Reduction in Apparent Permeability Owing to Surface Precipitation of Solutes by Drying Process and Its Effect on Geological Disposal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Column Preparation and Drying Experiment
2.2. Flow Experiments
3. Results and Discussion
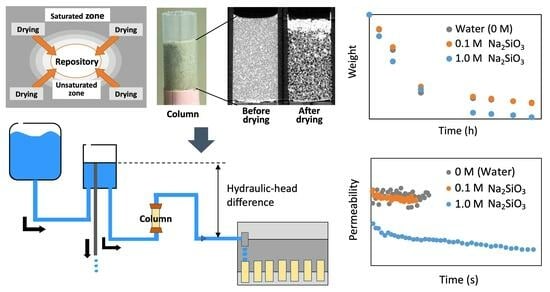
3.1. Weight Change and X-ray CT Analysis in Drying Experiments
3.1.1. Column Packed with GB
3.1.2. Column Packed with SS
3.2. Evaluation of Apparent Mass Transfer Coefficient Based on Water Evaporation Model
3.2.1. Concept of Water Evaporation Model
3.2.2. Estimation of the Apparent Mass Transfer Coefficient and Liquid Film Thickness
3.3. Permeability Change in the SS Columns after Drying
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NUMO. Safety of the Geological Disposal Project 2010-Safe Geological Disposal Based on Reliable Technologies. Available online: https://www.numo.or.jp/technology/technical_report/pdf/TR-13-05.pdf (accessed on 15 January 2024).
- NUMO. The NUMO Pre-Siting SDM-Based Safety Case. Available online: https://www.numo.or.jp/technology/technical_report/pdf/NUMO-TR21-01_rev220222.pdf (accessed on 15 January 2024).
- Keita, E.; Faure, P.; Rodts, S.; Coussot, P. MRI evidence for a receding-front effect in drying porous media. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2013, 87, 062303. [Google Scholar] [CrossRef] [PubMed]
- Keita, E.; Faure, P.; Rodts, S.; Coussot, P.; Weitz, D.; Faure, P. Evaporation from a capillary tube: Experiment and modelization. In Proceedings of the 5th International Conference on Porous Media and Their Applications in Science, Engineering and Industry, Kona, HI, USA, 22–27 June 2014. [Google Scholar]
- Atkinson, A.; Everitt, N.M.; Guppy, R.M. Time Depndence of pH in a cementitious repository. MRS Proc. 1988, 127, 439. [Google Scholar] [CrossRef]
- Natkunarajah, K.; Masilamani, K.; Maheswaran, S.; Lothenbach, B.; Amarasinghe, D.A.S.; Attygalle, D. Analysis of the trend of pH changes of concrete pore solution during the hydration by various analytical methods. Cem. Concr. Res. 2022, 156, 106780. [Google Scholar] [CrossRef]
- Roadcap, G.S.; Kelly, W.R.; Bethke, C.M. Geochemistry of extremely alkaline (pH > 12) ground water in slag-fill aquifers. Ground Water 2005, 43, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.V. Relation between rates of aluminosilicate mineral dissolution, pH, temperature, and surface charge. Am. J. Sci. 1996, 296, 693–728. [Google Scholar] [CrossRef]
- Dow, C.; Glasser, F.P. Calcium carbonate efflorescence on Portland cement and building materials. Cem. Concr. Res. 2003, 33, 147–154. [Google Scholar] [CrossRef]
- Pel, L.; Huinink, H.; Kopinga, K.; van Hees, R.P.J.; Adan, O.C.G. Efflorescence pathway diagram: Understanding salt weathering. Constr. Build. Mater. 2004, 18, 309–313. [Google Scholar] [CrossRef]
- Wang, J.B.; Li, F.S.; Zhou, Z.H.; Du, P.; Xu, D.Y.; Xie, N.; Cheng, X.; Liu, Y. Effect of zeolite on waste based alkali-activated inorganic binder efflorescence. Constr. Build. Mater. 2018, 158, 683–690. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ilić, T.; Ruiz-Agudo, E.; Elert, K. Carbonation mechanisms and kinetics of lime-based binders: An overview. Cem. Concr. Res. 2023, 173, 107301. [Google Scholar] [CrossRef]
- Chen, X.Y. Evaporation from a salt encrusted sediment surface—Field and laboratory studies. Soil Res. 1992, 30, 429–442. [Google Scholar] [CrossRef]
- Bringedal, C.; Schollenberger, T.; Pieters, G.J.M.; van Duijn, C.J.; Helmig, R. Evaporation-driven density instabilities in saturated porous media. Transp. Porous Media 2022, 143, 297–341. [Google Scholar] [CrossRef]
- Huinink, H.P.; Pel, L.; Michels, M.A.J. How ions distribute in a drying porous medium: A simple model. Phys. Fluids 2002, 14, 1389–1395. [Google Scholar] [CrossRef]
- Guglielmini, L.; Gontcharov, A.; Aldykiewicz, A.J.; Stone, H.A. Drying of salt solutions in porous materials: Intermediate-time dynamics and efflorescence. Phys. Fluids 2008, 20, 077101. [Google Scholar] [CrossRef]
- Li, X.H.; Guo, M. Experimental study of evaporation flux, salt precipitation, and surface temperature on homogeneous and heterogeneous porous media. Adv. Civ. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Eloukabi, H.; Sghaier, N.; Ben Nasrallah, S.; Prat, M. Experimental study of the effect of sodium chloride on drying of porous media: The crusty-patchy efflorescence transition. Int. J. Heat Mass Transf. 2013, 56, 80–93. [Google Scholar] [CrossRef]
- Tohoku KEISYA Co, Ltd. Web Page, Product Information. Available online: https://www.tohoku-keisya.co.jp/products/ (accessed on 15 January 2024).
- Rangel-German, E.R.; Kovscek, A.R. Experimental and analytical study of multidimensional imbibition in fractured porous media. J. Petrol. Sci. Eng. 2002, 36, 45–60. [Google Scholar] [CrossRef]
- Coussot, P. Scaling approach of the convective drying of a porous medium. Eur. Phys. J. B 2000, 15, 557–566. [Google Scholar] [CrossRef]
- Acree, W.E., Jr. Solubility Data Series Volume 59, Polycyclic Aromatic Hydrocarbons: Binary Non-Aqueous Systems, Part II: Solvents F-Z. 1995. Available online: https://iupac.github.io/SolubilityDataSeries/volumes/SDS-59.pdf (accessed on 15 January 2024).
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: New York, NY, USA, 1996. [Google Scholar]













| SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | TiO2 | Ig.loss | |
|---|---|---|---|---|---|---|---|---|
| % | 90.47 | 5.43 | 0.25 | 1.12 | 1.42 | 0.89 | 0.13 | 0.29 |
| Abbreviation | Column | Drying Experiment | Flow Experiment | |
|---|---|---|---|---|
| Packed Layer | Particle Size, φ | Filling Solution | Fluid | |
| GB φ0.5 | Glass bead | 0.5 mm | Pure water | - |
| 0.1 M CsCl | - | |||
| 1.0 M CsCl | - | |||
| GB φ1.0 | 1.0 mm | Pure water | - | |
| 0.1 M CsCl | - | |||
| 1.0 M CsCl | - | |||
| SS | Silica sand | 0.35 mm | Pure water | Pure water |
| 0.1 M CsCl | 0.1 M CsCl | |||
| 1.0 M CsCl | 1.0 M CsCl | |||
| 0.1 M Na2SiO3 | Pure water | |||
| 1.0 M Na2SiO3 | Pure water |
| Filling Solution | Fluid | Calculated kT (m2) |
|---|---|---|
| 0.1 M CsCl | 0.1 M CsCl | 2.62 × 10−12 |
| 1.0 M CsCl | 1.0 M CsCl | 2.21 × 10−12 |
| 0.1 M Na2SiO3 | Pure water | 2.87 × 10−12 |
| 1.0 M Na2SiO3 | Pure water | 2.28 × 10−12 |
| Condition (Filled Height) | (kg/(s·m2)) | δ (mm) |
|---|---|---|
| Pure water (10 mm) | 2.65 × 10−5 | 19.13 |
| Pure water (25 mm) | 1.56 × 10−5 | 32.41 |
| SS and pure water (25 mm) | 7.39 × 10−5 | 6.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, T.; Hongo, K.; Chida, T.; Niibori, Y. Reduction in Apparent Permeability Owing to Surface Precipitation of Solutes by Drying Process and Its Effect on Geological Disposal. Minerals 2024, 14, 428. https://doi.org/10.3390/min14040428
Seki T, Hongo K, Chida T, Niibori Y. Reduction in Apparent Permeability Owing to Surface Precipitation of Solutes by Drying Process and Its Effect on Geological Disposal. Minerals. 2024; 14(4):428. https://doi.org/10.3390/min14040428
Chicago/Turabian StyleSeki, Tsugumi, Kanta Hongo, Taiji Chida, and Yuichi Niibori. 2024. "Reduction in Apparent Permeability Owing to Surface Precipitation of Solutes by Drying Process and Its Effect on Geological Disposal" Minerals 14, no. 4: 428. https://doi.org/10.3390/min14040428