 2005, 39, 4093–4100. [Google Scholar] [CrossRef]
- Keck, L.; Wittmaack, K. Simplified approach to measuring semivolatile inorganic particulate matter using a denuded cellulose filter without backup filters. Atmos. Environ. 2006, 40, 7106–7114. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J.; Hering, S.V. Investigation of organic aerosol sampling artifacts in the Los Angeles Basin. Atmos. Environ. 1994, 28, 3061–3071. [Google Scholar] [CrossRef]
- Allen, G.; Rector, L.; Butcher, T.; Trojanowski, R. Evaluation of alternative filter media for particulate matter emission testing of residential wood heating devices. J. Air Waste Manag. Assoc. 2017, 67, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.J.; Chang, C.T.; Shih, B.H.; Aggarwal, S.G.; Li, S.N.; Chein, H.M.; Shih, T.S. The effect of environmental conditions and electrical charge on the weighing accuracy of different filter materials. Sci. Total Environ. 2002, 293, 201–206. [Google Scholar] [CrossRef]
- Chow, J.C. Summary of the 1995 A&WMA critical review: Measurement methods to determine compliance with ambient air quality standards for suspended particles. EM 1995, 1, 12–15. [Google Scholar]
- Stacey, P.; Kauffer, E.; Moulut, J.-C.; Dion, C.; Beauparlant, M.; Fernandez, P.; Key-Schwartz, R.; Friede, B.; Wake, D. An International Comparison of the Crystallinity of Calibration Materials for the Analysis of Respirable α-Quartz Using X-Ray Diffraction and a Comparison with Results from the Infrared KBr Disc Method. Ann. Occup. Hyg. 2009, 53, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J.G.; Bowen, J.L.; Chow, J.C.; Rogers, C.F.; Ruby, M.G.; Rood, M.J.; Egami, R.T. High volume measurement of size classified suspended particulate matter. In Methods of Air Sampling and Analysis, 3rd ed.; Lodge, J.P., Ed.; Lewis Publishers, Inc.: Chelsea, MI, USA, 1989; pp. 427–439. [Google Scholar]
- Ampian, S.G.; Virta, R.L. Crystalline Silica Overview: Occurrence and Analysis; U.S. Bureau of Mines: Washington, DC, USA, 1992.
- Abbasi, B.; Wang, X.L.; Chow, J.C.; Watson, J.G.; Peik, B.; Nasiri, V.; Riemenschnitter, K.B.; Elahifard, M. Review of respirable coal mine dust characterization for mass concentration, size distribution and chemical composition. Minerals 2021, 11, 426. [Google Scholar] [CrossRef]
- Kauffer, E.; Masson, A.; Moulut, J.C.; Lecaque, T.; Protois, J.C. Comparison of direct (X-ray diffraction and infrared spectrophotometry) and indirect (infrared spectrophotometry) methods for the analysis of alpha-quartz in airborne dusts. Ann. Occup. Hyg. 2005, 49, 661–671. [Google Scholar] [PubMed] [Green Version]
- Soo, J.C.; Lee, T.; Chisholm, W.P.; Farcas, D.; Schwegler-Berry, D.; Harper, M. Treated and untreated rock dust: Quartz content and physical characterization. J. Occup. Environ. Hyg. 2016, 13, D201–D207. [Google Scholar] [CrossRef] [PubMed]
- Key-Schwartz, R.; Baron, P.; Bartley, D.; Rice, F.; Schlecht, P. Chapter R: Determination of airborne crystalline silica. In NIOSH Manual of Analytical Methods; National Institute for Occupational Safety and Health (NIOSH): Cincinnati, OH, USA, 2003; pp. 260–280. [Google Scholar]
- Occupational Safety and Health Administration (OSHA). OSHA Method ID-142: Crystalline Silica: Quartz and Cristobalite; Occupational Safety and Health Administration (OSHA): Washington, DC, USA, 2016.
- Griffiths, P.R.; de Haseth, J.A. Fourior Transfer Infrared Spectrometry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Beer, A. Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten (Determination of the absorption of red light in colored liquids). Ann. Phys. Chem. 1852, 86, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, S.M. Infrared Analysis of Respirable Coal Mine Dust for Quartz: Thirty-Five Years; Harper, M., Lee, T., Eds.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 204–221. [Google Scholar]
- Anderson, C.C. Collaborative Tests of Two Methods for Determining Free Silica in Airborne Dust; Final Report; SRI International: Menlo Park, CA, USA, 1983. [Google Scholar]
- Lorberau, C. Investigation of the Determination of Respirable Quartz on Filter Media Using Fourier Transform Infrared Spectrophotometry; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 1989.
- Lorberau, C. Investigation of the determination of respirable quartz on filter media using Fourier Transform Infrared Spectrophotometry. Appl. Occup. Environ. Hyg. 1990, 5, 348–350. [Google Scholar] [CrossRef]
- Kingma, K.J.; Hemley, R.J. Raman spectroscopic study of microcrystalline silica. Am. Mineral. 1994, 79, 269–273. [Google Scholar]
- Araujo, C.F.; Nolasco, M.M.; Ribiero, A.M.P.; Ribiero-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Stacey, P.; Mader, K.T.; Sammon, C. Feasibility of the quantification of respirable crystalline silica by mass on aerosol sampling filters using Raman microscopy. J. Raman Spectrosc. 2017, 48, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Friedel, R.A.; Carlson, G.L. Difficult carbonaceous materials and their infra-red and Raman spectra. Reassignments for coal spectra. Fuel 1972, 51, 194–198. [Google Scholar] [CrossRef]
- Shin, K.; Chung, H. Wide area coverage Raman spectroscopy for reliable quantitative analysis and its applications. Analyst 2013, 138, 3335. [Google Scholar] [CrossRef]
- Zheng, L.N.; Kulkarni, P.; Birch, M.E.; Ashley, K.; Wei, S.J. Analysis of crystalline silica aerosol using portable Raman spectrometry: Feasibility of near real-time measurement. Anal. Chem. 2018, 90, 6229–6239. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Sampaio, C.H.; Guedes, A.; Fdez-Ortiz de Vallejuelo, S.; Madariaga, J.M. Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel 2012, 94, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Sanz, A.; Flores, D.; Noronha, F. Characterization of fly ash from a power plant and surroundings by micro-Raman spectroscopy. Int. J. Coal Geol. 2008, 73, 359–370. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Ryu, J.S.; Jeong, S.; Kim, J.H.; Jeong, H.Y.; Ra, K.T.; Yang, M.J.; Chang, H.J. Elemental and isotopic compositions in blank filters collecting atmospheric particulates. J. Anal. Sci. Technol. 2021, 12, 27. [Google Scholar] [CrossRef]
- Foster, R.D.; Walker, R.F. Quantitative determination of crystalline silica in respirable size dust samples by infrared spectrophotometry. Analyst 1984, 109, 1117–1127. [Google Scholar] [CrossRef]
- The National Institute of Occupational Safety and Health (NIOSH). SILICA, Respirable Crystalline, by IR (KBr Pellet); DHHS: Cincinnati, OH, USA, 2017.

| Sampler Type | Coal Mine Dust Personal Sampling Units (CMDPSU) | Personal Dust Monitor (PDM 3700) |
|---|---|---|
| Manufacturer | Zefon International, Ocala, FL, USA (zefon.com, accessed on 1 October 2022) | Thermo Fisher, Waltham, MA, USA (thermofisher.com, accessed on 1 October 2022) |
| Averaging Time | Integrated 8 h sample | Real time with 30 min average |
| Size-selective Inlet(d50 of 4 μm) | Dorr-Oliver nylon cyclone by Zefon | Higgins Dewell (HD) cyclone (Model BG14CP conductive plastic by Mesa Labs, Lakewood, CO, USA, mesalabs.com accessed on 1 October 2022) |
| Filter Cassette Assembly | 2, 3, or 4 piece conductive housing cassettes | 14 mm circular polypropylene base with a hollow axial stem |
| Flow Rate | 2.0 L/min (±5% in mine) for coal | 2.2 L/min (±2.5%) |
| Operating Temperature | 0 to 45 °C | −20 to +40 °C |
| Filter Type (Diameter) | 37 mm, 5 μm pore size polyvinyl chloride (PVC) | 13 mm Teflon-coated borosilicate glass-fiber filter (TX40HI20, Pall Corporation, East Hills, NY, USA, pall.com, accessed on 1 October 2022) |
| Filter Exposed Area | 784 mm2 | 132.7 mm2 |
| Face Velocity | 4.25 cm/s | 27.6 cm/s |
| Mass Loading Range | 0–200 mg | 0.1–4 mg |
| Mass Determination Method | Gravimetry | Real-time TEOM inertial microbalance |
| Accuracy | Accurate measurements are possible within ±5% | ±25% of the reference method for concentrations > 0.2 mg/m3 |
| Physical Dimensions | Sampling Tube: 92 cm Cyclone Assembly: 6 cm (d) × 15 cm (h) × 6 cm (w) Pump: 5.7 cm (d) × 10.8 cm (h) × 10.2 cm (w) | Sampling Tube: 92 cm Cyclone Assembly: 5.08 cm (w) × 4.32 cm (d) × 9.91 cm (h) Monitor: 24.31 cm (w) × 8.26 cm (d) × 17.15 cm (h) |
| Sampler Weight | Pump: 0.65 kg with battery pack | 2 kg |
| Pump Type | Escort ELF Pump | Internal sampling pump |
| Power Requirements | 48 volt battery pack of 4 NiMH cells | Lithium ion battery assembly |
| Filter Type | Filter Material (Manufacturer) | Pore Size (μm) | Filter Permeability Face Velocity a (cm/s) | Range of Collection Efficiency b |
|---|---|---|---|---|
| Teflon membrane | Fluoropore (PTFE-polyethylene reinforced, Millipore Sigma, Burlington, MA, USA) | 3 | 23.5 | 98.2%–99.8% |
| Teflon (Gelman Sciences, Hilliard, OH, USA) | 5 | 56.8 | 85%–99.9% | |
| Teflon (Ghia SKC, Eighty Four, PA, USA) | 2 | 23.4 | 99.89%–99.99% | |
| 3 | 24.2 | 92%–98.98% | ||
| Teflon (Zefluor, Millipore Sigma, Burlington, MA, USA) | 2 | 32.5 | 94.6%–99.96% | |
| 3 | 31.6 | 88%–99.9% | ||
| Silver membrane | Pure metallic silver (Sterlitech, Auburn, WA, USA) | 0.45 | 1.8 | 93.6%–99.98% |
| 0.8 | 6.2 | 90%–99.6% | ||
| 1.2 | 9.2 | 73%–99.7% | ||
| 5 | 19.0 | 25%–99.2% | ||
| Polyvinyl chloride (PVC) membrane | PVC (Metricel) | 0.8 | 2.7 | 99.96%–>99.99% |
| PVC (Metricel) | 5 | 51 | 49%–98.8% | |
| PVC (Millipore Sigma, Burlington, MA, USA) | 2 | 5 | 88%–99.9% | |
| PVC-5 (Millipore Sigma, Burlington, MA, USA) | 5 | 11 | 96.7%–>99.9% | |
| Cellulose acetate/nitrate membrane | MF-RA (Millipore Sigma, Burlington, MA, USA) | 1.2 | 6.2 | >99.9% |
| MF-SS (Millipore Sigma, Burlington, MA, USA) | 3 | 7.5 | 98.5%–99.9% | |
| MF-SM (V) | 5 | 10 | 98.1%–99.9% | |
| MF-SC (Millipore Sigma, Burlington, MA, USA) | 8 | 14.1 | 92%–99.9% | |
| Capillary pore membrane | Polycarbonate (Nuclepore, Whatman-Cytiva, Little Chalfont Buckinghamshire, UK) | 0.4 | 2.9 | 78%–99.99% |
| 0.6 | 2.1 | 53%–99.5% | ||
| 5 | 30.7 | 6%–90.7% | ||
| 8 | 21.2 | 1%–90.5% | ||
| Quartz fiber | 2500 QAO (Pallflex, Pall Corp., Duncan, SC, USA) | NA | 41 | 84%–99.9% |
| Teflon-coated glass fiber | TX40HI20 (Pall Corp., Duncan, SC, USA) | NA | 15.1 | 92.6%–99.6% |
| TX40HI20 (2nd lot, Pall Corp., Duncan, SC, USA) | NA | 9 | 98.9%–99.9% | |
| Cellulose fiber | Whatman 40 (Whatman-Cytiva, Little Chalfont Buckinghamshire, UK) | NA | 3.7 | 77%–99.99% |
| Whatman 41 (Whatman-Cytiva, Little Chalfont Buckinghamshire, UK) | NA | 16.9 | 43%–99.5% | |
| Glass fiber | Microquartz (Gelman Sciences, Hilliard, OH, USA) | NA | 14.1 | 98.5%–99.99% |
| GF/A (Whatman-Cytiva, Little Chalfont Buckinghamshire, UK) | NA | 14.5 | 99%–99.99% |
| Filter Type a | |||||
|---|---|---|---|---|---|
| PTFE | PVC | Silver Membrane | Polycarbonate | MCE | |
| No. of filters tested | 171 | 171 | 168 | 171 | 162 |
| Median | 99.86% | 99.74% | 96.07% | 98.01% | 99.99% |
| Mean ± standard deviation | 99.02 ± 2.25% | 98.85 ± 2.96% | 86.46 ± 20.3% | 85.32 ± 22.2% | 99.5 ± 4.76% |
| Lowest collection efficiency | 94.76% | 92.98% | 42.10% | 22.48% | 98.82% |
| Pore size b | 5 μm | 5 μm | 5 μm | 5 μm | 0.45 μm |
| Flow rate b | 1.7 L/min | 11.2 L/min | 4.4 L/min | 1.7 L/min | 2.5 L/min |
| Face velocity b,c | 3.11 cm/s | 20.5 cm/s | 8.06 cm/s | 3.11 cm/s | 4.58 cm/s |
| Vendor b | Pall | SKC | Sterlitech | Millipore | SKC |
| Average Absorbance b at Selected Quartz Bands c | |||||
|---|---|---|---|---|---|
| Filter Name | Filter Type a | Gravimetric Weight | 695 cm−1 | 779 cm−1 | 798 cm−1 |
| Nuclepore | PE | 4.90 mg | 0.057 ± 0.005 | 0.116 ± 0.004 | 0.157 ± 0.003 |
| Teflo | PTFE | 4.50 mg | 0.157 ± 0.021 | 0.074 ± 0.011 | 0.063 ± 0.010 |
| DM-450 (0.45 μm pore size) | PVC/A | 12.60 mg | 0.163 ± 0.003 | 0.078 ± 0.001 | 0.074 ± 0.001 |
| DM-800 (0.8 μm pore size) | PVC/A | 14.13 mg | 0.205 ± 0.005 | 0.111 ± 0.003 | 0.108 ± 0.003 |
| GLA-5000 | PVC/A | 5.31 mg | 0.222 ± 0.005 | 0.105 ± 0.006 | 0.112 ± 0.006 |
| LOD (μg) a | LOQ (μg) a | |||
|---|---|---|---|---|
| 47 mm Filter Type b (Pore Size) | MSHA c | NIOSH c | MSHA c | NIOSH c |
| DM-450 (0.45 μm) | 0.99 | 0.59 | 3.3 | 2 |
| PVC (5 μm) | 0.52 | 1.5 | 1.7 | 4.9 |
| Polypropylene (0.45 μm) | 0.72 | 1.2 | 2.4 | 4 |
| Nylon (0.45 μm) | 1.8 | 1.5 | 6 | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, J.C.; Watson, J.G.; Wang, X.; Abbasi, B.; Reed, W.R.; Parks, D. Review of Filters for Air Sampling and Chemical Analysis in Mining Workplaces. Minerals 2022, 12, 1314. https://doi.org/10.3390/min12101314
Chow JC, Watson JG, Wang X, Abbasi B, Reed WR, Parks D. Review of Filters for Air Sampling and Chemical Analysis in Mining Workplaces. Minerals. 2022; 12(10):1314. https://doi.org/10.3390/min12101314
Chicago/Turabian StyleChow, Judith C., John G. Watson, Xiaoliang Wang, Behrooz Abbasi, Wm. Randolph Reed, and David Parks. 2022. "Review of Filters for Air Sampling and Chemical Analysis in Mining Workplaces" Minerals 12, no. 10: 1314. https://doi.org/10.3390/min12101314





