Aggregation Mechanism of Particles: Effect of Ca2+ and Polyacrylamide on Coagulation and Flocculation of Coal Slime Water Containing Illite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods
2.2.1. Preparation of Coal Slime Water
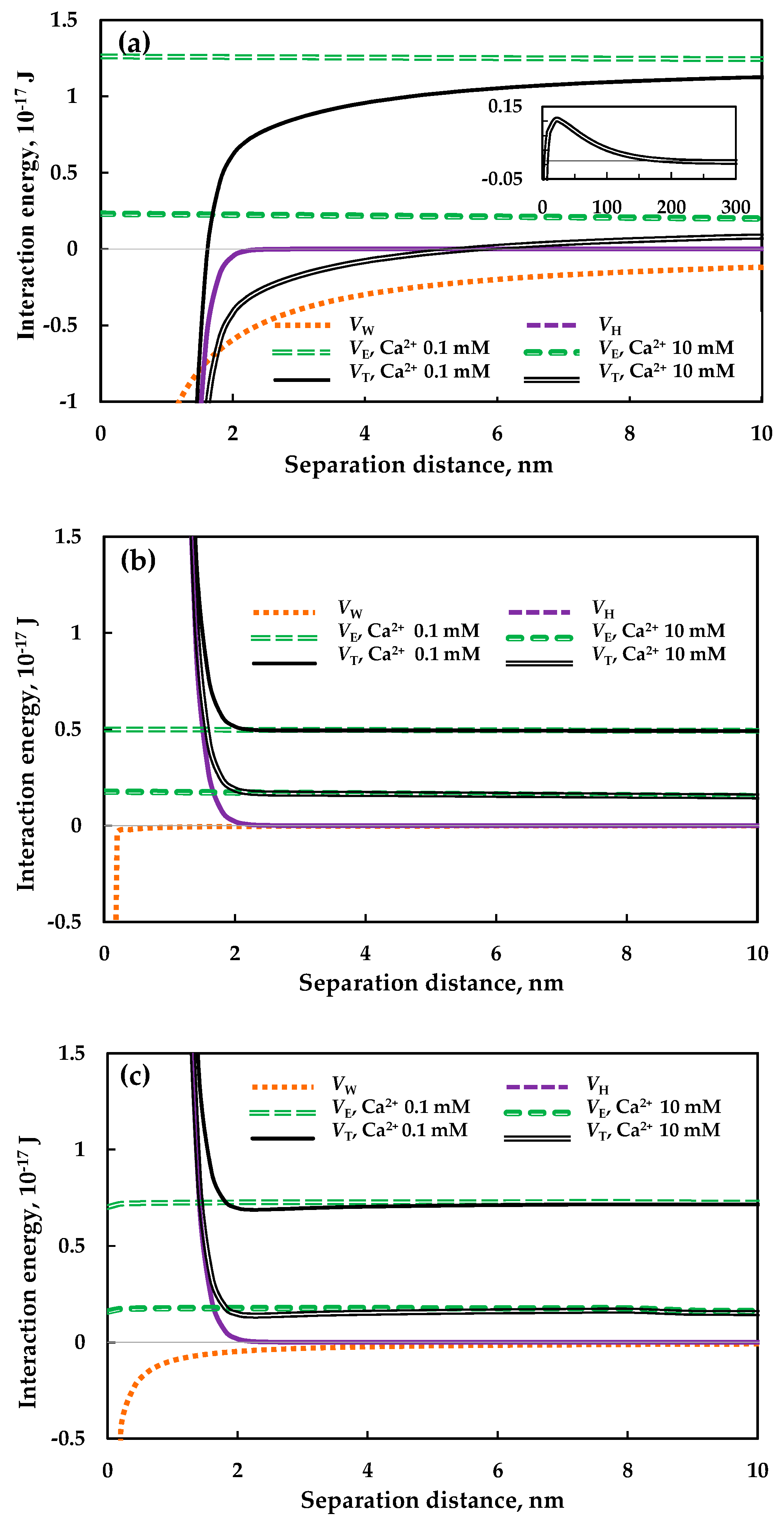
2.2.2. Theoretical Calculation of Particle Aggregation
2.2.3. Coagulation and Flocculation-Sedimentation of Coal Slime Water
3. Results and Discussion
3.1. Interaction between Particles
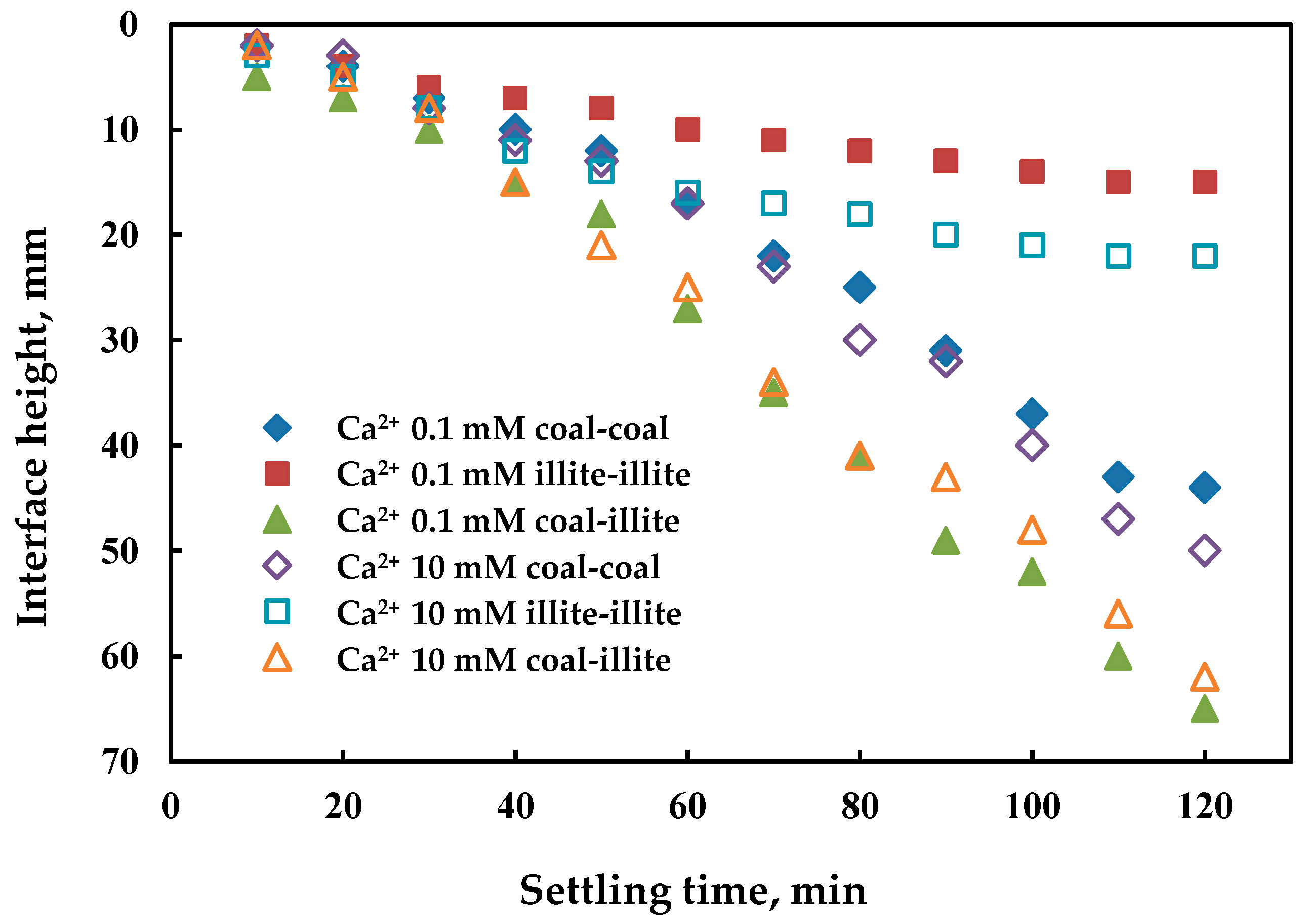
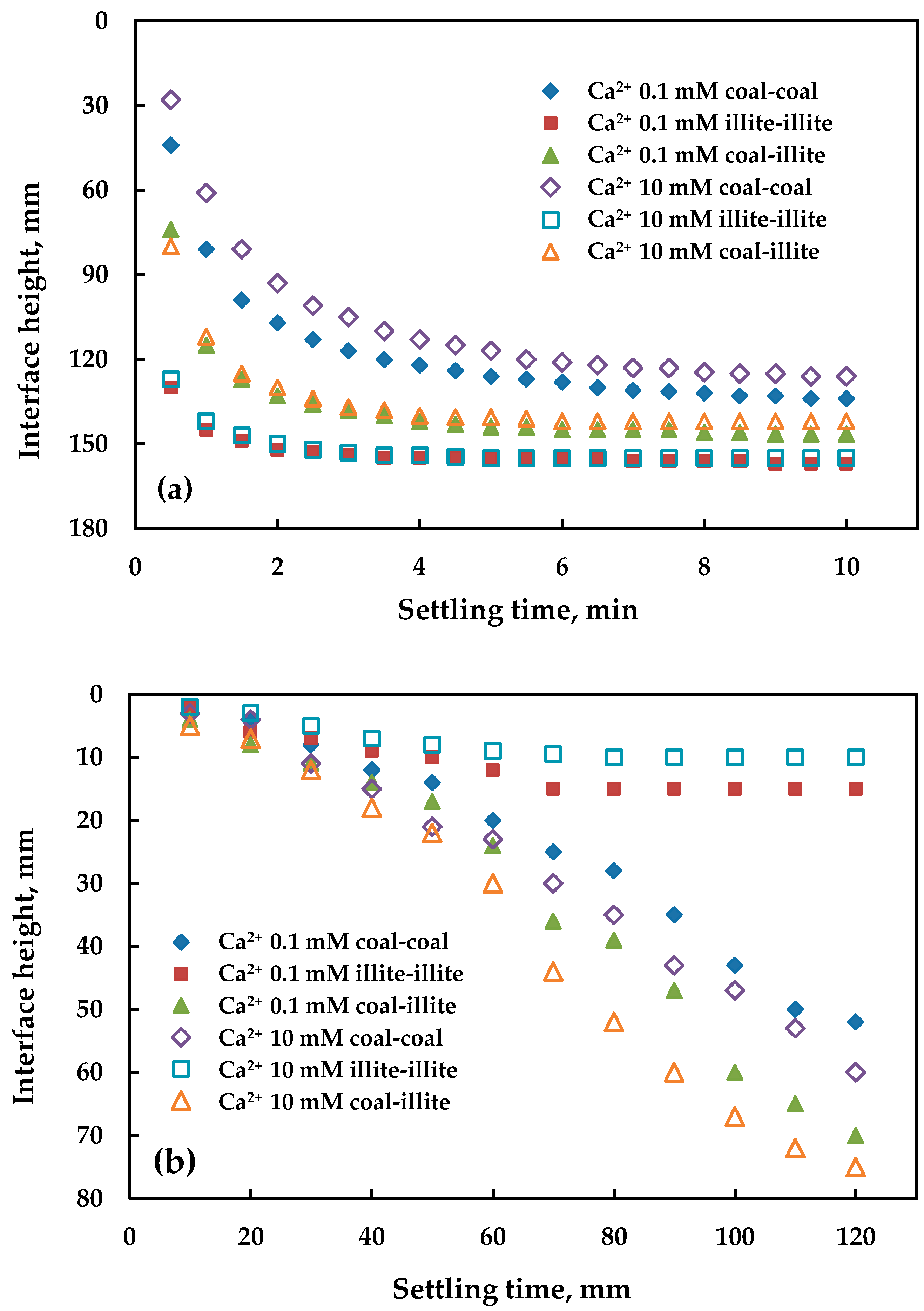
3.2. Coagulation Sedimentation of Particles
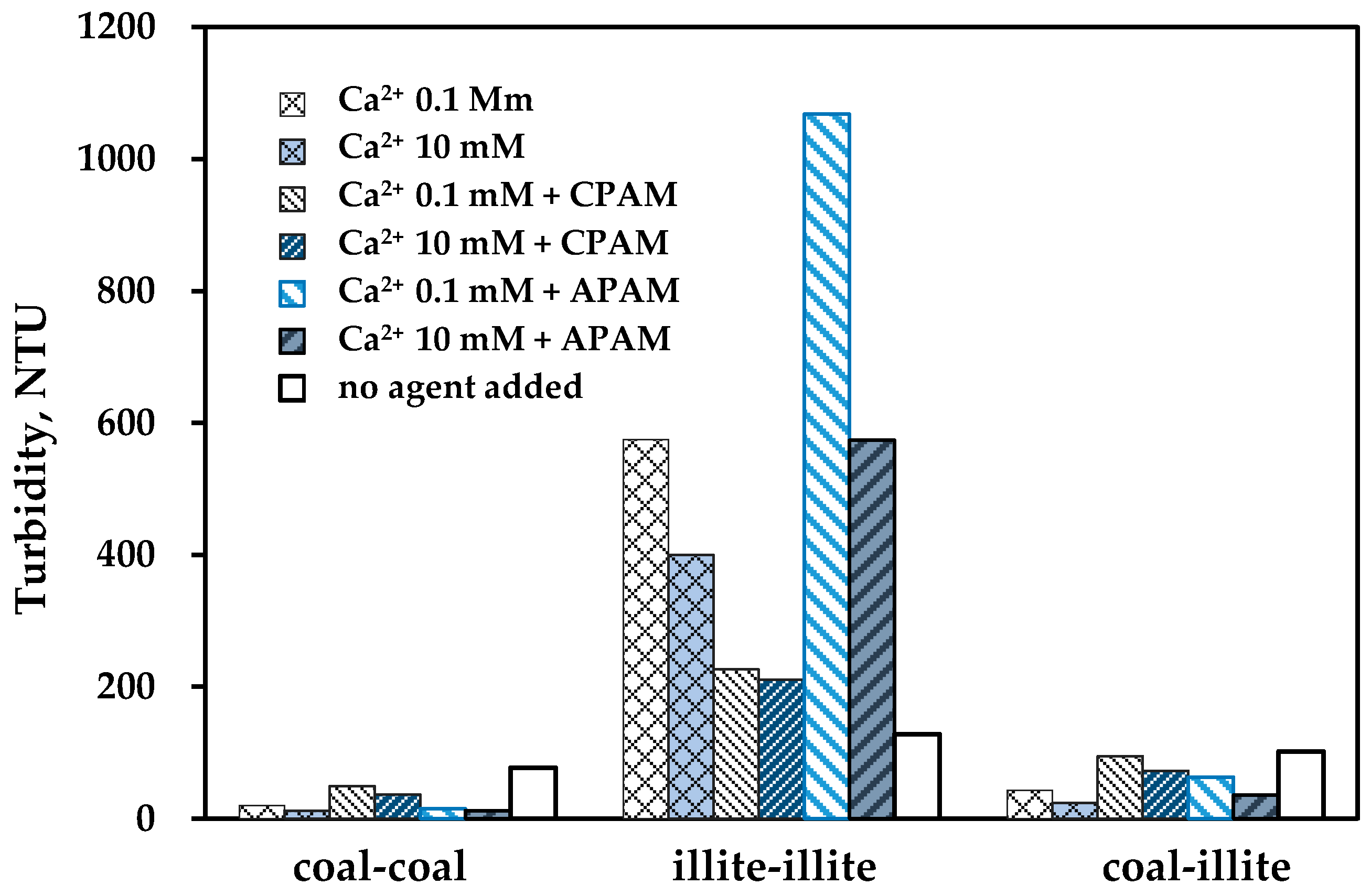
3.3. Flocculation Sedimentation of Particles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| A | Hamaker constant, A132 represents the effective Hamaker constant of the interaction between substance 1 and 2 in medium 3, A11 is the Hamaker constant for substance 1 in vacuum, so as A22. |
| c | Concentration for amount of substance of ions, mol/L |
| e | elementary charge, 1.602 × 10−19 C |
| H | surface distance between particles |
| h0 | relaxation length |
| K | Boltzmann constant, 1.38 × 10−23 J/K |
| NA | Avogadro’s number, 6.022 × 1023 |
| R | particle radius |
| r+ | surface energy electron acceptor component |
| r− | surface energy electron donor component |
| T | absolute temperature |
| VH0 | interfacial polar interaction energy constant |
| Z | ionic valence in solution |
| θ | contact angle |
| rd | surface energy dispersive component |
| εα | absolute dielectric constant of dispersion medium, F/m, the absolute dielectric constant of water is 7.172 × 10−10 F/m. [28] |
| κ−1 | length of Debye, m |
| ψ | surface potential. It was replaced by zeta potential in this study |
| ψ | surface potential. It was replaced by zeta potential in this study |
| Subscripts | |
| 1,2 | particles can be coal or illite in the present work |
| 3 | disperse medium (water here) |
| L | Liquid |
| S | Solid |
References
- Xu, H.X.; Cheng, G. A new method to evaluate the performance of slime water treatment. Energy Source 2016, 38, 777–782. [Google Scholar] [CrossRef]
- Gong, G.Q.; Zhang, Y.J.; Zheng, H.L. Experimental study on the transmittance of the supernatant of refractory slime water. J. Chem. Eng. Jpn. 2016, 49, 417–424. [Google Scholar] [CrossRef]
- Xie, W.; Cao, G.; Ren, X. Effect of flotation promoter on the rate of coal slime flotation. J. Min. Sci. 2014, 50, 601–607. [Google Scholar] [CrossRef]
- Tian, S.D.; Zhuo, Y.Q.; Zhan, Z.H.; Shu, X.Q.; Kang, Z.Z. Distribution of clay minerals in light coal fractions and the thermal reaction products of these clay minerals during combustion in a drop tube furnace. Energies 2016, 9, 428–442. [Google Scholar] [CrossRef]
- Liang, L.X.; Xiong, J.; Liu, X.J.; Luo, D.X. An investigation into the thermodynamic characteristics of methane adsorption on different clay minerals. J. Nat. Gas Sci. Eng. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Guo, S.H.; Hu, J.X. The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem. Eng. J. 2016, 286, 191–197. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Liu, J.T.; Wang, Y.T. Effects of water hardness on the dispersion of fine coal and kaolinite in coal slurry. J. China Coal Soc. 2008, 9, 1058–1062. [Google Scholar]
- Zhang, M.Q.; Liu, Q.; Liu, J.T. Extended DLVO theory applied to coal slime-water suspensions. J. Cent. South Univ. 2012, 19, 3558–3563. [Google Scholar] [CrossRef]
- Mcfarlane, A.J.; Bremmell, K.E.; Addai, M. Optimising the dewatering behaviour of clay tailings through interfacial chemistry, orthokinetic flocculation and controlled shear. Powder Technol. 2005, 160, 27–34. [Google Scholar] [CrossRef]
- Sabah, E.; Erkan, Z.E. Interaction mechanism of flocculants with coal waste slurry. Fuel 2006, 85, 350–359. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, C.; Shen, Z.Y.; Qi, X. The properties and sedimentation characteristics of extremely sliming coal slime water. J. China Coal Soc. 2010, 35, 312–315. [Google Scholar]
- Barraza, J.; Guerrero, J.; Pineres, J. Flotation of a refuse tailing fine coal slurry. Fuel Process. Technol. 2013, 106, 498–500. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chen, N.; Fu, G.; Yan, M.W.; Kim, Y.C. The safety attitudes of senior managers in the Chinese coal industry. Int. J. Environ. Res. Public Health 2016, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, J.F.; Bai, X.; Song, B.; Wang, R.D.; Zhou, T.H.; Jia, J.L.; Pu, H.X. Leaching behavior and potential environmental effects of trace elements in coal gangue of an open-cast coal mine area, Inner Mongolia, China. Minerals 2016, 6, 50–68. [Google Scholar] [CrossRef]
- Gui, X.H.; Xing, Y.W.; Rong, G.Q.; Cao, Y.J.; Liu, J.T. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Van Oss, C.J. Acid-base interfacial interactions in aqueous media. Colloids Surf. A 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Brant, J.A.; Childress, A.E. Assessing short-range membrane-colloid interactions using surface energetics. J. Membr. Sci. 2002, 203, 257–273. [Google Scholar] [CrossRef]
- Brant, J.A.; Childress, A.E. Membrane–colloid interactions: Comparison of extended DLVO predictions with AFM force measurements. Environ. Eng. Sci. 2002, 19, 413–427. [Google Scholar] [CrossRef]
- Churaev, N.V.; Derjaguin, B.V. Inclusion of structural forces in the theory of stability of colloids and films. J. Colloid Interface Sci. 1984, 2, 542–553. [Google Scholar] [CrossRef]
- Duran, J.D.G.; Ramos-Tejada, M.M.; Arroyo, F.J.; Gonzalez-Caballero, F. Rheological and electrokinetic properties of sodium montmorillonite suspensions: I. Rheological properties and interparticle energy of interaction. J. Colloid Interface Sci. 2000, 229, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hoek, E.M.V.; Agarwal, G.K. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 2006, 298, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nabweteme, R.; Yoo, M.; Kwon, H.S.; Kim, Y.J.; Hwang, G.; Lee, C.H.; Ahn, I.S. Application of the extended DLVO approach to mechanistically study the algal flocculation. J. Ind. Eng. Chem. 2015, 30, 289–294. [Google Scholar] [CrossRef]
- Peng, C.; Song, S.; Fort, T. Study of hydration layers near a hydrophilic surface in water through AFM imaging. Surf. Interface Anal. 2006, 38, 975–980. [Google Scholar] [CrossRef]
- Pashley, R.M.; Israelachvili, J.N. Molecular layering of water in thin films between mica surfaces and its relation to hydration forces. J. Colloid Interface Sci. 1984, 2, 511–523. [Google Scholar] [CrossRef]
- Yoon, R.H.; Vivek, S. Effects of short-chain alcohols and pyridine on the hydration forces between silica surfaces. J. Colloid Interface Sci. 1998, 204, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Żbik, M.; Horn, R.G. Hydrophobic attraction may contribute to aqueous flocculation of clays. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 323–328. [Google Scholar] [CrossRef]
- Duong, C.; Choung, J.; Xu, Z.; Szymanski, J. A novel process for recovering clean coal and water from coal tailings. Miner. Eng. 2000, 13, 173–181. [Google Scholar] [CrossRef]
- Harvey, P.A.; Nguyen, A.V.; Evans, G.M. Influence of electrical double-layer interaction on coal flotation. J. Colloid Interface Sci. 2004, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
| Equation No. | Formula | Literature |
|---|---|---|
| I | [16] | |
| Ia | [16] | |
| Ib | [16] | |
| II | [17] | |
| IIa | [17] | |
| IIb | [17] | |
| IIc | [17] | |
| III | [18,19] | |
| IIIa | [16] | |
| IIIb | [16] |
| Particle | Contact Angles, o | ||
|---|---|---|---|
| Water | Formaldehyde | Glycerol | |
| Coal | 79.0 | 75.3 | 77.8 |
| Illite | 25.5 | 20.6 | 17.5 |
| Concentration of Calcium, mM/L | ζcoal, mV | ζillite, mV |
|---|---|---|
| 0 | –19.33 | –21.93 |
| 0.1 | –14.98 | –13.32 |
| 10 | –6.41 | –7.91 |
| Liquid | rL | rLd | rL+ | rL− |
|---|---|---|---|---|
| Water | 72.8 | 21.8 | 25.5 | 25.5 |
| Glycerol | 64 | 34 | 4.92 | 57.4 |
| Formaldehyde | 58 | 39 | 2.28 | 39.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Li, P.; Hou, D.; Kuang, Y.; Wang, G. Aggregation Mechanism of Particles: Effect of Ca2+ and Polyacrylamide on Coagulation and Flocculation of Coal Slime Water Containing Illite. Minerals 2017, 7, 30. https://doi.org/10.3390/min7020030
Lin Z, Li P, Hou D, Kuang Y, Wang G. Aggregation Mechanism of Particles: Effect of Ca2+ and Polyacrylamide on Coagulation and Flocculation of Coal Slime Water Containing Illite. Minerals. 2017; 7(2):30. https://doi.org/10.3390/min7020030
Chicago/Turabian StyleLin, Zhe, Panting Li, Dou Hou, Yali Kuang, and Guanghui Wang. 2017. "Aggregation Mechanism of Particles: Effect of Ca2+ and Polyacrylamide on Coagulation and Flocculation of Coal Slime Water Containing Illite" Minerals 7, no. 2: 30. https://doi.org/10.3390/min7020030





