Crystallization of Jarosite with Variable Al3+ Content: The Transition to Alunite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Jarosite Formation
2.3. Characterisation of Solids
2.3.1. Vibrational Spectroscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Elemental Analysis
2.3.6. Dynamic Light Scattering (DLS)
2.4. Molecular Modelling
3. Results
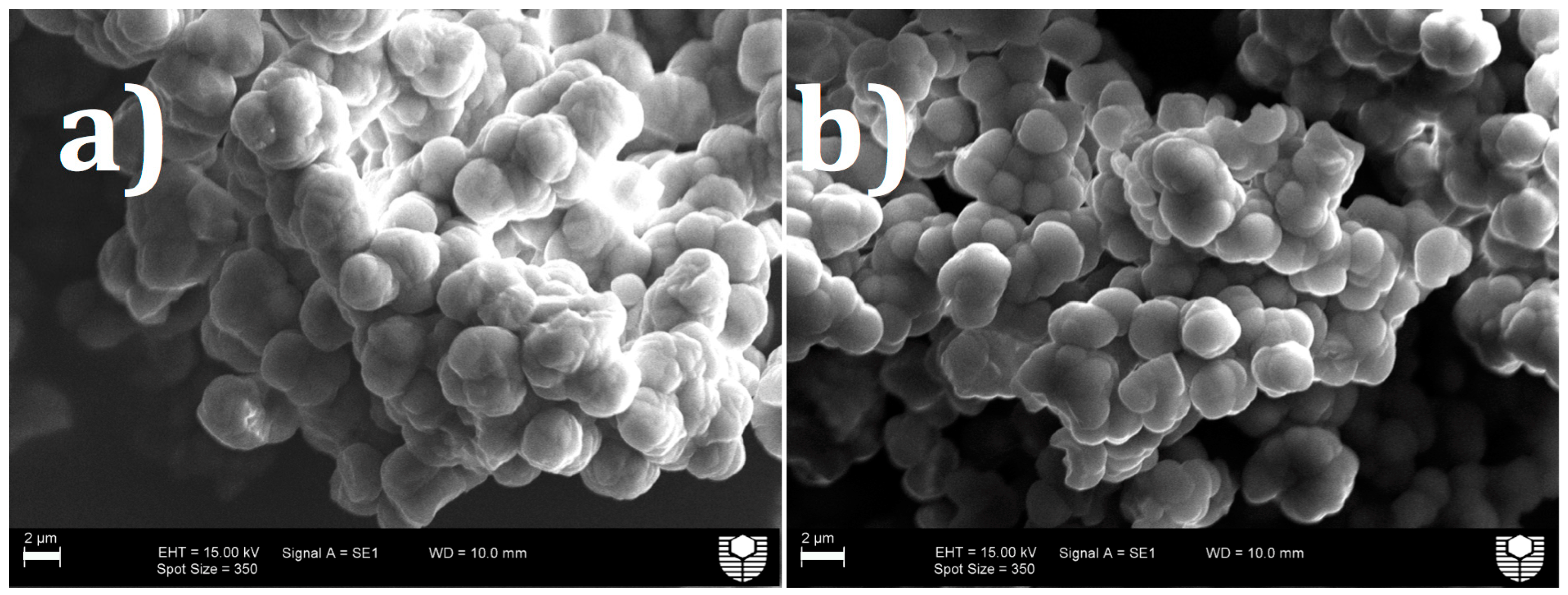
3.1. Morphology
3.2. Elemental Analysis
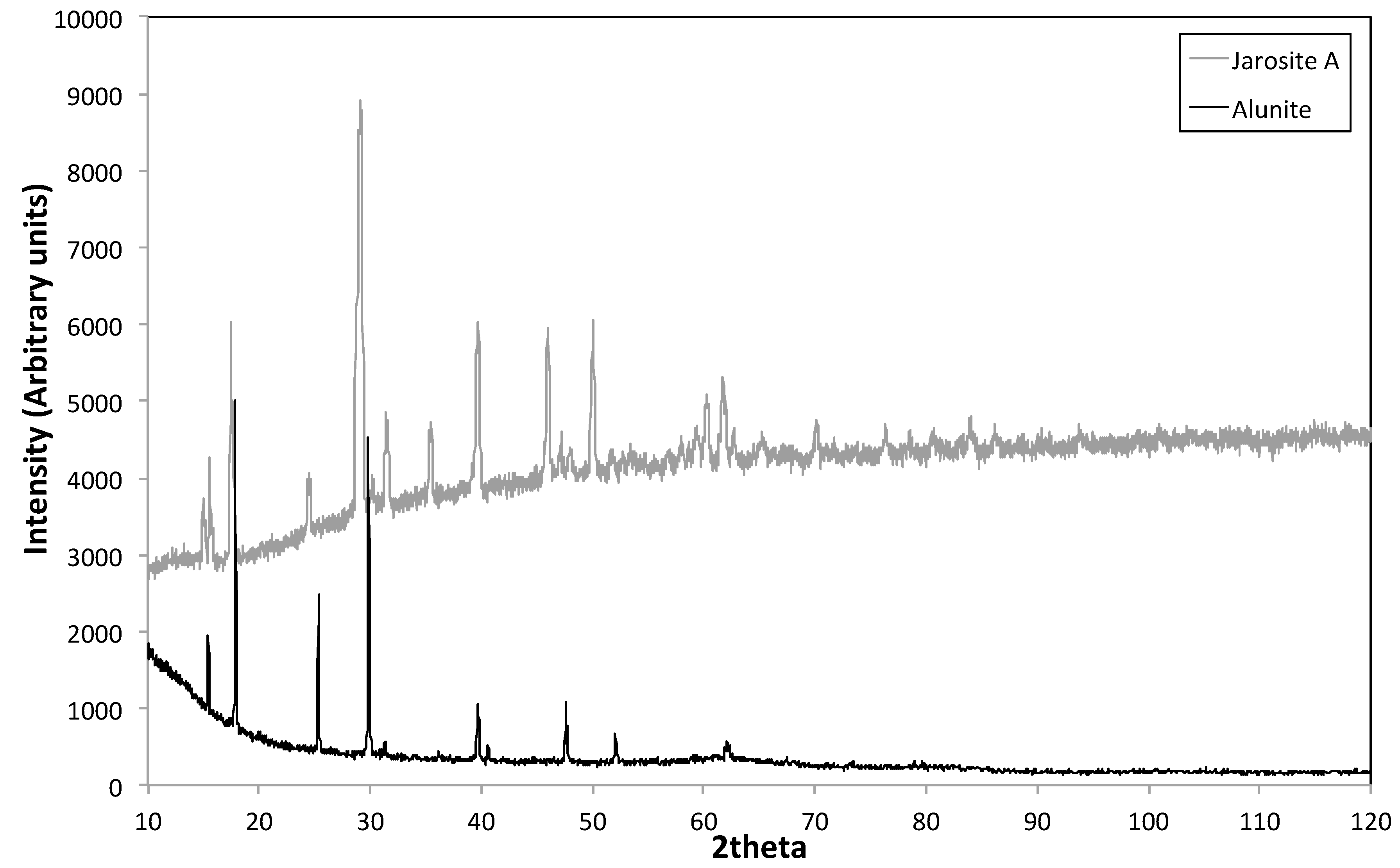
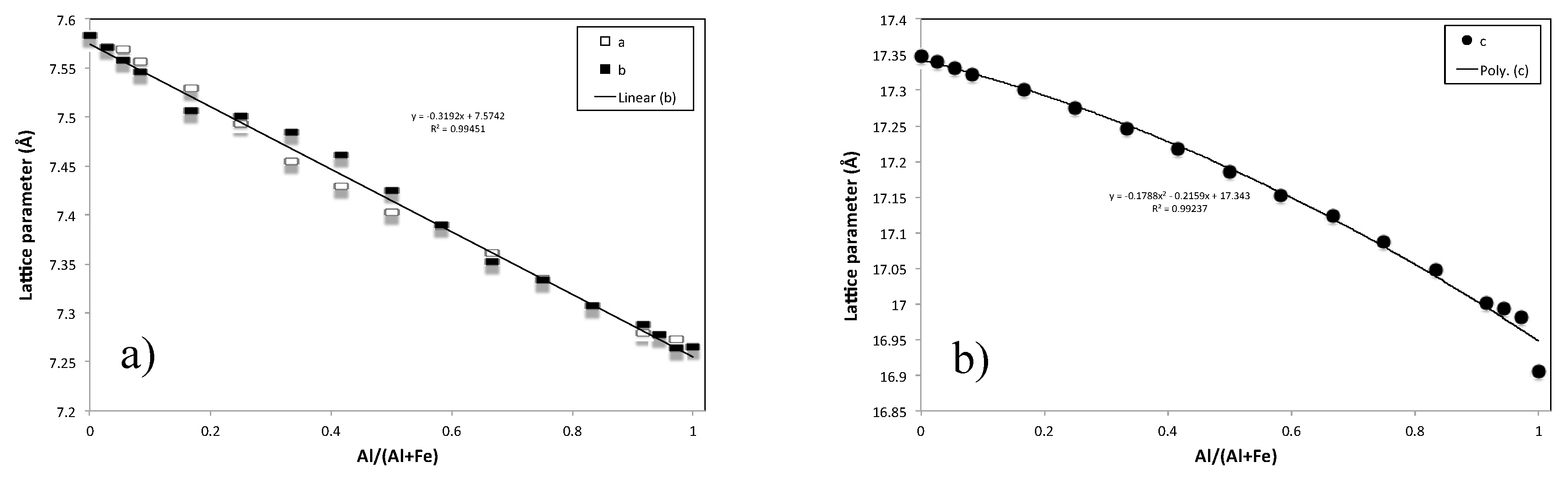
3.3. XRD
3.4. Vibrational Sepctroscopy
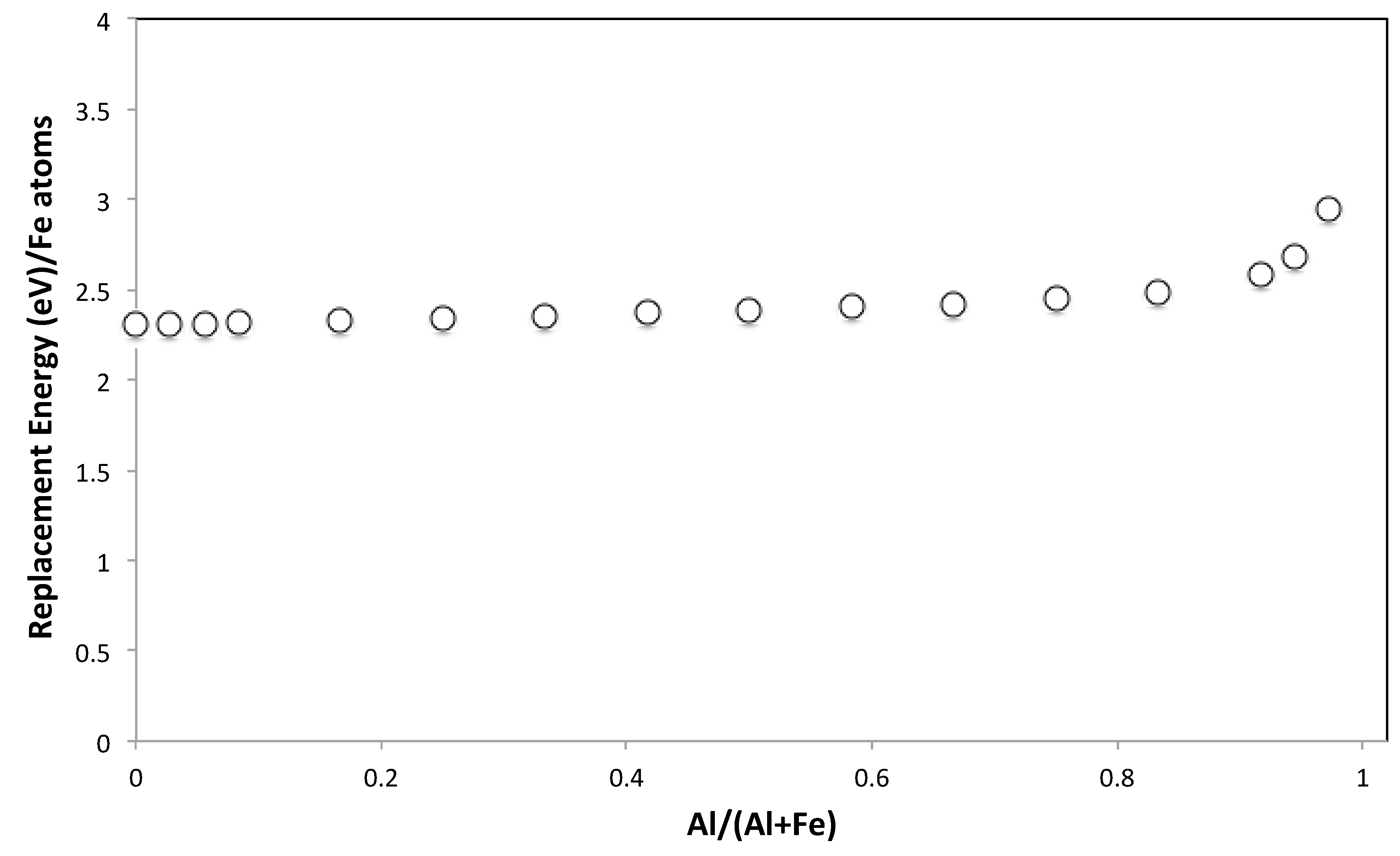
3.5. Molecular Modelling
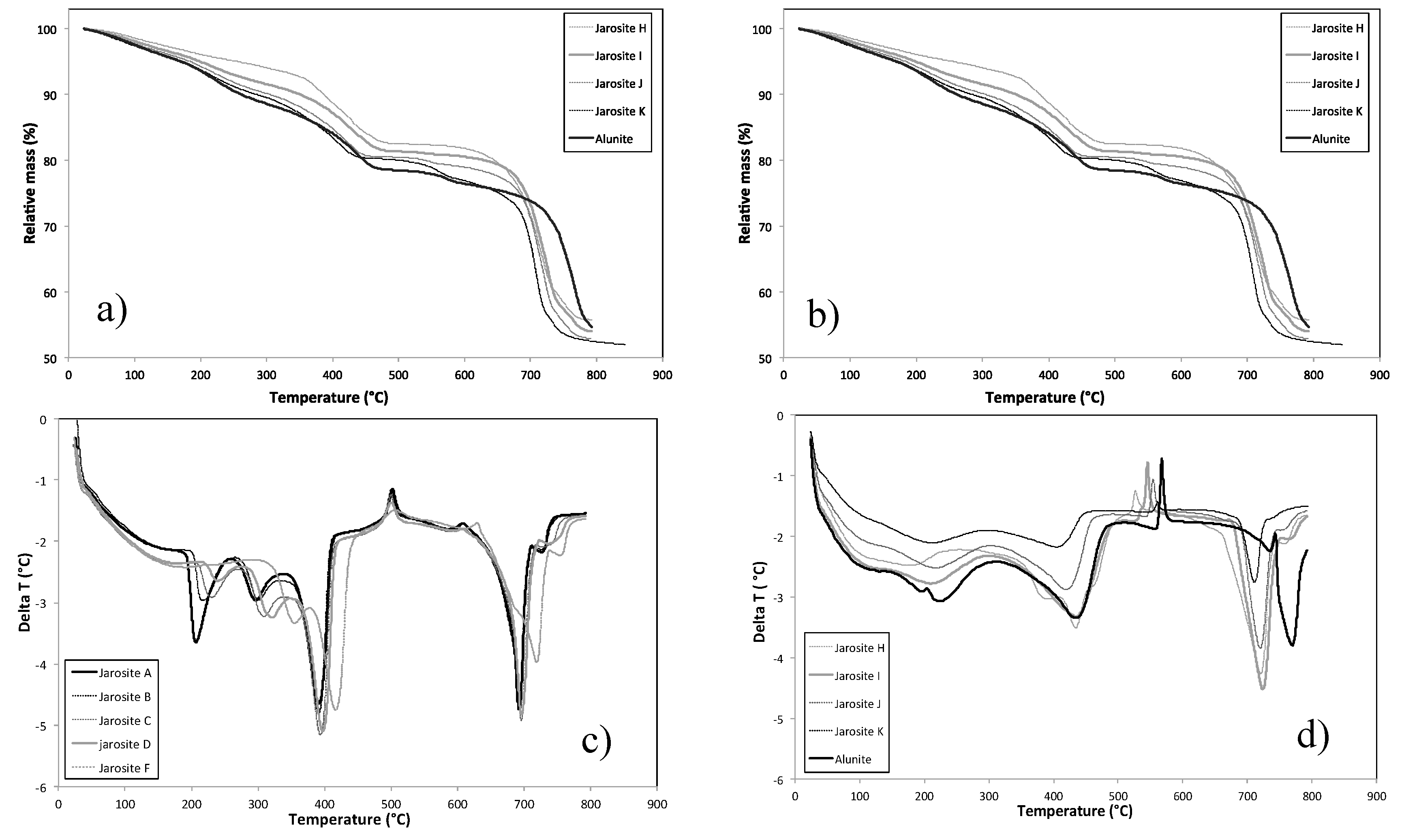
3.6. Thermal Behaviour
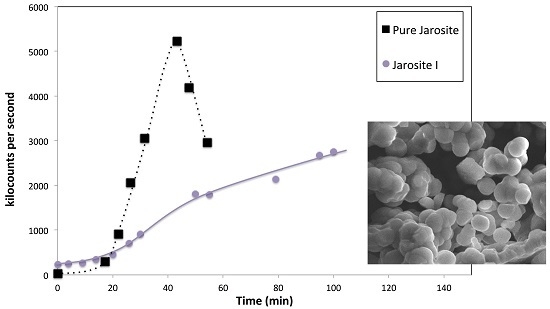
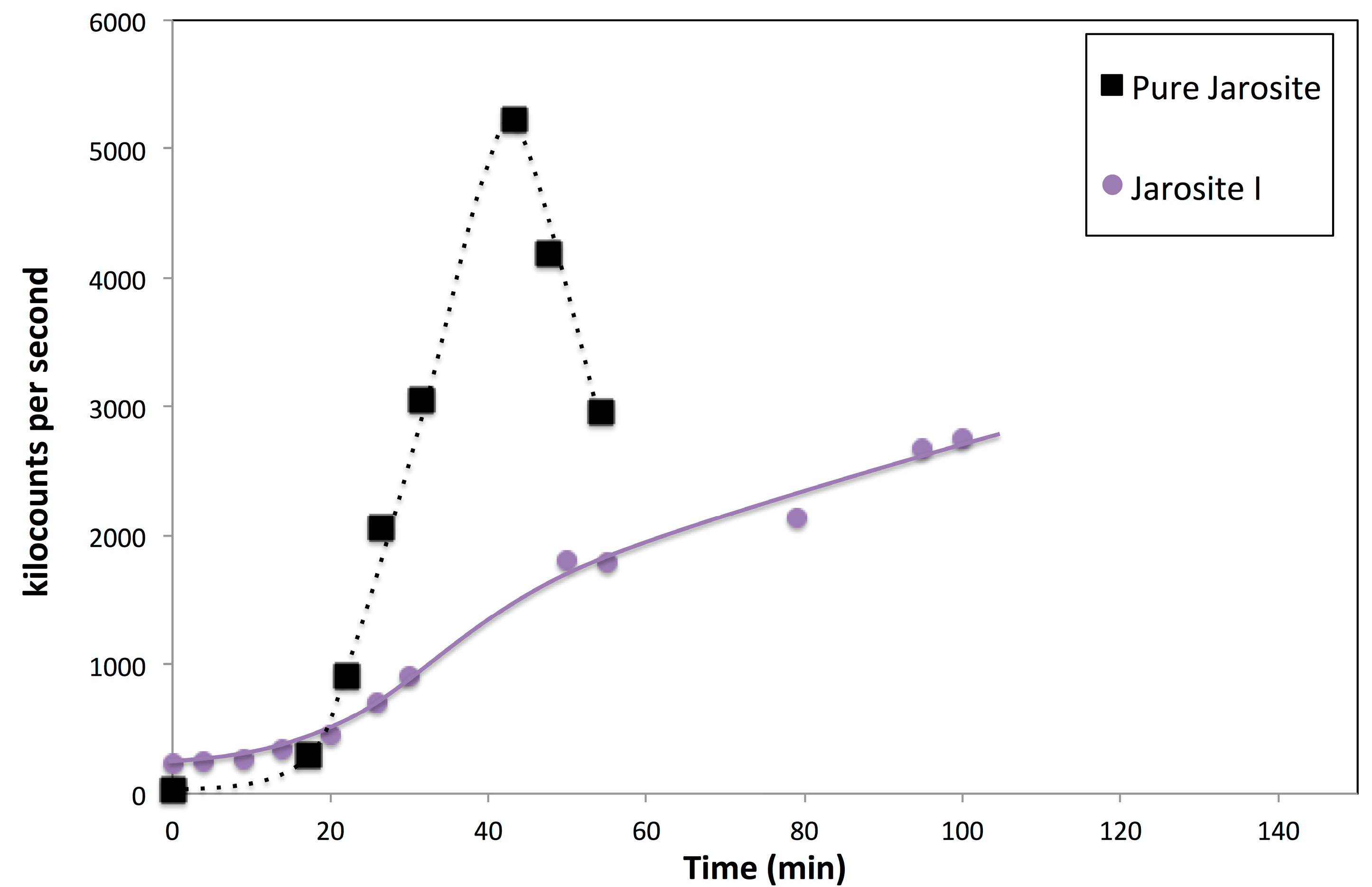
3.7. Nucleation Behaviour
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Bibi, I.; Singh, B.; Silvester, E. Akaganeite (beta-FeOOH) precipitation in inland acid sulfate soils of south-western New South Wales (NSW), Australia. Geochim. Cosmochim. Acta 2011, 75, 6429–6438. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar] [CrossRef]
- Long, D.T.; Fegan, N.E.; McKee, J.D.; Lyons, W.B.; Hines, M.E.; Macumber, P.G. Formation of alunite, jarosite and hydrous iron oxides in a hypersaline system: Lake Tyrell, Victoria. Aust. Chem. Geol. 1992, 96, 183–202. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Miner. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Klingelhofer, A.K.; Morris, R.V.; Bernhardt, B.; Schröder, C.; Rodinov, D.S.; de Souza Jnr, P.A.; Yen, A.; Gellert, R.; Evalanov, E.N.; Ming, D.W.; et al. Jarosite and hematite at Meridiani Planum from opportunity’s Mossbauer spectrometer. Science 2004, 306, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Comer, J.B.; Ennis, M.V.; Branam, T.B.; Butler, S.M.; Renton, P.M. Toxic Metals Removal in Acid mine Drainage Tratment Wetlands; Technical Report; Indiana Geological Survey: Bloomington, IN, USA, 2001. [Google Scholar]
- Figueiredo, M.-O.; da Silva, T.P. The positive environmental contribution of jarosite by retaining lead in acid mine drainage areas. Int. J. Environ. Res. Public Health 2011, 8, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K. Dissolution of Jarosite [KFe3(SO4)2(OH)6] at pH 2 and 8: Insights from Batch Experiments and Computational Modelling. Geochim. Cosmochim. Acta 2006, 70, 608–621. [Google Scholar] [CrossRef]
- Murphy, P.J.; Smith, A.M.L.; Hudson-Edwards, K.A.; Dubbin, W.E.; Wright, K. Raman and IR spectroscopic studies of alunite-supergroup compounds containing Al3+, Cr3+, Fe3+ and V3+ at the B site. Can. Mineral. 2009, 47, 663–681. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Freyer, B.J.; Weisener, C.G. Intracellular precipitation of Pb by Shewanella putrifaciens CN32 during the reductive dissolution of Pb-jarosite. Environ. Sci. Technol. 2009, 43, 8086–8091. [Google Scholar] [CrossRef] [PubMed]
- Leahy, M.J.; Schwarz, M.P. Modelling jarosite precipitation in isothermal chalcopyrite bioleaching columns. Hydrometallurgy 2009, 98, 181–191. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Hirajima, T. Effects of initial Fe2+ concentration and pulp density on the bioleaching of Cu from enargite by Acidianus brierleyi. Hydrometallurgy 2011, 109, 153–160. [Google Scholar] [CrossRef]
- Drouet, C.; Navrotsky, A. Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites. Geochim. Cosmochim. Acta 2003, 67, 2063–2076. [Google Scholar] [CrossRef]
- Drouet, C.; Pass, K.L.; Baron, D.; Drauker, S.; Navrotsky, A. Thermochemistry of jarosite-alunite and natrojarosite-natroalunite solid solutions. Geochim. Cosmochim. Acta 2004, 68, 2197–2205. [Google Scholar] [CrossRef]
- Grube, E.; Nielsen, U.G. The stoichiometry of synthetic alunite as a function of hydrothermal aging investigated by solid-state NMR spectroscopy, powder X-ray diffraction and infrared spectrscopy. Phys. Chem. Miner. 2015, 42, 337–345. [Google Scholar] [CrossRef]
- Stoffregen, R.E.; Alpers, C.N.; Jambor, J.L. Alunite-Jarosite crystallography, thermodynamics, and geochronology. Rev. Mineral. Geochem. 2012, 9, 454–479. [Google Scholar] [CrossRef]
- Alpers, C.N.; Rye, R.O.; Nordstrom, D.K.; White, L.D.; King, B.-S. Chemical, crystallographic and stable isotopic properties of alunite and jarosite from acid-hypersaline Australian lakes. Chem. Geol. 1992, 96, 203–226. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Swayze, G.A.; Milliken, R.E.; Mustard, J.F.; Clark, R.N.; Murchie, S.L.; Breit, G.N.; Wray, J.J.; Gondet, B.; Puolet, F.; et al. Discovery of alunite in Cross crater, Terra Sirenum, Mars: Evidence for acidic, sulforous waters. Am. Mineral. 2016, 101, 1527–1542. [Google Scholar] [CrossRef]
- Becker, U.; Gasharova, B. AFM observations and simulations of jarosite growth at the molecular scale: Probing the basis for the incorporation of foreign ions into jarosite as a storage material. Phys. Chem. Miner. 2001, 28, 545–556. [Google Scholar] [CrossRef]
- Desborough, G.A.; Smith, K.S.; Lowers, H.A.; Swayze, G.A.; Hammarstrom, J.M.; Diehl, S.F.; Leinz, R.W.; Driscoll, R.L. Mineralogical and chemical characteristics of some natural jarosites. Geochim. Cosmochim. Acta 2010, 74, 1041–1056. [Google Scholar] [CrossRef]
- Scott, K.M. Solid solution in, and classification of, gossan-derived members of the alunite-jarosite family, northwest Queensland, Australia. Am. Mineral. 1987, 72, 178–187. [Google Scholar]
- Scott, K.M. Origin of alunite and jarosite-group minerals in the Mt. Leyshon epithermal gold deposit, northeast Queensland, Australia. Am. Mineral. 1990, 75, 1176–1181. [Google Scholar]
- Brophy, G.P.; Scott, E.S.; Snellgrove, R.A. Sulfate studies II. Solid solution between alunite and jarosite. Am. Mineral. 1962, 47, 112–126. [Google Scholar]
- Basciano, L.C.; Peterson, R.C. Crystal chemistry of the natrojarosite-jarosite and natrojarosite-hydronium jarosite solid-solution series: A synthetic study with full Fe site occupancy. Am. Mineral. 2008, 93, 853–862. [Google Scholar] [CrossRef]
- Nielsen, U.G.; Majzlan, J.; Grey, C.P. Determination and quantification of the local environments in stoichiometric and defect jarosite by solid-state H-2 NMR spectroscopy. Chem. Mater. 2008, 20, 2234–2241. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Grey, I.E.; Brand, H.E.A. Ordering of iron vacancies in monoclinic jarosites. Am. Mineral. 2010, 95, 1590–1593. [Google Scholar] [CrossRef]
- Maubec, N.; Lahfid, A.; Lerouge, C.; Wille, G.; Michel, K. Characterization of alunite supergroup minerals by Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Spratt, H.J.; Rintoul, L.; Avdeev, M.; Martens, W.N. The crystal structure and vibrational spectroscopy of jarosite and alunite minerals. Am. Mineral. 2013, 98, 1633–1643. [Google Scholar] [CrossRef]
- Rudolph, W.W.; Mason, R.; Schmidt, P. Synthetic alunites of the potassium-oxonium solid solution series and some other members of the group: synthesis, thermal and X-ray characterization. Eur. J. Mineral. 2003, 15, 913–924. [Google Scholar] [CrossRef]
- Crabbe, H.; Fernandez, N.; Jones, F. Crystallization of jarosite in the presence of amino acids. J. Cryst. Growth 2015, 416, 28–33. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Dinardo, O.; Kaiman, S. Factors affecting lead jarosite formation. Hydrometallurgy 1980, 5, 305–324. [Google Scholar] [CrossRef]
- Mullin, J.W. Nucleation. In Crystallization, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1961; pp. 172–201. [Google Scholar]
- Acero, P.; Hudson-Edwards, K.A.; Gale, J.D. Influence of pH and temperature on alunite dissolution: Rates, products and insights on mechanisms from atomistic simulations. Chem. Geol. 2015, 419, 1–9. [Google Scholar] [CrossRef]
- Bunney, K.; Freeman, S.R.; Ogden, M.I.; Richmond, W.R.; Rohl, A.L.; Jones, F. Effect of La3+ on the crystal growth of barium sulfate. Cryst. Growth Des. 2014, 14, 1650–1658. [Google Scholar] [CrossRef]
- Jones, F.; Richmond, W.R.; Rohl, A.L. Molecular modelling of phosphonate molecules onto barium sulfate terraced surfaces. J. Phys. Chem. B. 2006, 110, 7414–7424. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Rohl, A.L. GDIS: A visualization program for molecular and periodic systems. Zeitschrift für Kristallographie 2005, 220, 580–584. [Google Scholar] [CrossRef]
- Gale, J.D.; Rohl, A.L. The General Utility Lattice Program. Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions Part 5. Gibbs Free energy of hydration at 298.15K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Sasaki, K. Raman study of the microbially mediated dissolution of pyrite by Thiobacillus ferrooxidans. Can. Mineral. 1997, 35, 999–1008. [Google Scholar]
- Sasaki, K.; Konno, H. Morphology of jarosite-group compounds precipitated from biologically and chemically oxidized Fe ions. Can. Mineral. 2000, 38, 45–56. [Google Scholar] [CrossRef]
- Majzlan, J.; Speziale, S.; Duffy, T.S.; Burns, P.C. Single-crystal elastic properties of alunite, KAl3(SO4)2(OH)6. Phys. Chem. Miner. 2006, 33, 567–573. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Zeitschrift für Physik 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Kubisz, J. Studies on synthetic alkali-hydronium jarosites II: Thermal investigations. Mineral. Pol. 1971, 2, 51–59. [Google Scholar]
- Kuçuk, F.; Yildiz, K. The decomposition kinetics of mechanically activated alunite ore in air atmosphere by thermogravimetry. Thermochim. Acta 2006, 448, 107–110. [Google Scholar] [CrossRef]
- Piana, S.; Jones, F.; Gale, J.D. Assisted desolvation as a key kinetic step for crystal growth. J. Am. Chem. Soc. 2006, 128, 13668–13674. [Google Scholar] [CrossRef] [PubMed]

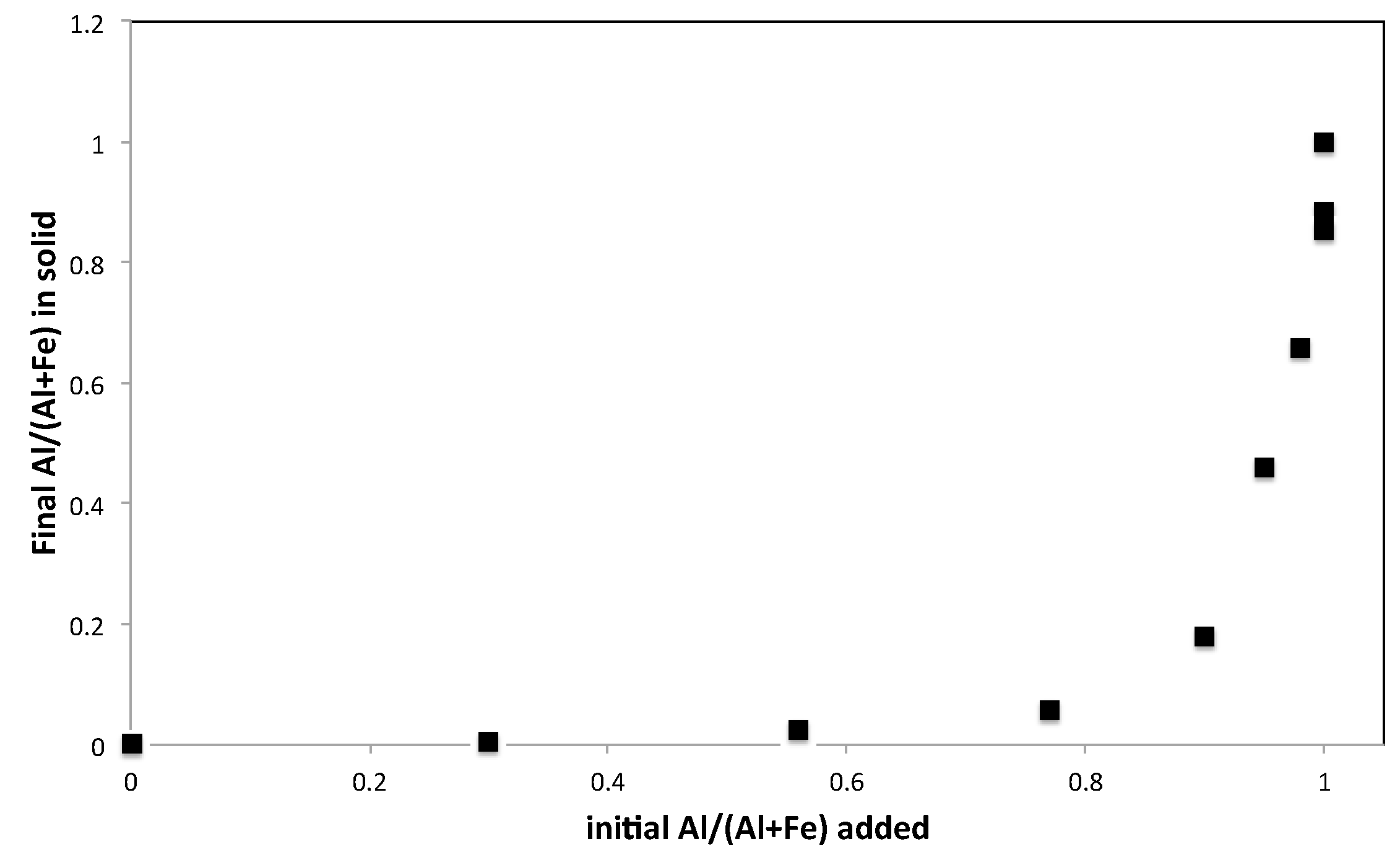
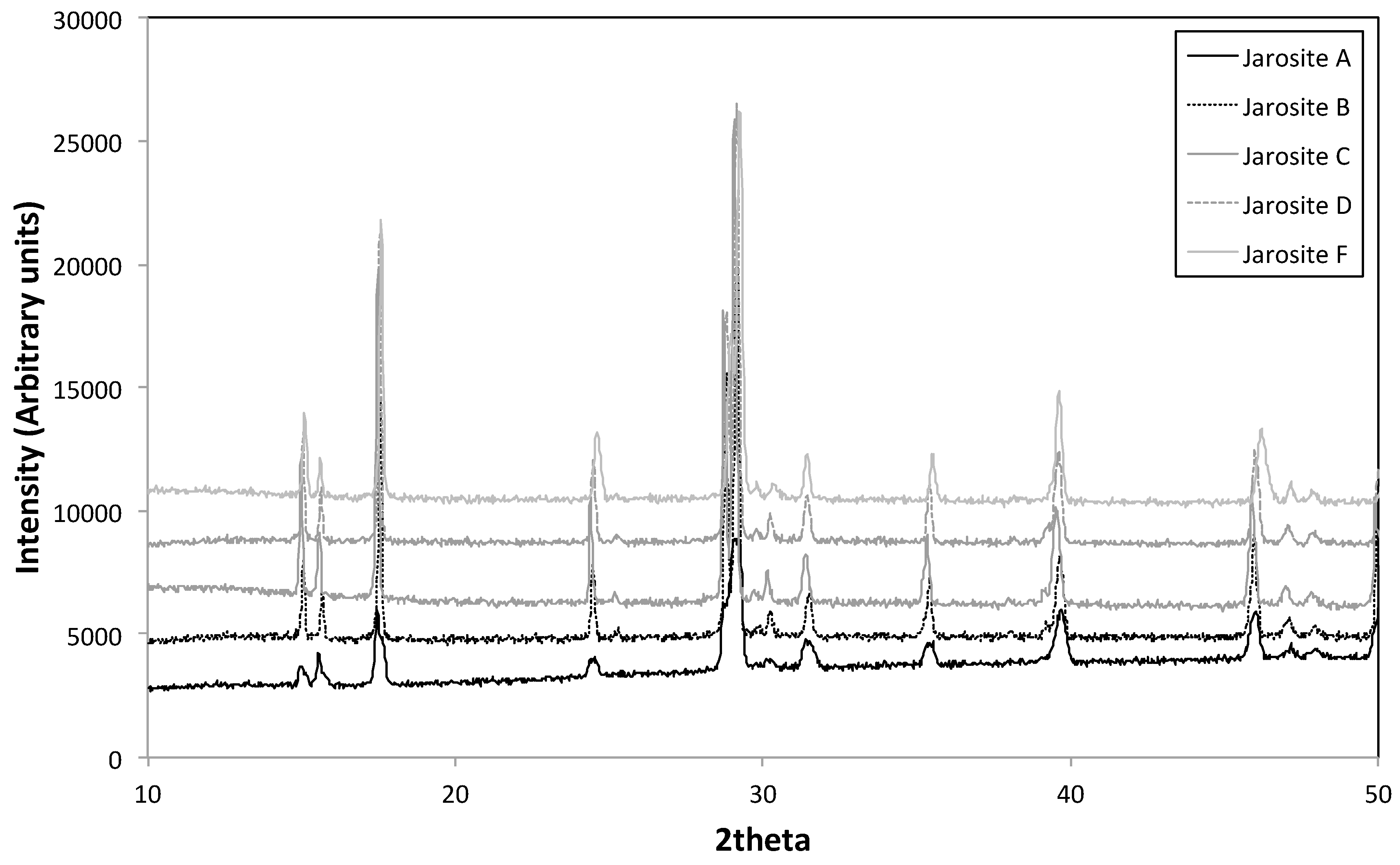
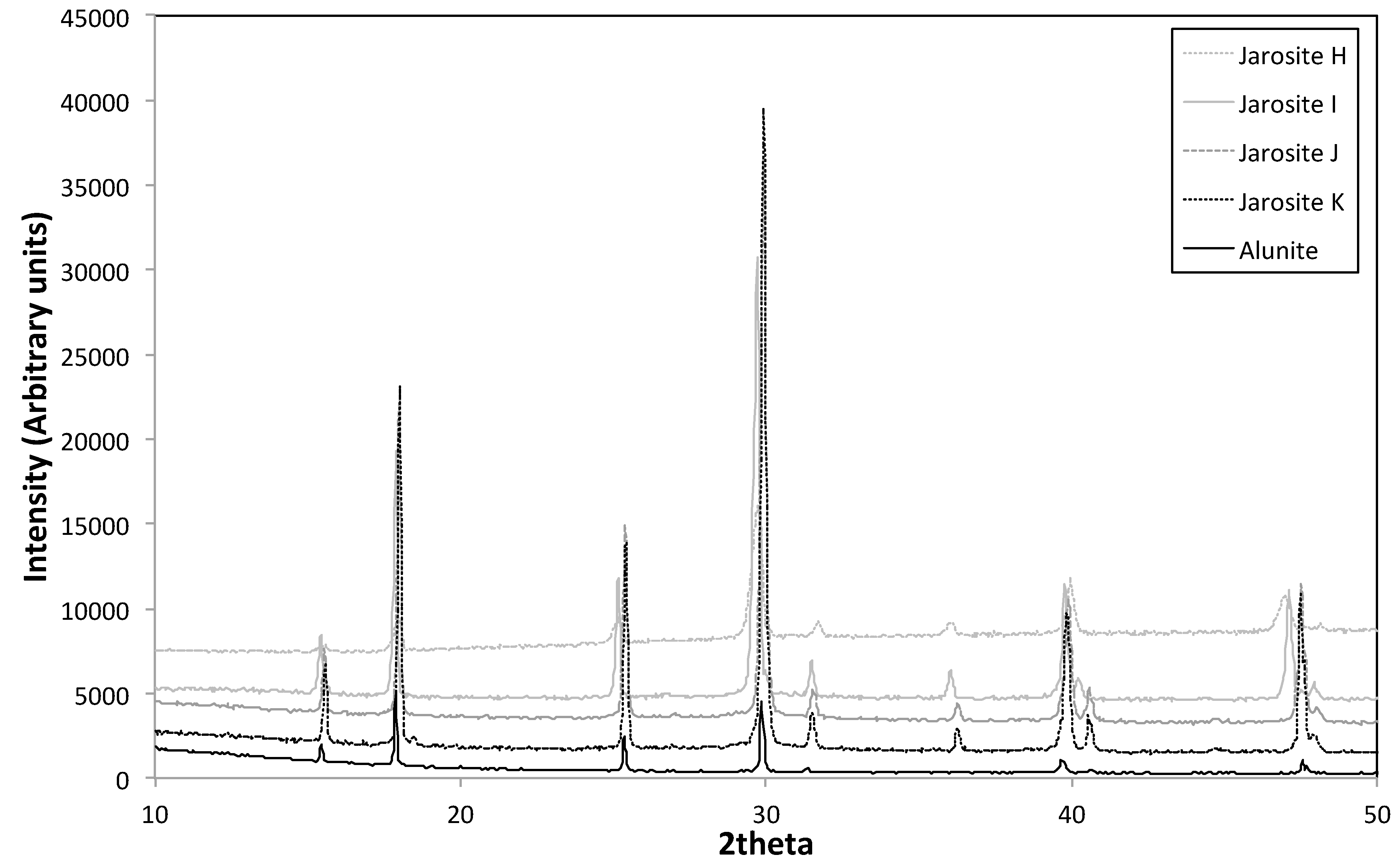


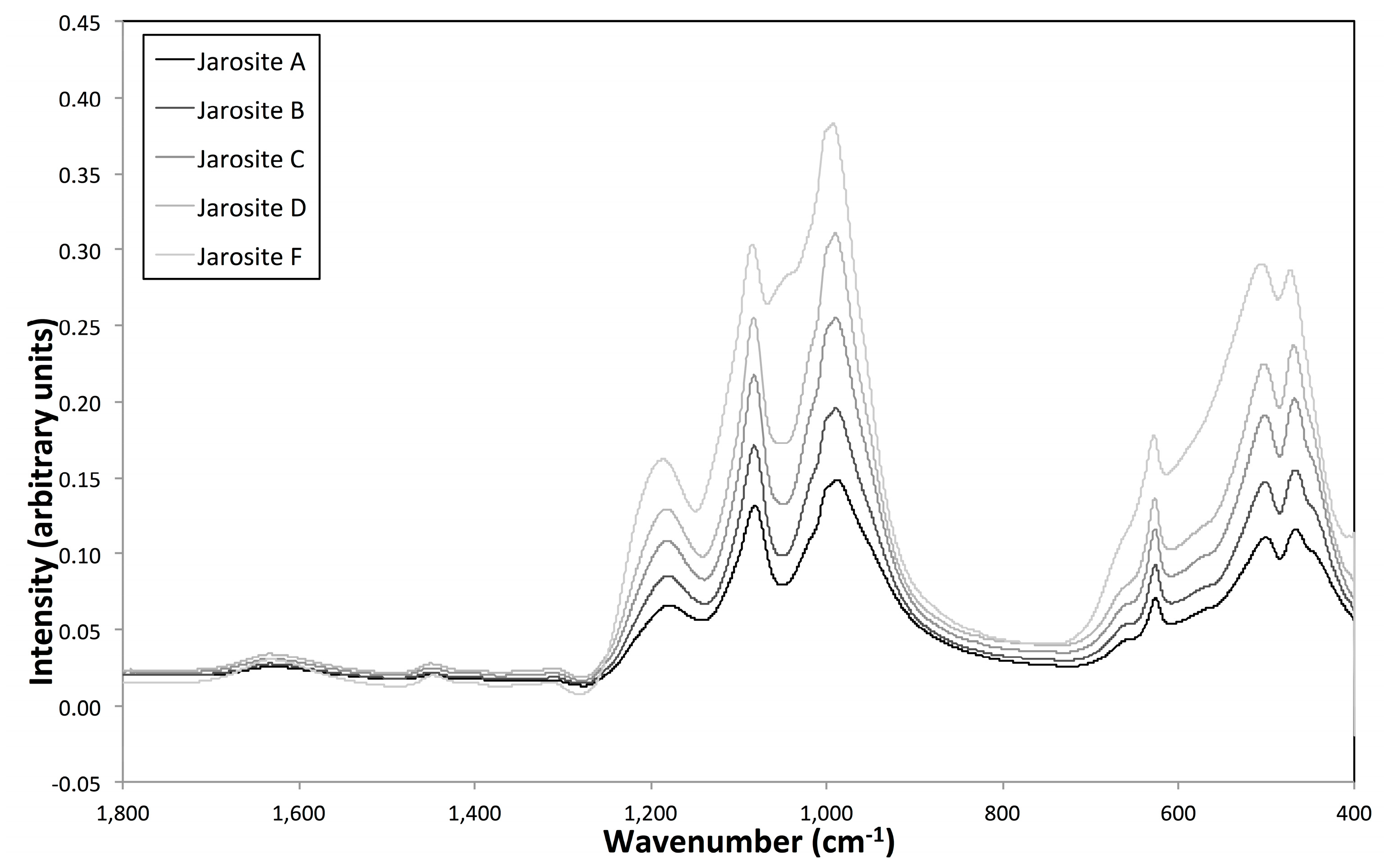
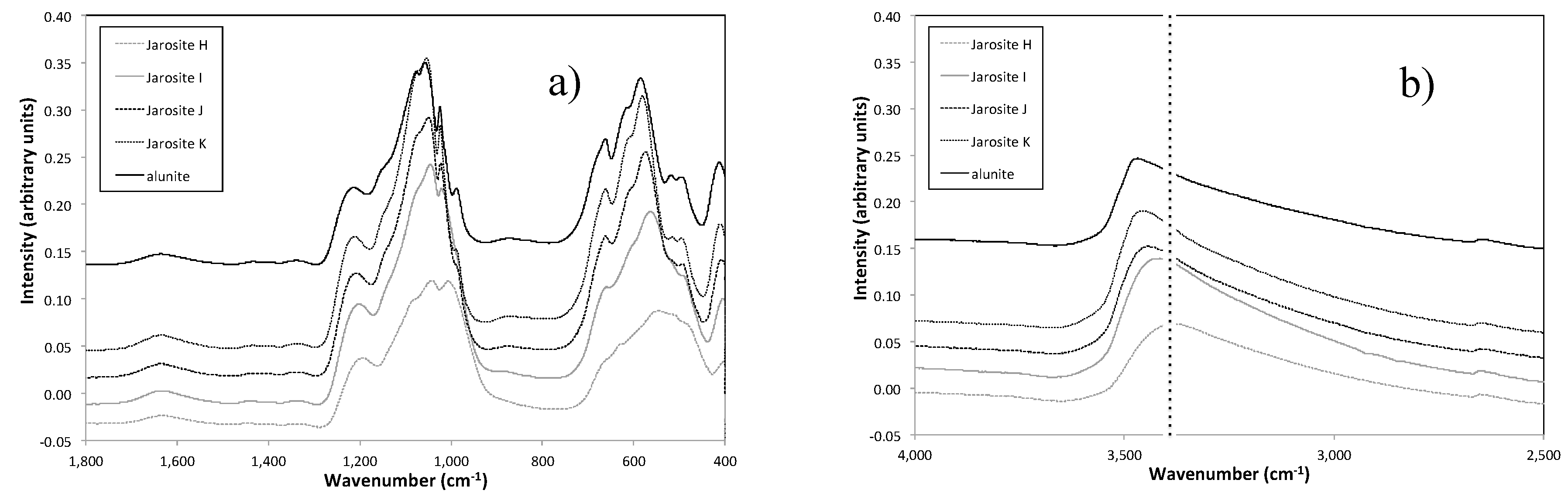
| Sample Name | Fe Moles (×10−3) | Al Moles (×10−3) | Fe/Al Molar Ratio | InitialAl/(Al + Fe) |
|---|---|---|---|---|
| Jarosite A | 2.85 | 0 | - | 0 |
| Jarosite B | 1.85 | 0.78 | 2.37 | 0.30 |
| Jarosite C | 1.28 | 1.65 | 0.77 | 0.56 |
| Jarosite D | 0.69 | 2.28 | 0.30 | 0.77 |
| Jarosite E (no solids obtained) | 0 | 3.09 | 0 | 1.00 |
| Jarosite F | 0.74 | 6.53 | 0.11 | 0.90 |
| Jarosite H | 2.22 | 44.0 | 0.05 | 0.95 |
| Jarosite I | 0.89 | 44.0 | 0.02 | 0.98 |
| Jarosite J | 0.04 | 44.0 | 0.01 | 1.00 |
| Jarosite K | 0.02 | 44.0 | 0.005 | 1.00 |
| (Jarosite G) Alunite | 0 | 44.0 | 0 | 1.00 |
| Sample Name | Fe | Al | K | S | OH− | H2O | H3O+ |
|---|---|---|---|---|---|---|---|
| Jarosite A | 2.61 | 0.00 | 0.88 | 2 | 5.91 | 0.09 | 0.12 |
| Jarosite B | 2.65 | 0.02 | 0.90 | 2 | 5.89 | 0.11 | 0.10 |
| Jarosite C | 2.44 | 0.06 | 0.87 | 2 | 5.63 | 0.37 | 0.13 |
| Jarosite D | 2.33 | 0.15 | 0.84 | 2 | 5.82 | 0.18 | 0.16 |
| Jarosite F | 2.02 | 0.44 | 0.91 | 2 | 5.18 | 0.82 | 0.09 |
| Jarosite H | 1.28 | 1.09 | 0.89 | 2 | 5.02 | 0.98 | 0.11 |
| Jarosite I | 0.80 | 1.55 | 0.86 | 2 | 5.23 | 0.77 | 0.14 |
| Jarosite J | 0.27 | 2.02 | 0.92 | 2 | 4.52 | 1.48 | 0.08 |
| Jarosite K | 0.33 | 1.90 | 0.82 | 2 | 5.16 | 0.84 | 0.18 |
| Alunite | 0.00 | 2.23 | 0.95 | 2 | 4.11 | 1.89 | 0.06 |
| Sample Name | Alunite | Jarosite |
|---|---|---|
| Jarosite A | a = 7.306 ± 0.004; c = 17.100 ± 0.008 | |
| Jarosite B | a = 7.3139 ± 0.0005; c = 17.116 ± 0.001 | |
| Jarosite C | a = 7.3068 ± 0.0003; c = 17.1157 ± 0.0008 | |
| Jarosite D | a = 7.2994 ± 0.0003; c = 17.1258 ± 0.0008 | |
| Jarosite F | a = 7.2614 ± 0.0003; c = 17.125 ± 0.001 | |
| Jarosite H | a = 7.173 ± 0.003; c = 17.104 ± 0.007 | |
| Jarosite I | a = 7.1193 ± 0.0001; c = 17.1205 ± 0.0003 | |
| Jarosite J | a = 7.055 ± 0.0005; c = 17.088 ± 0.001 | |
| Jarosite K | a = 7.0579 ± 0.0003; c = 17.1210 ± 0.0008 | |
| (Jarosite G) Alunite | a = 7.0172 ± 0.0003; c = 17.1204 ± 0.0008 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, F. Crystallization of Jarosite with Variable Al3+ Content: The Transition to Alunite. Minerals 2017, 7, 90. https://doi.org/10.3390/min7060090
Jones F. Crystallization of Jarosite with Variable Al3+ Content: The Transition to Alunite. Minerals. 2017; 7(6):90. https://doi.org/10.3390/min7060090
Chicago/Turabian StyleJones, Franca. 2017. "Crystallization of Jarosite with Variable Al3+ Content: The Transition to Alunite" Minerals 7, no. 6: 90. https://doi.org/10.3390/min7060090