Cultivation of Microalgae (Scenedesmus sp.) Using Coal Mining Wastewater and Separation via Coagulation/Flocculation and Dissolved Air Flotation (DAF)
Abstract
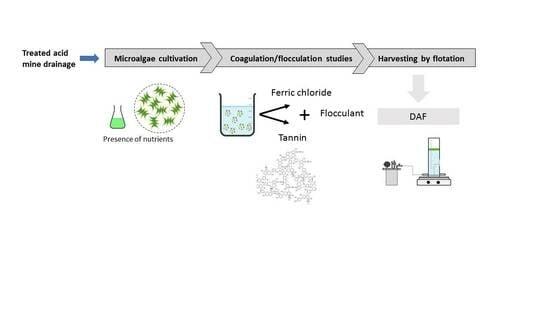
:1. Introduction
2. Experimental
2.1. Mine Water
2.2. Reagents
2.3. Algae Growth
2.4. Microalgae Destabilization
2.5. Algae Separation by Dissolved Air Flotation (DAF)
- 1.3—air density (mg mL−1);
- A/S—air/solids ratio (mg mg−1);
- Sair—air solubility in the water (20 mL L−1 at 25 °C and 1 atm of pressure);
- f—efficiency of gas dissolved at a given pressure (90%);
- P—pressure (atm);
- TSS—total suspended solids (mg L−1);
- q—recirculation ratio (recirculation flowrate for air saturation per effluent flowrate).

2.6. Analytical Methods
3. Results and Discussion
3.1. Microalgae Biomass Production
3.2. Microalgae Removal
3.2.1. Microalgae Removal via Sedimentation
3.2.2. Microalgae Removal by Dissolved Air Flotation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control, and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Skousen, J.; Rose, A.; Geidel, G.; Foreman, J.; Evans, R.; Hellier, W. Handbook of Technologies for Avoidance and Remediation of Acid Mine Drainage; The National Mine Land Reclamation Center, West Virginia University: Morgantown, WV, USA, 1998. [Google Scholar]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Thomashausen, S.; Maennling, N.; Mebratu-Tsegaye, T. A comparative overview of legal frameworks governing water use and wastewater discharge in the mining sector. Resour. Policy 2018, 55, 143–151. [Google Scholar] [CrossRef]
- da Silveira, A.N.; Silva, R.; Rubio, J. Treatment of Acid Mine Drainage (AMD) in South Brazil: Comparative active processes and water reuse. Int. J. Miner. Process. 2009, 93, 103–109. [Google Scholar] [CrossRef]
- Palma, H.; Killoran, E.; Sheehan, M.; Berner, F.; Heimann, K. Assessment of microalga biofilms for simultaneous remediation and biofuel generation in mine tailings water. Bioresour. Technol. 2017, 234, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Sustainable iron recovery and biodiesel yield by acid-adapted microalgae, Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3, grown in synthetic acid mine drainage. ACS Omega 2020, 5, 6888–6894. [Google Scholar] [CrossRef]
- Urrutia, C.; Yañez-Mansilla, E.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; dos Santos, K.B.; Lautert-Dutra, W.; de Souza Teodoro, L.; de Almeida, V.O.; Weiler, J.; Schneider, I.A.H.; Bogo, M.R. Acid mine drainage (AMD) treatment by neutralization: Evaluation of physical-chemical performance and ecotoxicological effects on zebrafish (Danio rerio) development. Chemosphere 2020, 253, 126665. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Jaiyeola, A.T.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- dos Santos, K.B.; de Almeida, V.O.; Weiler, J.; Schneider, I.A.H. Removal of pollutants from an AMD from a coal mine by neutralization/precipitation followed by “In Vivo” biosorption step with the microalgae Scenedesmus sp. Minerals 2020, 10, 711. [Google Scholar] [CrossRef]
- Praveen, K.; Abinandan, S.; Venkateswarlu, K.; Megharaj, M. Sustainability evaluation of immobilized acid-adapted microalgal technology in acid mine drainage remediation following emergy and carbon footprint analysis. Molecules 2022, 27, 1015. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Rahimpour, M.R.; Ghasemi, Y.; Raeissi, S. The biodiesel of microalgae as a solution for diesel. Energies 2018, 11, 950. [Google Scholar] [CrossRef]
- Leite, G.B.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Algal biofuels: Challenges and opportunities. Bioresour. Technol. 2013, 145, 134–141. [Google Scholar] [CrossRef]
- Sim, T.-S.; Goh, A.; Becker, E.W. Comparision of centrifugation, dissolved air flotation and drum filtration techniques for harvesting sewage-grown algae. Biomass 1988, 16, 51–62. [Google Scholar] [CrossRef]
- Ide, W.R.; Ide, C.N.; Ribeiro, M.L.; Campos, M.; Boncz, M.A. Uso da Flotação por Ar Dissolvido (FAD) para colheita de microalgas presentes em lagoas de polimento. Rev. AIDIS Ing. Cienc. Ambient. Investig. Desarro. Práctica 2016, 9, 125–138. [Google Scholar]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Van Haver, L.; Nayar, S. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017, 24, 167–180. [Google Scholar] [CrossRef]
- Özacar, M.; Sengil, A. Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 2003, 229, 85–96. [Google Scholar] [CrossRef]
- Teixeira, M.S.; Speranza, L.G.; Silva, I.C.; Moruzzi, R.B.; Silva, G.H.R. Tannin-based coagulant for harvesting microalgae cultivated in wastewater: Efficiency, floc morphology and products characterization. Sci. Total Environ. 2022, 807, 150776. [Google Scholar] [CrossRef] [PubMed]
- Barrado-Moreno, M.M.; Beltrán-Heredia, J.; Martín-Gallardo, J. Removal of Oocystis algae from freshwater by means of tannin-based coagulant. J. Appl. Phycol. 2016, 28, 1589–1595. [Google Scholar] [CrossRef]
- Barrado-Moreno, M.M.; Beltrán-Heredia, J.; Martín-Gallardo, J. Microalgal removal with natural coagulants. Phycologia 2016, 55, 688–695. [Google Scholar] [CrossRef]
- Roselet, F.; Vandamme, D.; Roselet, M.; Muilaert, K.; Abreu, P.C. Screening of commercial natural and synthetic cationic polymers for flocculation of freshwater and marine microalgae and effects of molecular weight and charge density. Algal Res. 2015, 10, 183–188. [Google Scholar] [CrossRef]
- Grehs, W.; Lopes, A.; Moreira, N.; Fenandes, T.; Linton, M.; Silva, A.; Manaia, C.; Carissimi, E.; Nunces, O. Removal of microorganisms and antibiotic resistance genes from treated urban wastewater: A comparison between aluminium sulphate and tannin coagulants. Water Res. 2019, 166, 115056. [Google Scholar] [CrossRef] [PubMed]
- Amaral Filho, J.R.; Schneider, I.A.H.; Brum, I.A.S.; Sampaio, C.H.; Miltzarek, G.; Schneider, C. Characterization of coal tailing deposit for integrated mine waste management in the Brazilian coal field of Santa Catarina. REM Rev. Esc. Minas 2013, 66, 347–353. [Google Scholar] [CrossRef]
- Amaral Filho, J.R.; Weiler, J.; Broadhurst, J.L.; Schneider, I.A.H. The use of static and humidity cell tests to assess the effectiveness of coal waste desulfurization on acid rock drainage risk. Mine Water Environ. 2017, 36, 429–435. [Google Scholar] [CrossRef]
- Weiler, J.; Amaral Filho, J.R.; Schneider, I.A.H. Coal waste processing to reduce costs related to acid mine drainage treatment—Case study in the Carboniferous District of Santa Catarina. Eng. Sanit. Ambient. 2016, 21, 337–345. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente, Conselho Nacional do Meio Ambiente, Brasil. Resolução CONAMA No. 430/2011; Conselho Nacional do Meio Ambiente, Ministério do Meio Ambiente, Brasil: Brasília, Brazil, 2011. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, H.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Rodrigues, R.T.; Rubio, J. New basis for measuring the size distribution of bubbles. Miner. Eng. 2003, 16, 757–765. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists; Willian Horwitz: Washington, DC, USA, 1980. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.; Greenbaerg, A.E.; Franson, M.A.H. Standard Methods for Examination of Water and Wastewater: Centennial Edition; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Schroeder, L.; Scherer, M.D.; Balmant, W.; Vargas, J.V.C.; Mariano, A.B. Energy analysis of lipid extraction of Scenedesmus sp. produced in pilot scale. Eng. Térmica (Therm. Eng.) 2015, 14, 22–33. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.W.; Park, C.S.; Kim, S.J.; Jeune, K.H.; Chang, M.U.; Acreman, J. Enhanced production of Scenedesmus spp. (green microalgae) using a new medium containing fermented swine wastewater. Bioresour. Technol. 2007, 98, 2220–2228. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina Grima, E. Influence of culture conditions on the lutein content of the new strain Scenedesmus almeriensi. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Çetinkaya Dönmez, G.; Aksu, Z.; Öztürk, A.; Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem. 1999, 34, 885–892. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Adams, C.; Gofrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growthand lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Yuan, C.; Li, T.; Li, A. Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply. J. Appl. Phycol. 2019, 31, 2797–2809. [Google Scholar] [CrossRef]
- Guastalci, A.C.; Aparecida, A.H. Calcium phosphates of biological interest: Importance as biomaterials, properties and methods for coatings obtaining. Química Nova 2010, 33, 1352–1358. [Google Scholar]
- Fitch, E.B.; Stevenson, D.G. Gravity separation equipment. In Solid/Liquid Separation Equipment Scale-Up; Purchas, D.B., Ed.; Upland Press: Croydon, UK, 1977; pp. 81–153. [Google Scholar]
- Oliveira, C.; Rubio, J. A short overview of the formation of aerated flocs and their applications in solid/liquid separation by flotation. Miner. Eng. 2012, 39, 124–132. [Google Scholar] [CrossRef]
- Edzwald, J.K. Dissolved air flotation and me. Water Res. 2009, 44, 2077–2106. [Google Scholar] [CrossRef] [PubMed]
- Edzwald, J.K.; Haarhoff, J. Dissolved Air Flotation for Water Clarification, 1st ed.; American Water Works Association and McGraw Hill: Denver, CO, USA, 2010. [Google Scholar]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Gregory, R.; Edzwald, J.K. Sedimentation and flotation. In Water Quality and Treatment, 6th ed.; Edzwald, J.K., Ed.; McGraw Hill: New York, NY, USA, 2010. [Google Scholar]
- Edzwald, J.K. Algae, bubbles, coagulants, and dissolved air flotation. Water Sci. Technol. 1993, 27, 67–81. [Google Scholar] [CrossRef]
- Rubio, J.; Carissimi, E.; Rosa, J.J. Flotation in water and wastewater treatment and reuse: Recent trends in Brazil. Int. J. Environ. Pollut. 2007, 30, 197–212. [Google Scholar] [CrossRef]
- Associação Brasileira de Carvão Mineral. Dados Estatísticos 2022. Available online: https://www.siecesc.com.br/pdf/dados_estatisticos_ano_2022.pdf (accessed on 29 February 2024).
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Wastewater treatment high rate algal ponds (WWT HRAP) for low-cost biofuel production. Bioresour. Technol. 2015, 184, 202–214. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Utln. 2012, 3, 89–100. [Google Scholar]








| Parameter | AMD | AMD after Neutralisation with Ca(OH)2 | Emission Standards CONAMA 430/2011 [31] |
|---|---|---|---|
| pH | 2.9 | 7.0 | 5–9 |
| Conductivity (µS cm−1) | 784 | 568 | - |
| Na (mg L−1) | 2.1 | 0.4 | - |
| K (mg L−1) | 0.4 | 1.3 | - |
| Ca (mg L−1) | 1.5 | 116.7 | - |
| Mg (mg L−1) | 0.4 | 1.2 | - |
| Zn (mg L−1) | 0.64 | <0.01 | 5 |
| Fe (mg L−1) | 323.3 | 0.53 | 15 |
| Mn (mg L−1) | 0.05 | <0.01 | 1 |
| Al (mg L−1) | 67.2 | <0.01 | - |
| Sulphates (mg L−1) | 490.6 | 302.5 | - |
| Salt | Concentration (g L−1) |
|---|---|
| CaCl2·2H2O | 36.76 |
| MgSO4·7H2O | 36.97 |
| NaHCO3 | 12.6 |
| K2HPO4 | 8.71 |
| NaNO3 | 85.01 |
| Na2SiO3·7H2O | 28.42 |
| Condition | pH Range | Algae Removal (%) | Residual Turbidity Range (NTU) | Description |
|---|---|---|---|---|
| 50–200 mg L−1 of FeCl3·6H2O 1–3 mg L−1 of an anionic polyacrylamide flocculant | 5–10 | ≈98 | 8–13 | Excellent flocculation. Good sedimentation. However, there is the presence of small residual floccules suspended in the effluent. |
| 0.2–0.3 mL L−1 of Tanfloc SG 1–3 mg L−1 of an anionic polyacrylamide flocculant | 5–9 | ≈99 | 6–13 |
| Condition | pH | Algae Removal (%) | Residual Turbidity Range (NTU) | Description |
|---|---|---|---|---|
| 150 mg L−1 of FeCl3·6H2O 2 mg L−1 anionic polyacrylamide flocculant Air/solids ratio: 0.036 mg mg−1 | 8.0 ± 0.1 | 100 | 8–13 | Excellent flocculation and flocs separation. Complete removal of algae flocs. |
| 0.3 mL L−1 of Tanfloc 2 mg L−1 anionic polyacrylamide flocculant Air/solids ratio: 0.034 mg mg−1 | 8.0 ± 0.1 | 100 | 6–13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicknig, M.A.; Azevedo, A.C.d.; de Oliveira, H.A.; Schneider, I.A.H. Cultivation of Microalgae (Scenedesmus sp.) Using Coal Mining Wastewater and Separation via Coagulation/Flocculation and Dissolved Air Flotation (DAF). Minerals 2024, 14, 426. https://doi.org/10.3390/min14040426
Nicknig MA, Azevedo ACd, de Oliveira HA, Schneider IAH. Cultivation of Microalgae (Scenedesmus sp.) Using Coal Mining Wastewater and Separation via Coagulation/Flocculation and Dissolved Air Flotation (DAF). Minerals. 2024; 14(4):426. https://doi.org/10.3390/min14040426
Chicago/Turabian StyleNicknig, Marcio Alexandre, André Camargo de Azevedo, Henrique Alberton de Oliveira, and Ivo André Homrich Schneider. 2024. "Cultivation of Microalgae (Scenedesmus sp.) Using Coal Mining Wastewater and Separation via Coagulation/Flocculation and Dissolved Air Flotation (DAF)" Minerals 14, no. 4: 426. https://doi.org/10.3390/min14040426