Origin of Minerals and Critical Metals in an Argillized Tuff from the Huayingshan Coalfield, Southwestern China
Abstract
:1. Introduction
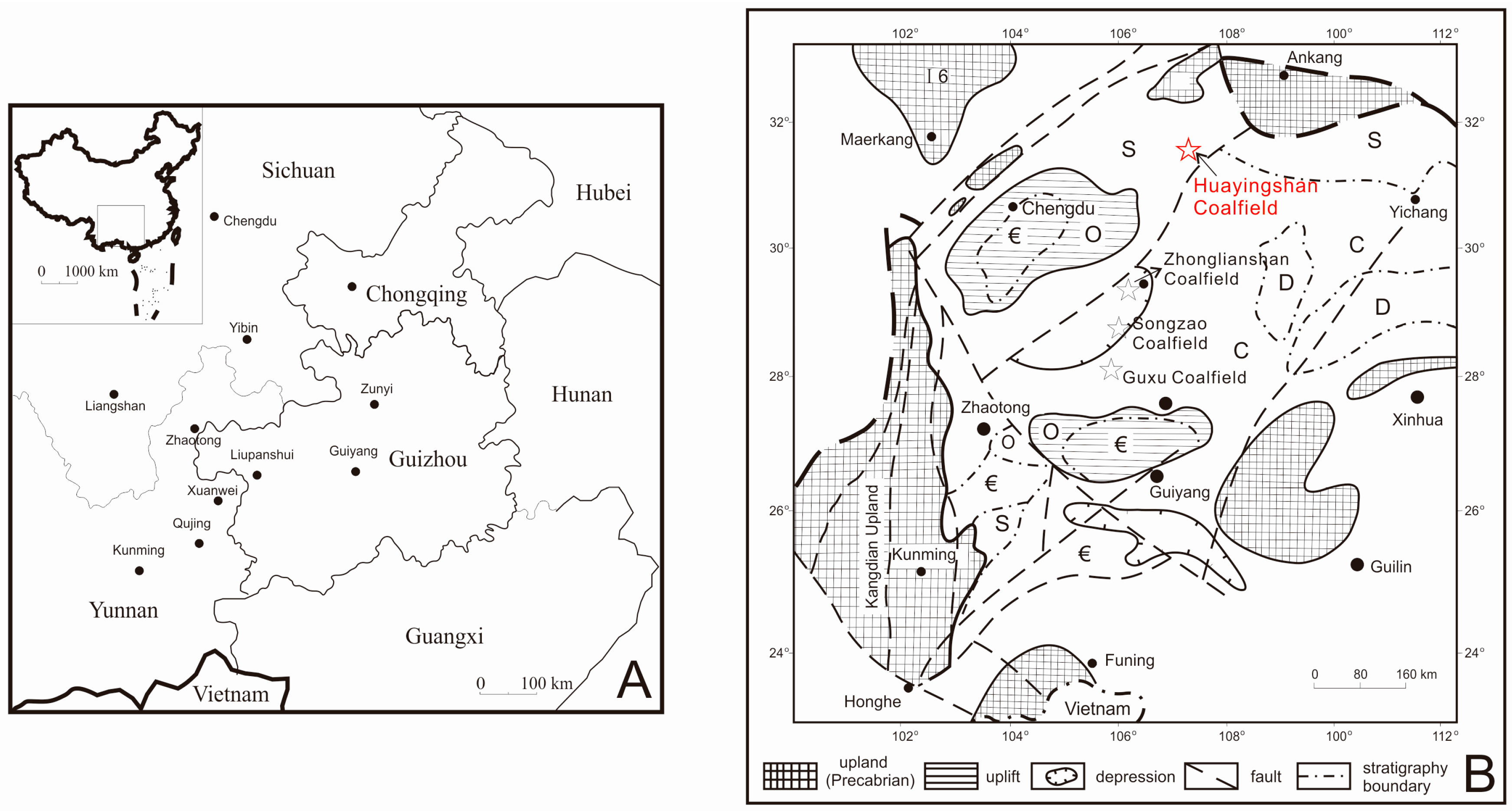
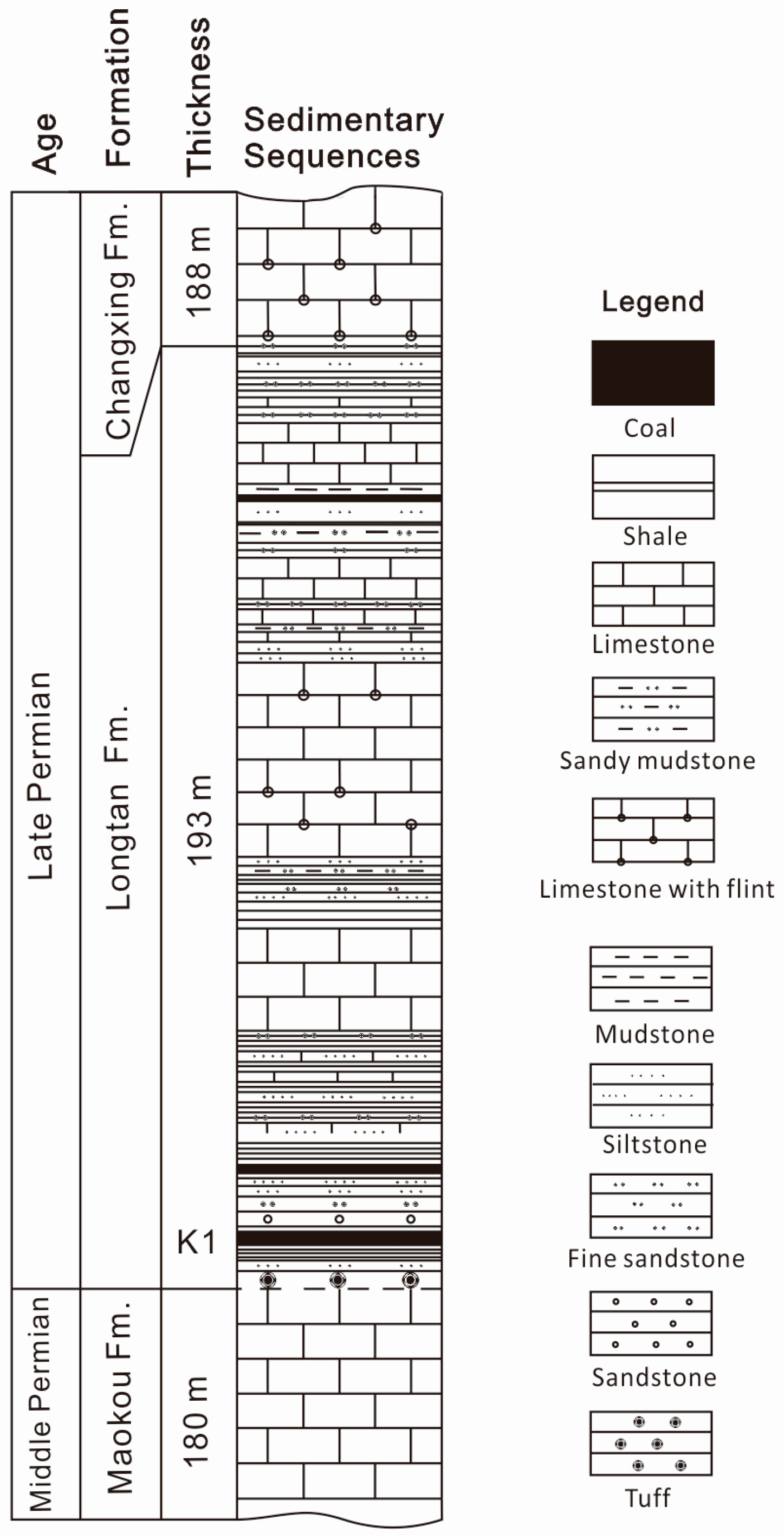
2. Geological Setting
3. Samples and Analytical Techniques
4. Results and Discussion
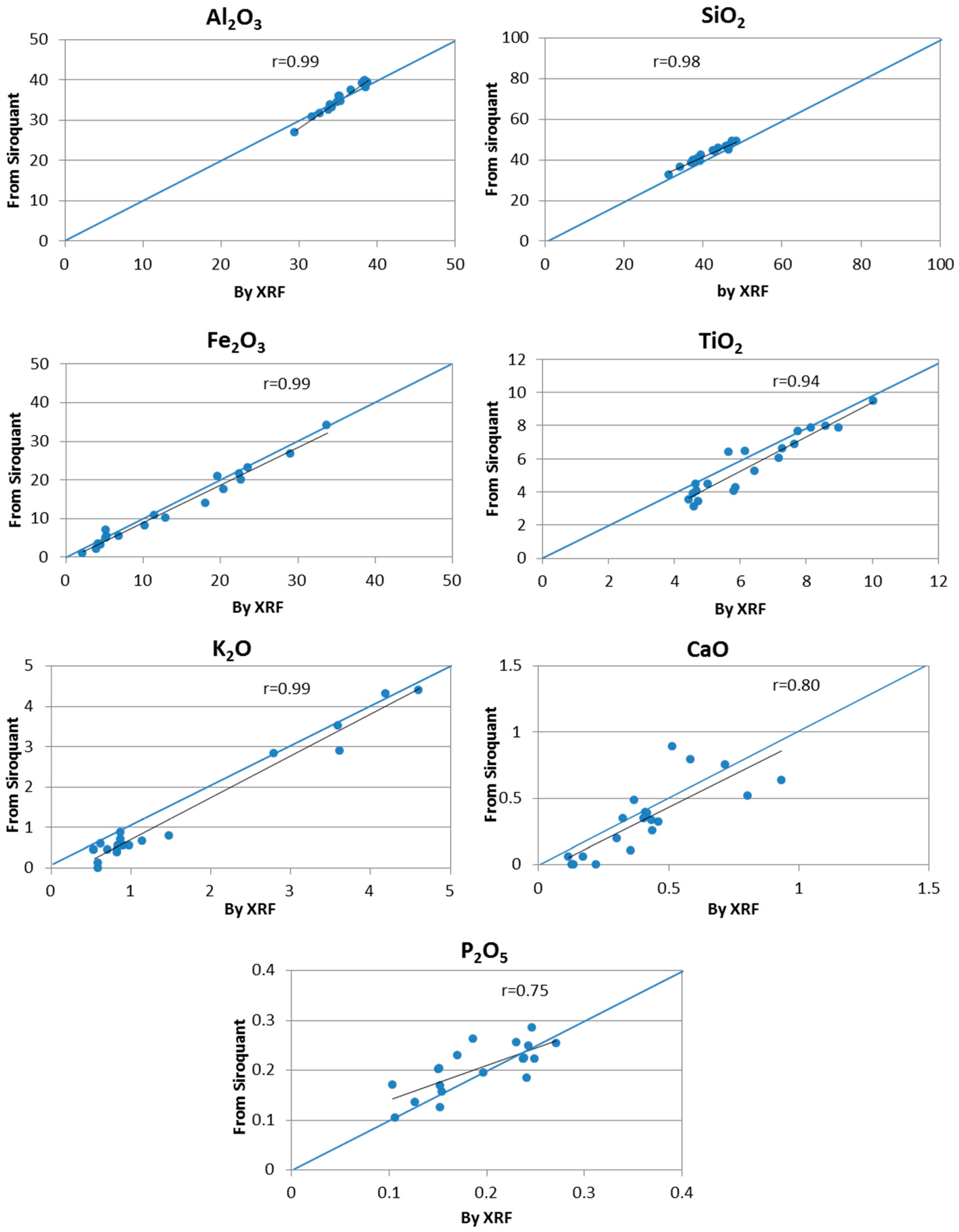
4.1. Comparison between Mineralogical and Chemical Data
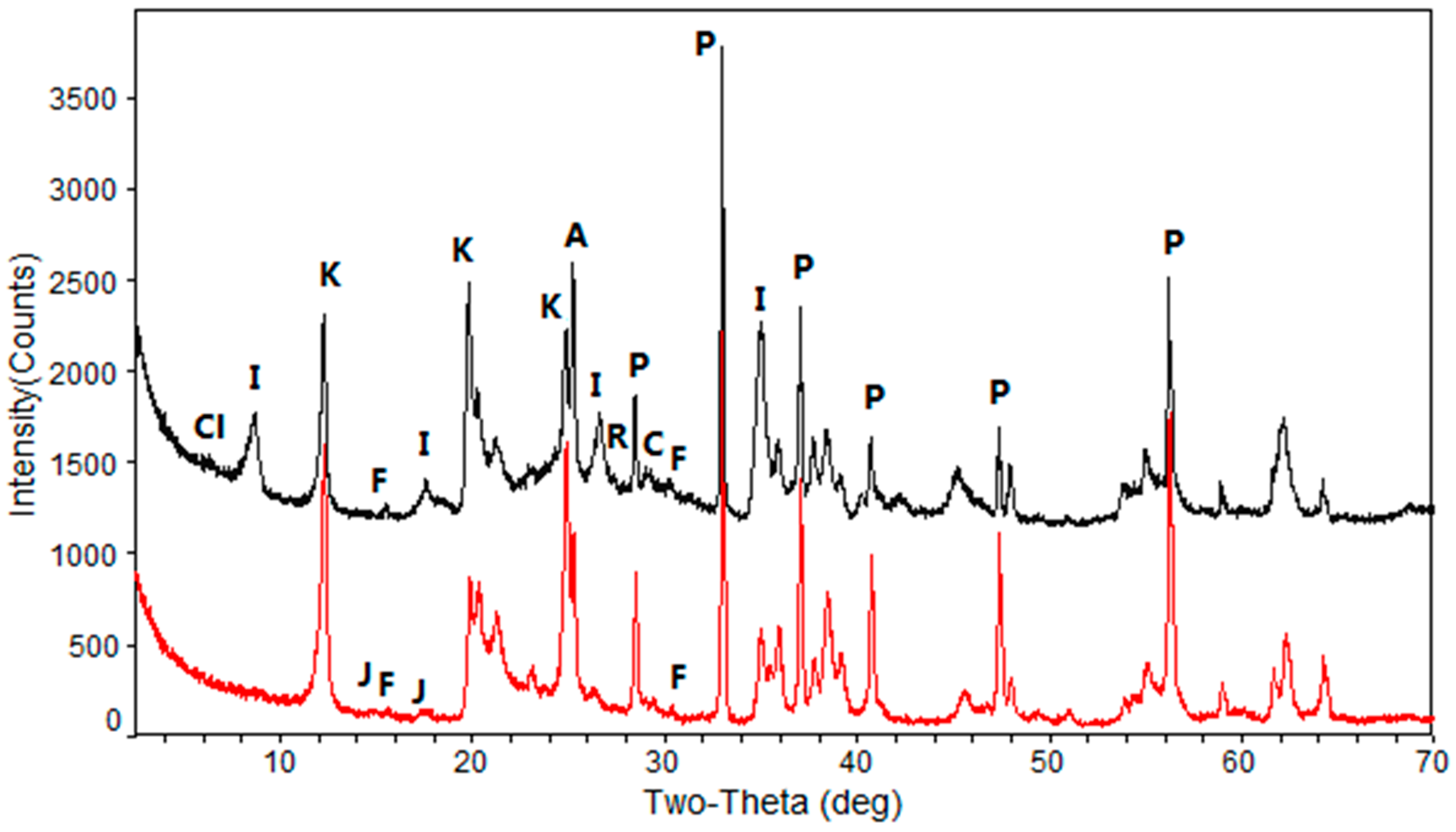
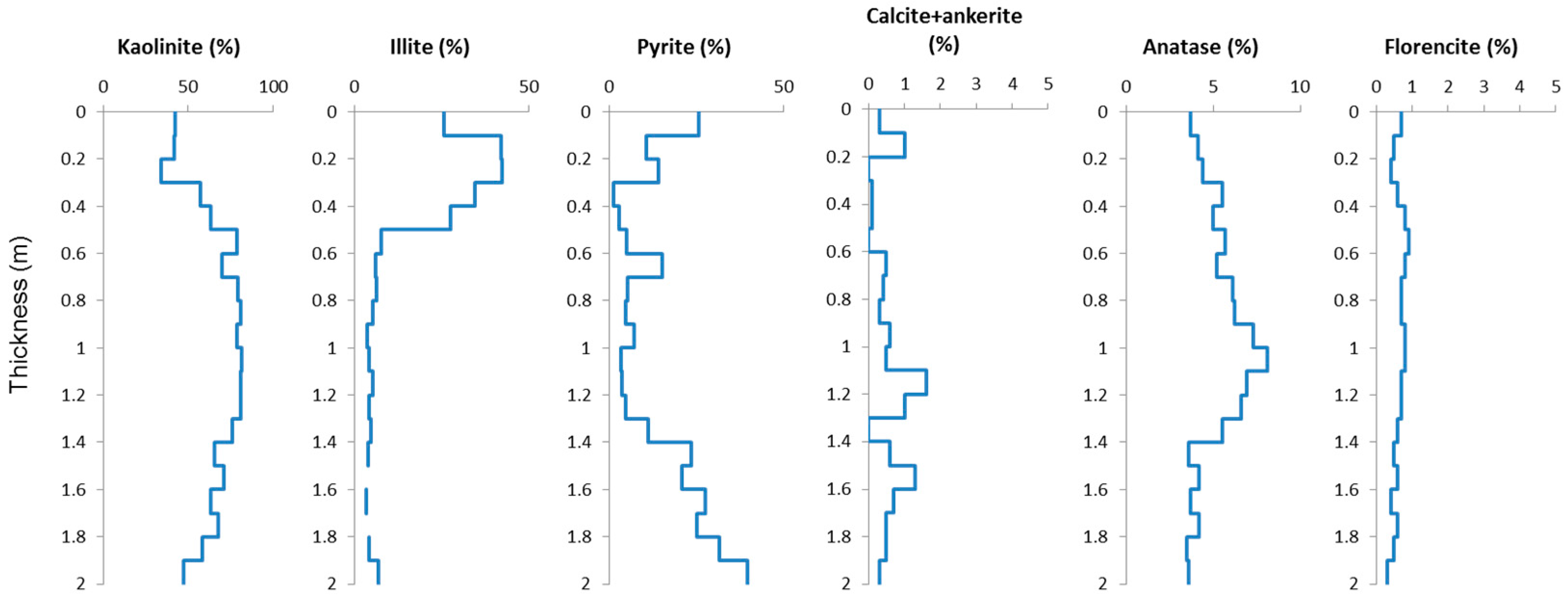
4.2. Minerals in the Samples
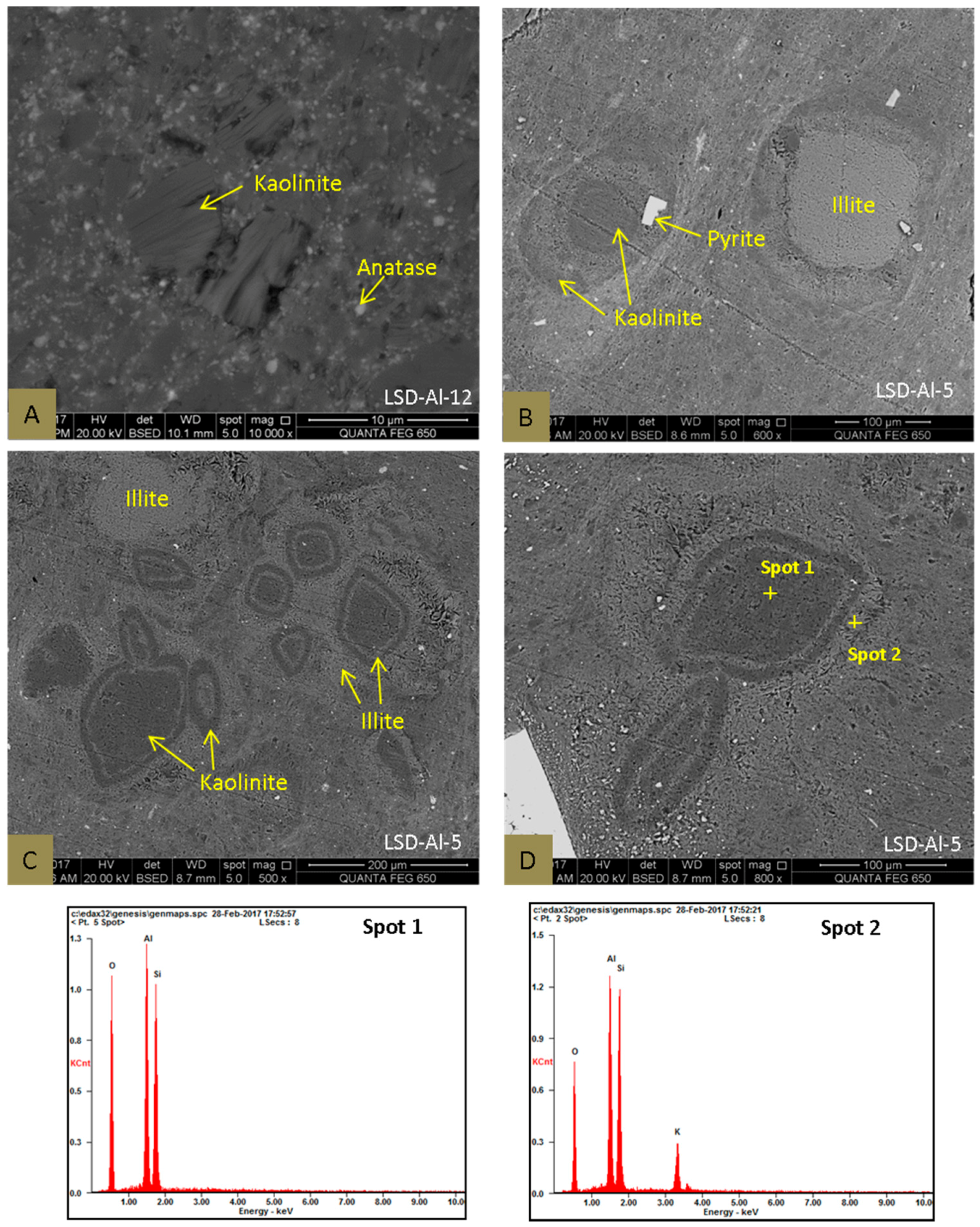
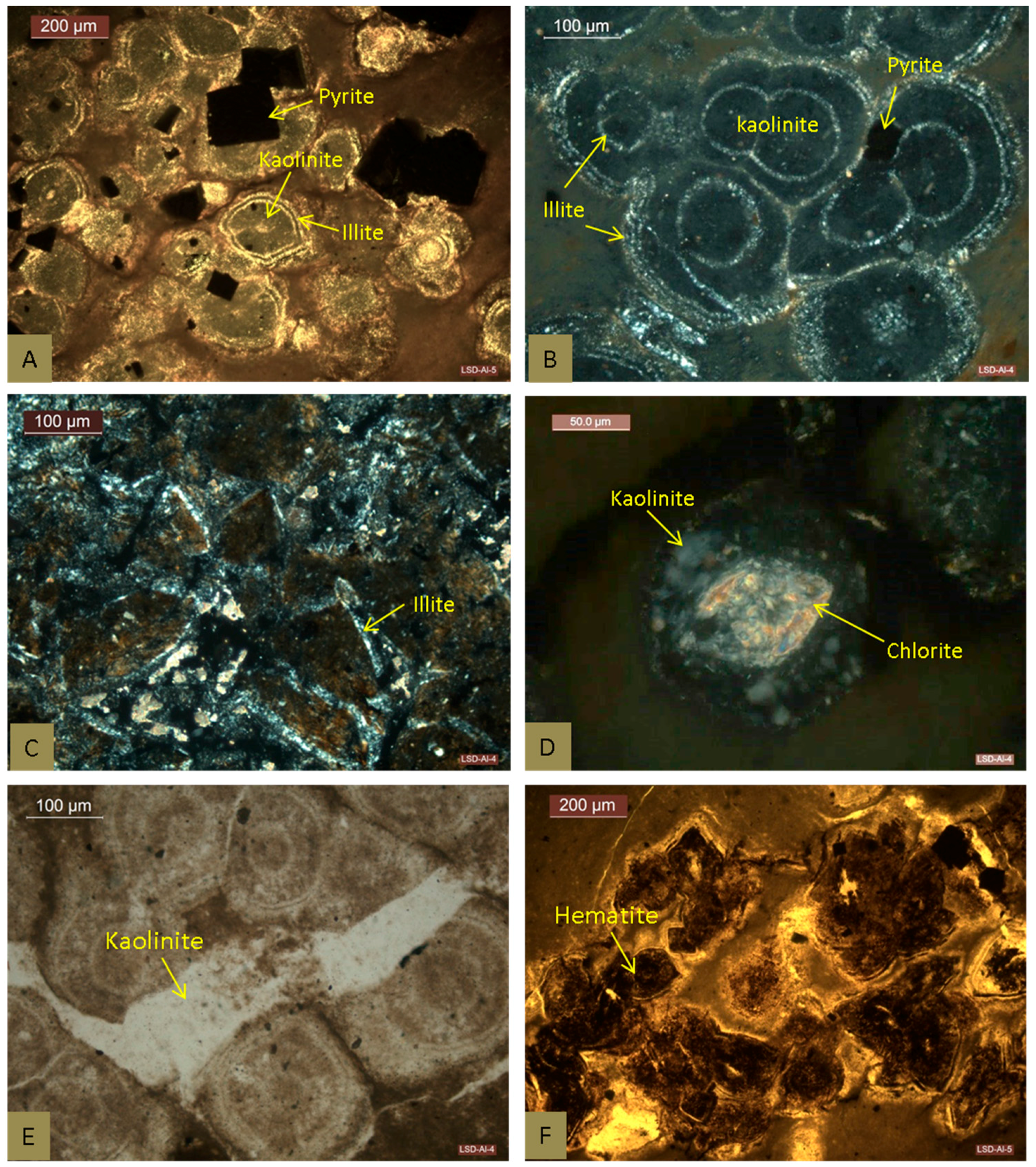
4.2.1. Clay Minerals
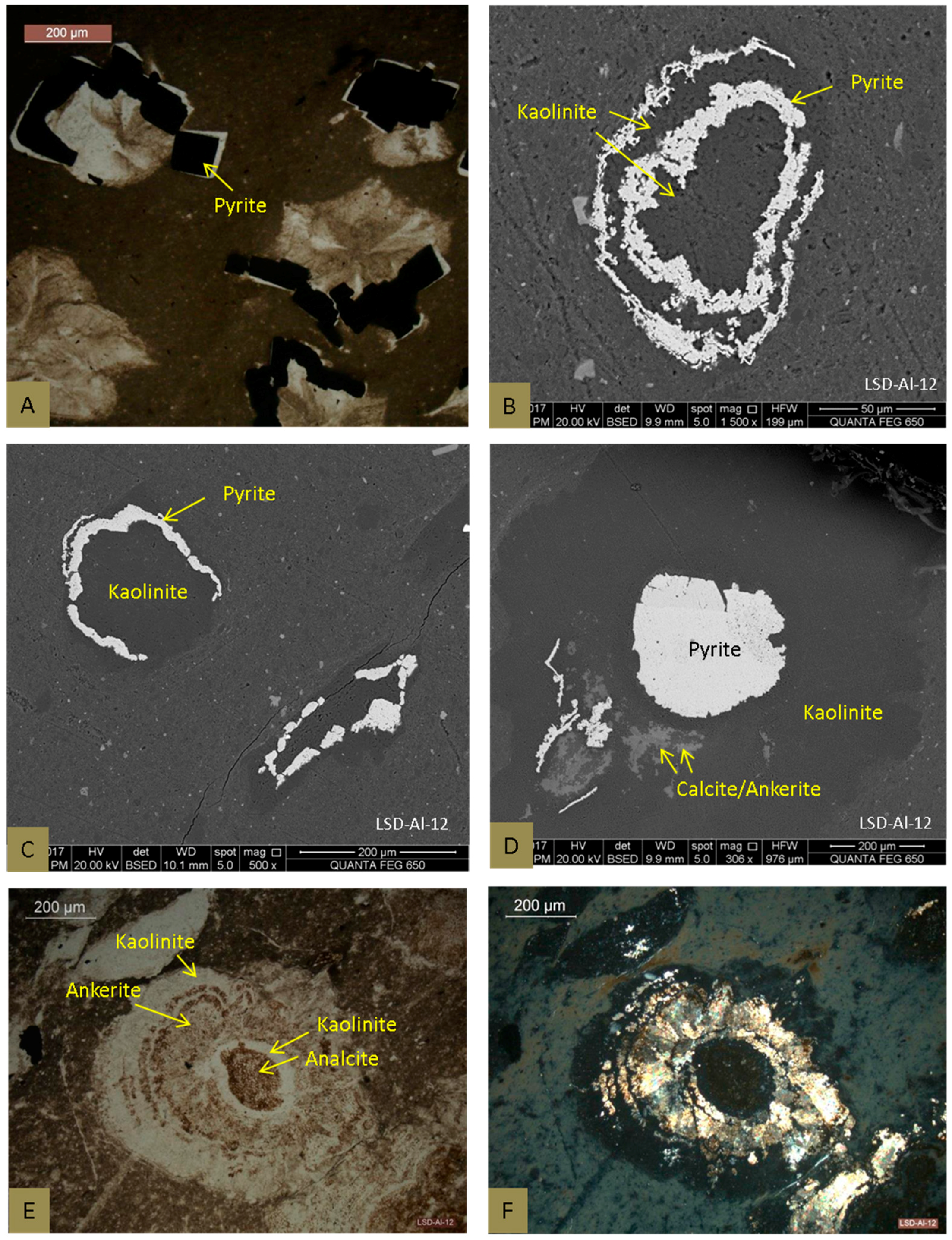
4.2.2. Pyrite
4.2.3. Carbonate Minerals
4.2.4. Anatase and Rutile
4.2.5. Florencite and Other Minerals
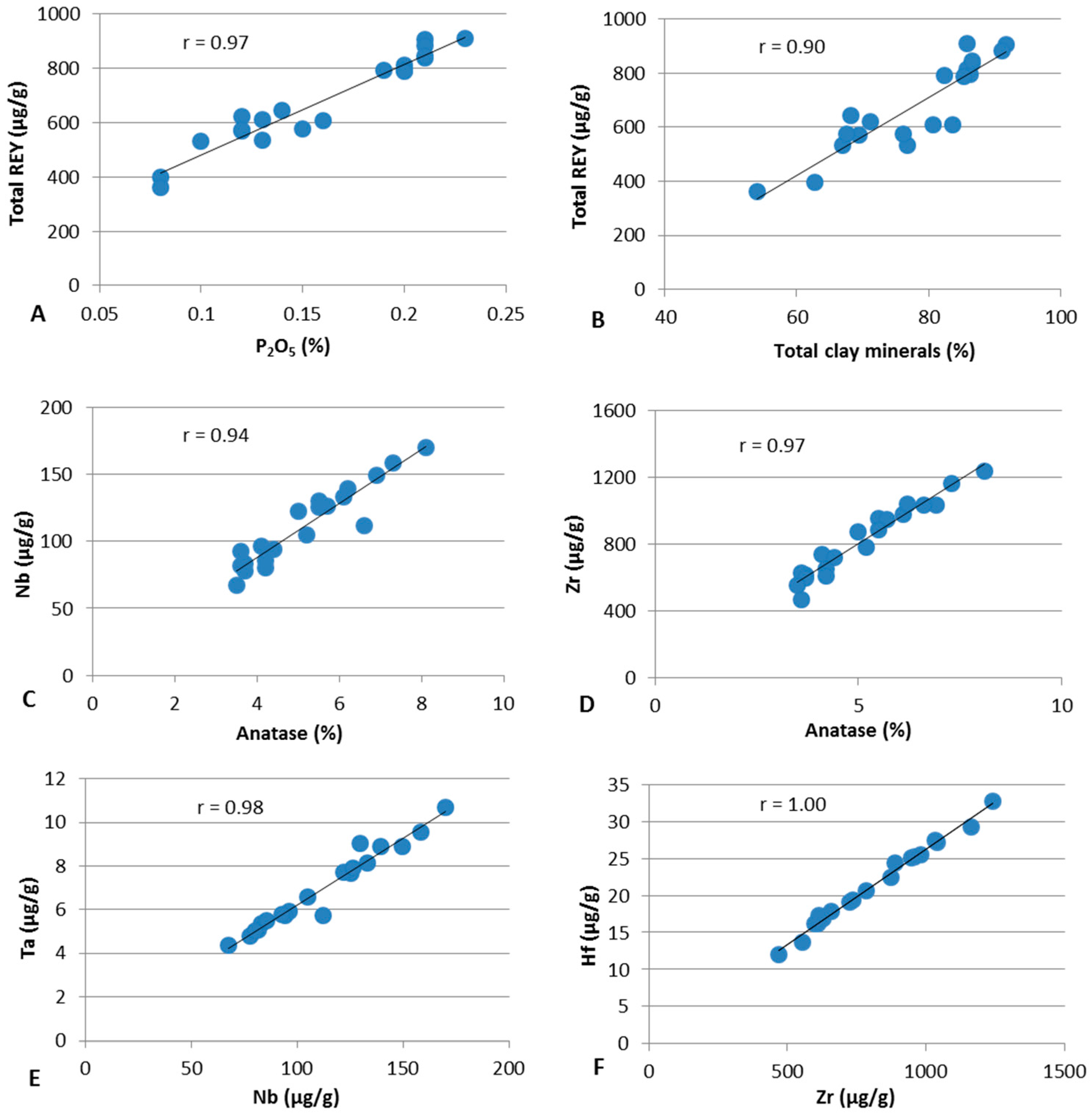
4.3. Elements in the Samples
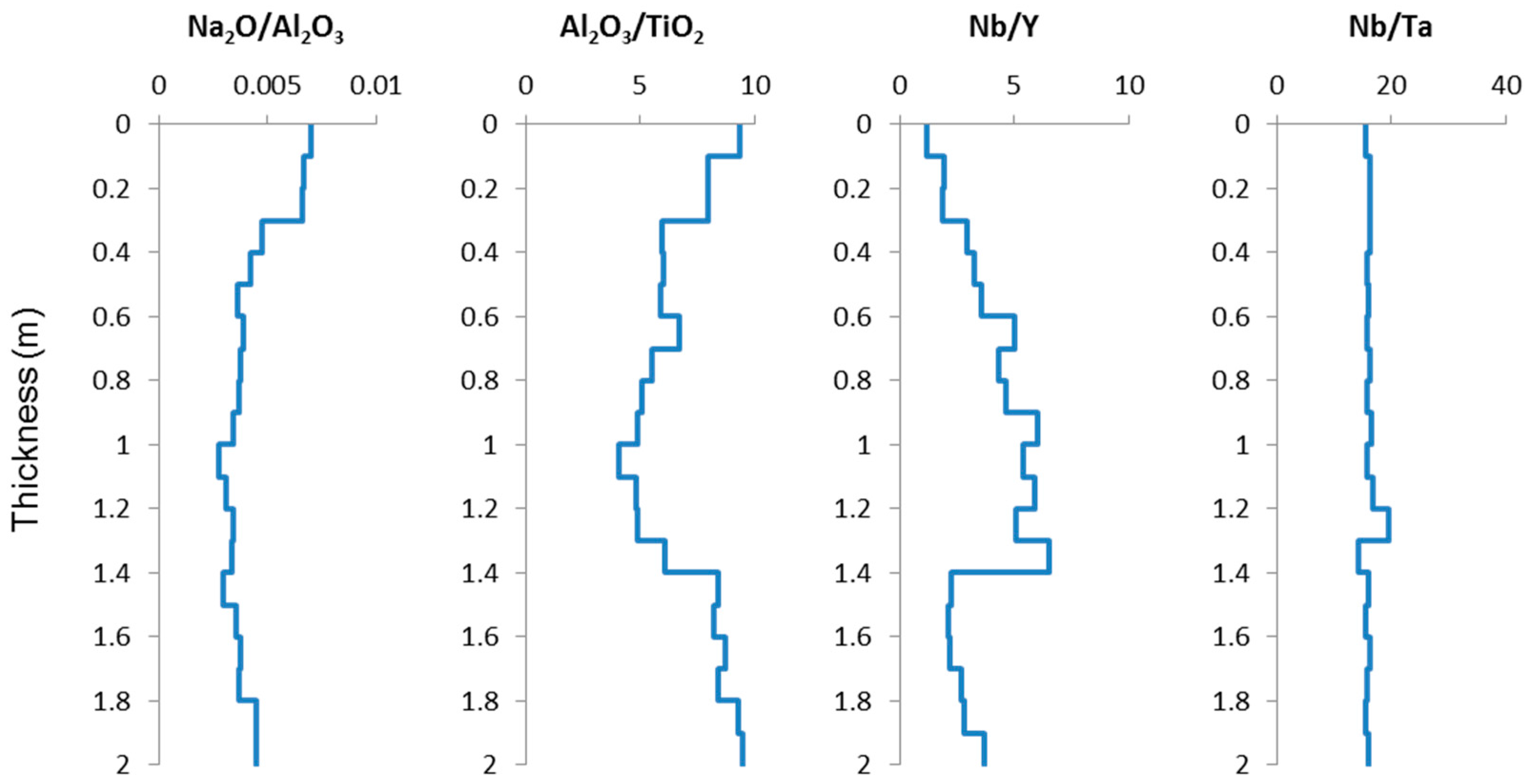
4.3.1. Concentrations of Selected Major and Trace Elements
4.3.2. Modes of Occurrence of Critical Metals
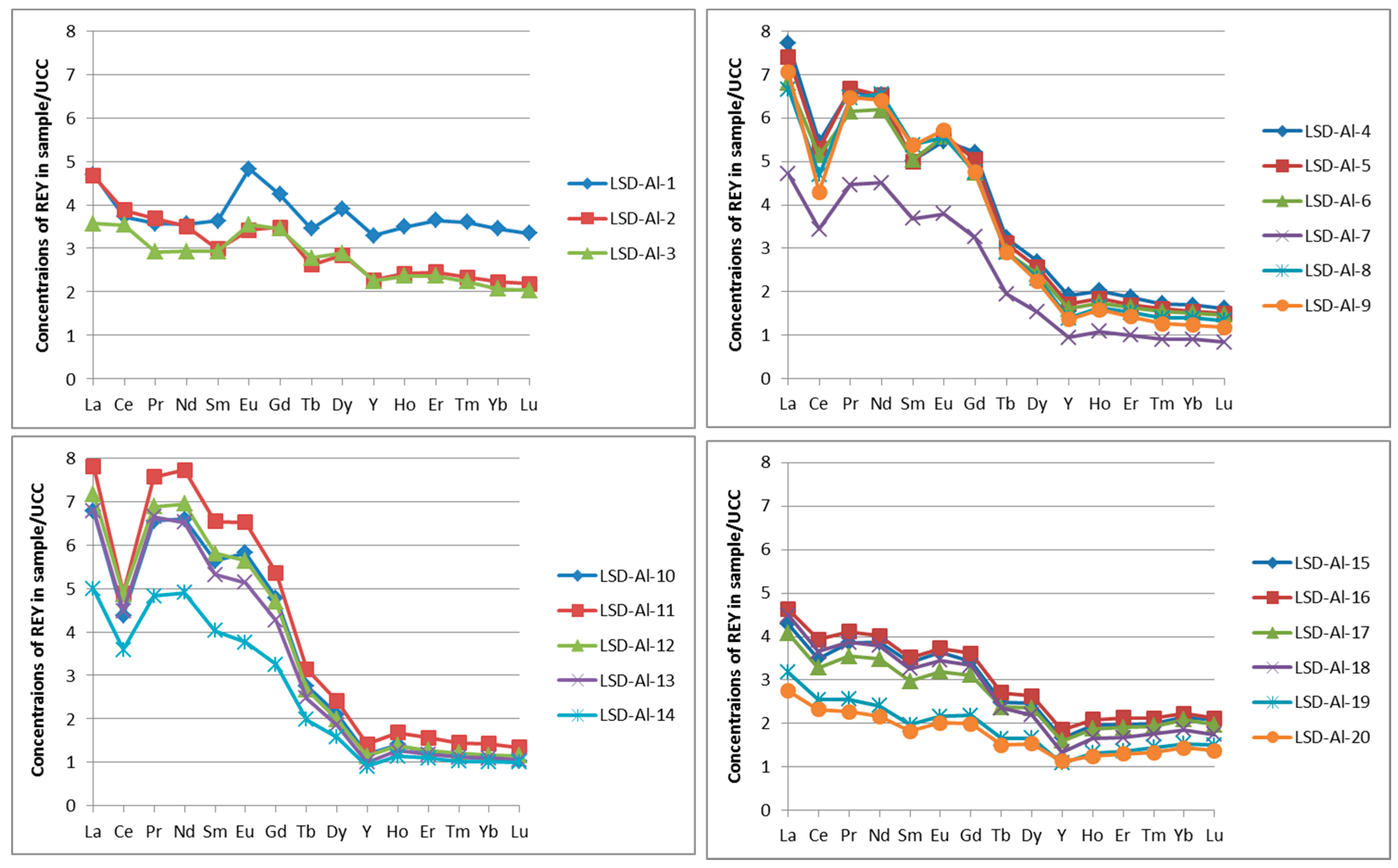
4.3.3. REY Distribution Patterns and Anomalies
5. Discussion
5.1. Formation of the Minerals
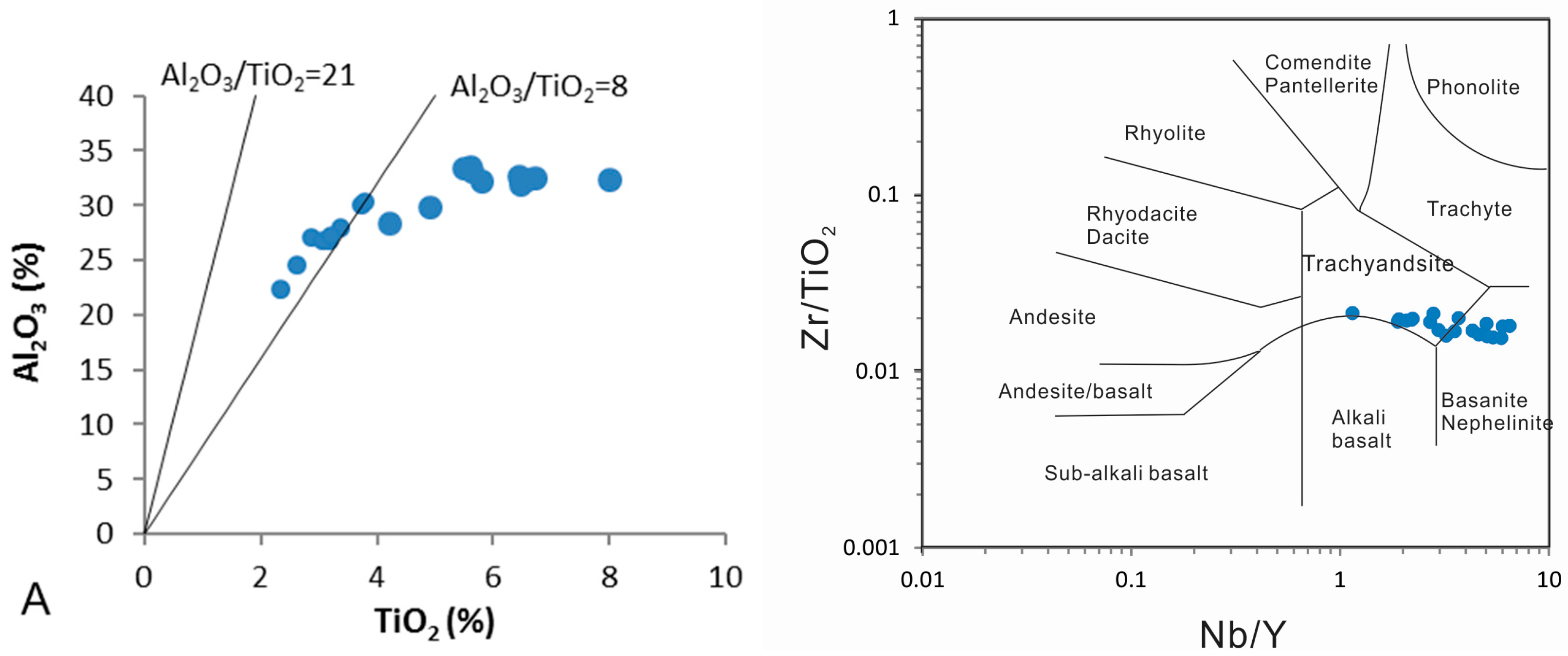
5.2. Chemical Composition of the Original Volcanic Ash
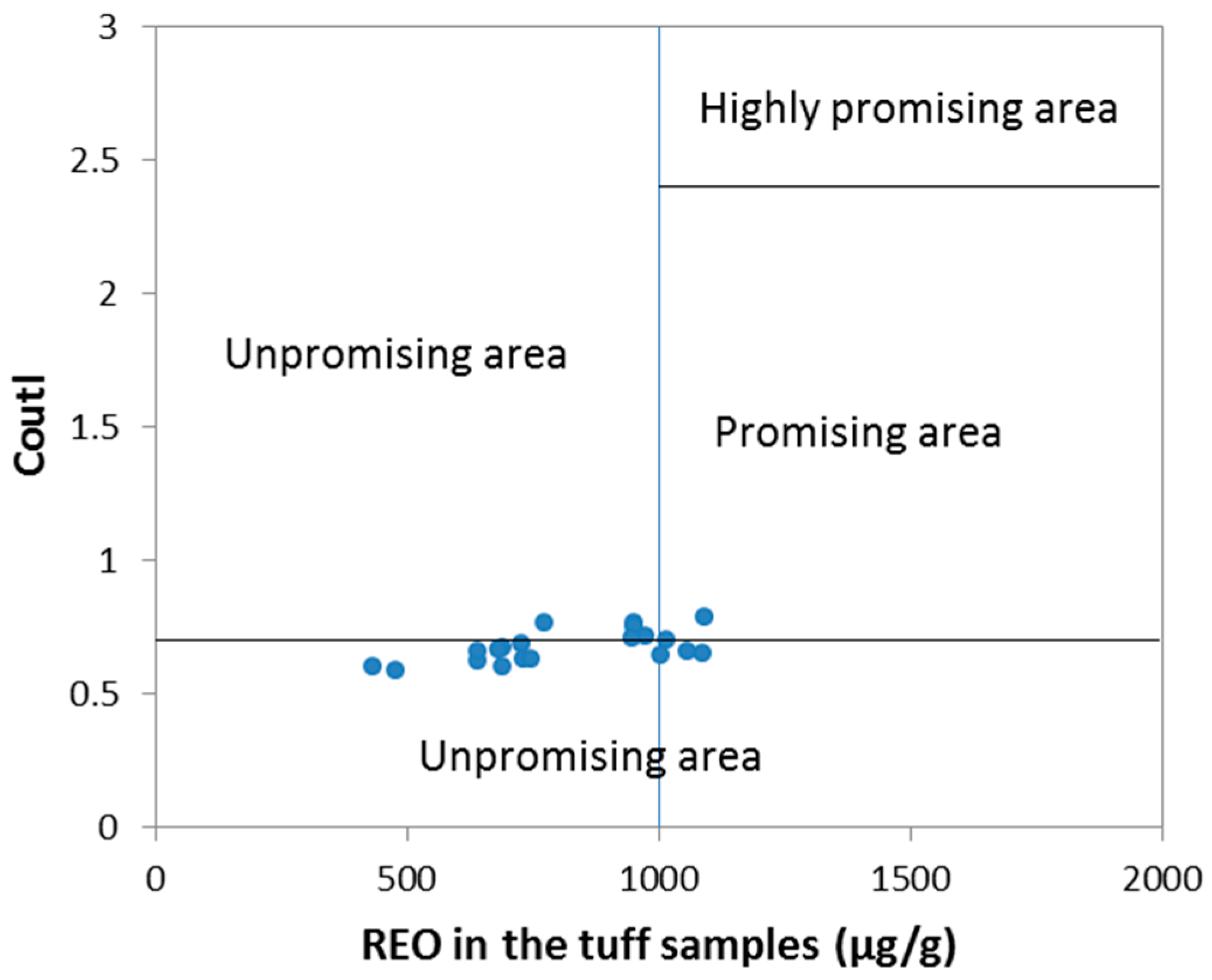
5.3. Potential Economic Significance of the Rare Metals in the Tuffs
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, S.-L.; Jahn, B.-M. Plume-lithosphere interaction in generation of the Emeishan flood basalts at the Permian-Triassic boundary. Geology 1995, 23, 889–892. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, Y.G.; Mei, H.J.; Zheng, Y.F.; He, B.; Pirajno, F. Distinct mantle sources of low-Ti and high-Ti basalts from the western Emeishan large igneous province, SW China: Implications for plume–lithosphere interaction. Earth Planet. Sci. Lett. 2004, 228, 525–546. [Google Scholar] [CrossRef]
- He, B.; Xu, Y.-G.; Chung, S.-L.; Xiao, L.; Wang, Y. Sedimentary evidence for a rapid, kilometer-scale crustal doming prior to the eruption of the Emeishan flood basalts. Earth Planet. Sci. Lett. 2003, 213, 391–405. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Chen, W.; Li, D.; Chou, C.-L.; Zhou, Y.; Zhu, C.; Li, H.; Zhu, X.; Xing, Y.; et al. A high-pyrite semianthracite of late Permian age in the Songzao Coalfield, southwestern China: Mineralogical and geochemical relations with underlying mafic tuffs. Int. J. Coal Geol. 2010, 83, 430–445. [Google Scholar] [CrossRef]
- Kramer, W.; Weatherall, G.; Offler, R. Origin and correlation of tuffs in the Permian Newcastle and Wollombi Coal Measures, NSW, Australia, using chemical fingerprinting. Int. J. Coal Geol. 2001, 47, 115–135. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Graham, I.T.; Li, X.; Liu, H.; Song, X.; Hower, J.C.; Zhou, Y. Cryptic sediment-hosted critical element mineralization from eastern Yunnan Province, southwestern China: Mineralogy, geochemistry, relationship to Emeishan alkaline magmatism and possible origin. Ore Geol. Rev. 2017, 80, 116–140. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Zhang, M.; Wang, X.; Wang, J.; Song, X.; Jiang, Y.; Luo, Y.; Song, Z.; Yang, Z.; et al. A new type of Nb(Ta)–Zr(Hf)–REE–Ga polymetallic deposit in the Late Permian coal-bearing strata, eastern Yunnan, southwestern China: Possible economic significance and genetic implications. Int. J. Coal Geol. 2010, 83, 55–63. [Google Scholar] [CrossRef]
- Hower, J.C.; Ruppert, L.F.; Eble, C.F. Lanthanide, yttrium, and zirconium anomalies in the Fire Clay coal bed, Eastern Kentucky. Int. J. Coal Geol. 1999, 39, 141–153. [Google Scholar] [CrossRef]
- Dai, S.; Luo, Y.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhao, L.; Liu, S.; Zhao, C.; Tian, H.; Zou, J. Revisiting the Late Permian coal from the Huayingshan, Sichuan, southwestern China: Enrichment and occurrence modes of minerals and trace elements. Int. J. Coal Geol. 2014, 122, 110–128. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; French, D.; Jia, S.; Hood, M.M.; Garrison, T.M. Mineralogical and geochemical compositions of Late Permian coals and host rocks from the Guxu Coalfield, Sichuan Province, China, with emphasis on enrichment of rare metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Jia, S.; Ward, C.R.; Hower, J.C.; Yan, X.; French, D. Enrichment of U-Re-V-Cr-Se and rare earth elements in the Late Permian coals of the Moxinpo Coalfield, Chongqing, China: Genetic implications from geochemical and mineralogical data. Ore Geol. Rev. 2017, 80, 1–17. [Google Scholar] [CrossRef]
- Zhuang, X.; Su, S.; Xiao, M.; Li, J.; Alastuey, A.; Querol, X. Mineralogy and geochemistry of the Late Permian coals in the Huayingshan coal-bearing area, Sichuan Province, China. Int. J. Coal Geol. 2012, 94, 271–282. [Google Scholar] [CrossRef]
- China Coal Geology Bureau. Sedimentary Environments and Coal Accumulation of Late Permian Coal Formation in Western Guizhou, Southern Sichuan, and Eastern Yunnan, China; Chongqing University Press: Chongqing, China, 1996; p. 216.
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef]
- Ward, C.R.; Taylor, J.C.; Matulis, C.E.; Dale, L.S. Quantification of mineral matter in the argonne premium coals using interactive Rietveld-based X-ray diffraction. Int. J. Coal Geol. 2001, 46, 67–82. [Google Scholar] [CrossRef]
- Kelloway, S.J.; Ward, C.R.; Marjo, C.E.; Wainwright, I.E.; Cohen, D.R. Quantitative chemical profiling of coal using core-scanning X-ray fluorescence techniques. Int. J. Coal Geol. 2014, 128–129, 55–67. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, J.; Guo, W.; Wang, P.; Ji, D. Mineralogy of the Pennsylvanian coal seam in the Datanhao mine, Daqingshan Coalfield, Inner Mongolia, China: Genetic implications for mineral matter in coal deposited in an intermontane basin. Int. J. Coal Geol. 2016, 167, 201–214. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Zhou, Y.; Hower, J.C.; Li, D.; Chen, W.; Zhu, X.; Zou, J. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the Late Permian coals from the Songzao Coalfield, Chongqing, southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Ward, C.R.; Spears, D.A.; Booth, C.A.; Staton, I.; Gurba, L.W. Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int. J. Coal Geol. 1999, 40, 281–308. [Google Scholar] [CrossRef]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I.T. Mineralogical composition of Late Permian coal seams in the Songzao Coalfield, southwestern China. Int. J. Coal Geol. 2013, 116–117, 208–226. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Zou, J.; Tian, H.; Li, T. Geochemistry and mineralogy of tuff in Zhongliangshan mine, Chongqing, southwestern China. Minerals 2016, 6, 47. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; p. 312. [Google Scholar]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanidesand yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Murray, R.W.; Buchholtz, T.; Brink, M.R.; Jones, D.L.; Gerlach, D.C.; Russ, G.P. Rare earth elements as indicators of different marine depositional environments in chert and shale. Geology 1990, 18, 268–272. [Google Scholar] [CrossRef]
- Alibo, D.; Nozaki, Y. Rare earth elements in seawater: Particle association, shalenormalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Altaner, S.P.; Hower, J.; Whitney, G.; Aronson, J.L. Model for K-bentonite formation: Evidence from zoned K-bentonites in the disturbed belt, Montana. Geology 1984, 12, 412–415. [Google Scholar] [CrossRef]
- Amouric, M.; Olives, J. Illitization of smectite as seen by high-resolution transmission electron microscopy. Eur. J. Mineral. 1991, 3, 831–835. [Google Scholar] [CrossRef]
- Hower, J.; Eslinger, E.V.; Hower, M.E.; Perry, E.A. Mechanism of burial metamorphism of argillaceous sediment: 1. Mineralogical and chemical evidence. Geol. Soc. Am. Bull. 1976, 87, 725–737. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Graham, I.; Wang, P. Clay mineralogy of coal-hosted Nb-Zr-REE-Ga mineralized beds from Late Permian strata, eastern Yunnan, SW China: Implications for paleotemperature and origin of the micro-quartz. Minerals 2016, 6, 45. [Google Scholar] [CrossRef]
- Bohor, B.F.; Triplehorn, D.M. Tonsteins: Altered Volcanic-Ash Layers in Coal-Bearing Sequences; Special Paper; Geological Society of America: Boulder, CO, USA, 1993; Volume 285, p. 40. [Google Scholar]
- Zielinski, R.A. Element mobility during alteration of silicic ash to kaolinite-a study of tonstein. Sedimentology 1985, 32, 567–579. [Google Scholar] [CrossRef]
- Kiipli, T.; Hints, R.; Kallaste, T.; Verš, E.; Voolma, M. Immobile and mobile elements during the transition of volcanic ash to bentonite—An example from the Early Palaeozoic sedimentary section of the Baltic Basin. Sediment. Geol. 2017, 347, 148–159. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of ~1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Price, N.B.; Duff, P.M.D. Mineralogy and chemistry of tonsteins from Carboniferous sequences in Great Britain. Sedimentology 1969, 13, 45–69. [Google Scholar] [CrossRef]
- Spears, D.A.; Rice, C.M. An Upper Carboniferous tonstein of volcanic origin. Sedimentology 1973, 20, 281–294. [Google Scholar] [CrossRef]
- Spears, D.A.; Kanaris-Sotiriou, R. Titanium in some Carboniferous sediments from Great Britain. Geochim. Cosmochim. Acta 1976, 40, 345–351. [Google Scholar] [CrossRef]
- Loughnan, F.C. Chemical Weathering of the Silicate Minerals; American Elsevier Pub. Co.: Atlanta, GA, USA, 1969. [Google Scholar]
- Zhao, L.; Ward, C.; French, D.; Graham, I. Major and trace element geochemistry of coals and intra-seam claystones from the Songzao Coalfield, SW China. Minerals 2015, 5, 870–893. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
| Sample | Quartz | Kaolinite | Illite | Chlorite | Pyrite | Calcite | Ankerite | Hematite | Analcite | Anatase | Rutile | Florencite | Jarosite | Bassanite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 42.5 | 25.7 | - | 25.6 | 0.3 | - | - | 0.6 | 3.7 | - | 0.7 | 1 | - |
| 2 | - | 41.7 | 42 | - | 10.7 | 1 | - | - | - | 4.1 | - | 0.5 | - | - |
| 3 | 0.3 | 34.4 | 42.4 | 3.8 | 14.2 | - | - | - | - | 4.4 | - | 0.4 | 0.1 | - |
| 4 | - | 57.1 | 34.7 | - | 1.4 | 0.1 | - | - | 0.5 | 5.5 | - | 0.6 | - | - |
| 5 | - | 63.5 | 27.7 | - | 2.9 | 0.1 | - | - | - | 5 | - | 0.8 | - | - |
| 6 | - | 79 | 7.6 | - | 5.1 | - | - | 1.3 | - | 5.7 | 0.6 | 0.9 | - | - |
| 7 | - | 70.2 | 6 | - | 15.2 | 0.5 | - | 1.3 | - | 5.2 | 0.2 | 0.8 | 0.5 | - |
| 8 | - | 79.6 | 6.3 | - | 5.3 | 0.4 | - | 1.1 | - | 6.1 | 0.5 | 0.7 | - | - |
| 9 | - | 80.9 | 5.4 | - | 4.7 | - | 0.3 | 1.2 | - | 6.2 | 0.5 | 0.7 | - | - |
| 10 | - | 78.7 | 3.7 | - | 7.1 | 0.6 | - | 1.3 | - | 7.3 | 0.4 | 0.8 | - | - |
| 11 | - | 81.6 | 4.3 | - | 3.4 | 0.5 | - | 0.8 | - | 8.1 | 0.6 | 0.8 | - | - |
| 12 | - | 81.2 | 5.4 | - | 3.8 | 0.4 | 1.2 | - | - | 6.9 | 0.5 | 0.7 | - | - |
| 13 | 0.1 | 81 | 4.3 | - | 4.8 | 0.4 | 0.6 | 1 | - | 6.6 | 0.4 | 0.7 | - | - |
| 14 | 0.1 | 76 | 4.7 | - | 11.1 | - | - | 0.9 | - | 5.5 | 0.6 | 0.6 | 0.5 | - |
| 15 | - | 65.5 | 4 | - | 23.7 | 0.6 | - | - | - | 3.6 | 0.2 | 0.5 | 1.1 | 0.8 |
| 16 | 0.3 | 71.2 | - | - | 20.8 | 1.3 | - | - | - | 4.2 | 0.6 | 0.6 | 1.1 | - |
| 17 | - | 63.5 | 3.5 | - | 27.7 | 0.7 | - | - | - | 3.7 | - | 0.4 | 0.6 | - |
| 18 | - | 67.6 | - | - | 25.3 | 0.5 | - | - | - | 4.2 | 0.5 | 0.6 | 1.1 | - |
| 19 | - | 58.4 | 4.3 | - | 31.6 | 0.5 | - | - | - | 3.5 | 0.3 | 0.5 | 0.9 | - |
| 20 | 0.2 | 47.2 | 6.8 | - | 39.6 | 0.3 | - | - | - | 3.6 | - | 0.3 | 0.7 | 1.1 |
| Sample | LOI | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | MnO | Na2O | K2O | P2O5 | Total | Li | Be | F | Sc | V | Cr | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16.7 | 32.4 | 2.9 | 27.0 | 16.2 | 0.35 | 0.25 | 0.02 | 0.19 | 2.99 | 0.14 | 82.5 | 994 | 4.9 | 796 | 23 | 298 | 267 | 77 | 154 | 62 | 22 |
| 2 | 13.8 | 37.1 | 3.8 | 29.9 | 8.8 | 0.37 | 0.79 | 0.05 | 0.2 | 3.55 | 0.13 | 84.5 | 1348 | 4.9 | 718 | 25 | 391 | 302 | 68 | 113 | 197 | 30 |
| 3 | 13.7 | 36.7 | 3.8 | 30.3 | 9.9 | 0.37 | 0.11 | 0.003 | 0.2 | 3.94 | 0.13 | 85.4 | 2231 | 4.5 | 637 | 22 | 348 | 252 | 37 | 67 | 221 | 33 |
| 4 | 11.8 | 42.2 | 5.6 | 33.5 | 1.9 | 0.26 | 0.15 | 0.005 | 0.16 | 3.14 | 0.21 | 87.2 | 1826 | 5.7 | 625 | 21 | 562 | 746 | 32 | 67 | 346 | 35 |
| 5 | 12.6 | 41.1 | 5.5 | 33.3 | 3.5 | 0.21 | 0.1 | 0.003 | 0.14 | 2.42 | 0.21 | 86.4 | 1628 | 5.8 | 580 | 19 | 355 | 629 | 38 | 72 | 520 | 16 |
| 6 | 13.9 | 39.9 | 5.6 | 33.1 | 4.6 | 0.16 | 0.19 | 0.004 | 0.12 | 1.27 | 0.21 | 85.1 | 1420 | 5.8 | 566 | 19 | 595 | 520 | 51 | 103 | 49 | 40 |
| 7 | 18.4 | 31.8 | 4.2 | 28.3 | 14.6 | 0.18 | 0.35 | 0.05 | 0.11 | 0.71 | 0.15 | 80.5 | 968 | 4.6 | 401 | 11 | 451 | 468 | 60 | 110 | 163 | 93 |
| 8 | 14.6 | 38.6 | 5.8 | 32.1 | 5.8 | 0.21 | 0.37 | 0.05 | 0.12 | 0.97 | 0.2 | 84.3 | 1073 | 5.6 | 511 | 17 | 583 | 568 | 65 | 106 | 130 | 73 |
| 9 | 14.6 | 39.1 | 6.5 | 32.6 | 4.4 | 0.19 | 0.3 | 0.02 | 0.12 | 0.83 | 0.2 | 84.3 | 1131 | 5.9 | 522 | 16 | 514 | 453 | 100 | 225 | 276 | 100 |
| 10 | 16.0 | 38.3 | 6.5 | 31.8 | 4.3 | 0.17 | 0.34 | 0.02 | 0.11 | 0.69 | 0.19 | 82.5 | 1000 | 5.3 | 451 | 14 | 582 | 473 | 96 | 249 | 265 | 118 |
| 11 | 14.0 | 39.2 | 8.0 | 32.4 | 3.6 | 0.15 | 0.39 | 0.02 | 0.09 | 0.61 | 0.23 | 84.6 | 1029 | 5.4 | 412 | 19 | 655 | 512 | 62 | 147 | 314 | 73 |
| 12 | 14.1 | 39.3 | 6.7 | 32.4 | 3.9 | 0.23 | 0.68 | 0.04 | 0.1 | 0.77 | 0.21 | 84.4 | 951 | 5.1 | 390 | 19 | 452 | 471 | 45 | 123 | 252 | 73 |
| 13 | 14.5 | 38.9 | 6.6 | 32.4 | 4.4 | 0.18 | 0.35 | 0.02 | 0.11 | 0.7 | 0.2 | 83.9 | 944 | 5.1 | 380 | 17 | 594 | 377 | 48 | 139 | 338 | 70 |
| 14 | 17.9 | 34.7 | 4.9 | 29.9 | 10.6 | 0.14 | 0.11 | 0.003 | 0.1 | 0.69 | 0.16 | 81.2 | 927 | 4.3 | 338 | 14 | 500 | 710 | 24 | 91 | 243 | 142 |
| 15 | 20.4 | 29.6 | 3.2 | 26.8 | 17.9 | 0.13 | 0.46 | 0.01 | 0.08 | 0.43 | 0.12 | 78.6 | 365 | 3.2 | 414 | 20 | 314 | 453 | 25 | 109 | 208 | 123 |
| 16 | 19.3 | 30.8 | 3.4 | 27.9 | 16.3 | 0.14 | 0.41 | 0.01 | 0.1 | 0.48 | 0.12 | 79.7 | 946 | 3.6 | 414 | 26 | 328 | 510 | 32 | 169 | 205 | 120 |
| 17 | 20.4 | 29.3 | 3.1 | 26.7 | 18.6 | 0.14 | 0.29 | 0.01 | 0.1 | 0.43 | 0.1 | 78.8 | 824 | 3.4 | 390 | 24 | 307 | 514 | 28 | 179 | 185 | 71 |
| 18 | 20.0 | 29.8 | 3.2 | 27.1 | 17.8 | 0.15 | 0.32 | 0.01 | 0.1 | 0.47 | 0.12 | 79.1 | 637 | 3.3 | 429 | 25 | 281 | 415 | 29 | 148 | 196 | 70 |
| 19 | 22.2 | 26.3 | 2.6 | 24.5 | 22.4 | 0.18 | 0.25 | 0.005 | 0.11 | 0.49 | 0.08 | 77.0 | 443 | 2.9 | 351 | 21 | 252 | 436 | 18 | 125 | 173 | 356 |
| 20 | 23.5 | 23.7 | 2.4 | 22.2 | 25.5 | 0.19 | 0.54 | 0.01 | 0.1 | 0.66 | 0.08 | 75.3 | 370 | 2.2 | 318 | 19 | 251 | 432 | 11 | 78 | 147 | 233 |
| Sample | Ga | Ge | Rb | Sr | Y | Zr | Nb | Mo | Cd | In | Sn | Sb | Cs | Ba | Hf | Ta | Hg | Tl | Pb | Bi | Th | U |
| 1 | 43 | 2.5 | 38.7 | 730 | 72 | 614 | 83 | 0.67 | 0.91 | 0.25 | 7.9 | 1.77 | 4.2 | 99 | 17 | 5.4 | 190 | 0.37 | 30 | 1.57 | 19 | 5.6 |
| 2 | 51 | 3.7 | 31.5 | 242 | 50 | 738 | 96 | 0.59 | 1 | 0.55 | 9.5 | 1.78 | 3.7 | 59 | 19 | 5.9 | 202 | 0.17 | 23 | 1.11 | 16 | 2.7 |
| 3 | 53 | 3.9 | 46.9 | 534 | 50 | 724 | 94 | 0.38 | 0.96 | 0.75 | 8.8 | 0.6 | 3.5 | 78 | 19 | 5.8 | 65 | 0.1 | 9 | 1.63 | 14 | 2.3 |
| 4 | 46 | 3.8 | 35.5 | 569 | 42 | 958 | 125 | 0.52 | 1.23 | 0.57 | 10.3 | 0.93 | 4.0 | 86 | 25 | 7.7 | 85 | 0.04 | 13 | 0.97 | 16 | 3.0 |
| 5 | 41 | 2.7 | 23.2 | 186 | 38 | 873 | 122 | 0.02 | 1.17 | 0.69 | 2.1 | 0.36 | 3.8 | 51 | 22 | 7.7 | 84 | 0.04 | 11 | 1.26 | 16 | 3.0 |
| 6 | 42 | 2.3 | 14.7 | 543 | 36 | 948 | 126 | 1.22 | 1.24 | 0.25 | 9.3 | 2.58 | 3.4 | 90 | 25 | 7.9 | 255 | 0.13 | 13 | 1.94 | 11 | 5.0 |
| 7 | 37 | 3.4 | 10.0 | 647 | 21 | 784 | 105 | 0.81 | 1.4 | 0.36 | 9.0 | 3.58 | 3.0 | 100 | 21 | 6.6 | 249 | 0.45 | 19 | 1.05 | 10 | 3.2 |
| 8 | 47 | 3.1 | 10.9 | 464 | 31 | 983 | 133 | 0.85 | 1.55 | 0.39 | 10.8 | 3.51 | 3.4 | 92 | 25 | 8.1 | 167 | 0.11 | 16 | 1.09 | 16 | 4.8 |
| 9 | 49 | 3.2 | 9.8 | 388 | 30 | 1041 | 140 | 0.73 | 1.72 | 0.5 | 13.5 | 3.74 | 3.3 | 95 | 27 | 8.9 | 158 | 0.08 | 36 | 1.48 | 16 | 3.1 |
| 10 | 44 | 3.5 | 7.5 | 429 | 26 | 1162 | 158 | 0.49 | 1.95 | 0.51 | 13.3 | 2.97 | 2.9 | 104 | 29 | 9.6 | 162 | 0.07 | 28 | 1.53 | 15 | 2.9 |
| 11 | 51 | 3.7 | 5.5 | 221 | 31 | 1241 | 170 | 0.88 | 1.88 | 0.62 | 13.4 | 2.16 | 2.9 | 58 | 33 | 10.7 | 118 | 0.06 | 26 | 1.54 | 19 | 3.6 |
| 12 | 50 | 2.4 | 4.6 | 325 | 25 | 1034 | 150 | 0.02 | 1.65 | 0.55 | 2.4 | 0.15 | 2.7 | 70 | 27 | 8.9 | 108 | 0.04 | 23 | 0.86 | 16 | 3.1 |
| 13 | 50 | 4.0 | 5.2 | 247 | 22 | 1037 | 112 | 0.53 | 1.58 | 0.49 | 11.2 | 1.45 | 2.8 | 57 | 27 | 5.7 | 106 | 0.05 | 18 | 1.1 | 10 | 3.0 |
| 14 | 43 | 4.0 | 8.1 | 357 | 20 | 888 | 130 | 0.71 | 1.83 | 0.66 | 11.7 | 1.32 | 2.9 | 73 | 24 | 9.0 | 157 | 1.37 | 29 | 1.23 | 13 | 2.9 |
| 15 | 31 | 2.0 | 6.8 | 482 | 37 | 628 | 82 | 0.43 | 1.37 | 0.46 | 8.7 | 1 | 2.5 | 82 | 17 | 5.1 | 254 | 7.53 | 28 | 2.11 | 20 | 3.0 |
| 16 | 35 | 1.9 | 7.8 | 518 | 41 | 657 | 86 | 1.51 | 1.42 | 0.4 | 9.0 | 2.87 | 2.9 | 90 | 18 | 5.5 | 153 | 2.35 | 80 | 1.51 | 21 | 3.6 |
| 17 | 32 | 1.8 | 7.3 | 430 | 35 | 600 | 78 | 0.51 | 1.14 | 0.38 | 7.8 | 2.63 | 2.9 | 75 | 16 | 4.8 | 196 | 2.72 | 94 | 1.58 | 21 | 3.8 |
| 18 | 33 | 1.7 | 7.7 | 498 | 30 | 609 | 80 | 0.55 | 1.21 | 0.35 | 8.4 | 2.42 | 2.9 | 96 | 16 | 5.1 | 204 | 5.24 | 67 | 2.88 | 19 | 3.3 |
| 19 | 28 | 1.7 | 7.9 | 320 | 24 | 555 | 68 | 0.56 | 2.25 | 0.62 | 6.8 | 1.92 | 2.5 | 63 | 14 | 4.4 | 194 | 2.56 | 274 | 0.68 | 16 | 4.0 |
| 20 | 27 | 1.2 | 8.4 | 334 | 25 | 469 | 93 | 0.91 | 3.95 | 0.39 | 5.6 | 2.4 | 1.8 | 57 | 12 | 5.8 | 127 | 0.82 | 66 | 0.61 | 14 | 3.3 |
| Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | EuN/EuN* | CeN/CeN* | (Y/Ho)N | (La/Yb)N | REO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.70 | 3.73 | 3.57 | 3.56 | 3.64 | 4.83 | 4.24 | 3.45 | 3.91 | 3.29 | 3.50 | 3.64 | 3.61 | 3.45 | 3.34 | 1.35 | 0.90 | 0.94 | 1.36 | 927 |
| 2 | 4.66 | 3.88 | 3.69 | 3.51 | 2.99 | 3.42 | 3.48 | 2.63 | 2.84 | 2.28 | 2.43 | 2.45 | 2.33 | 2.23 | 2.19 | 1.19 | 0.93 | 0.94 | 2.09 | 880 |
| 3 | 3.57 | 3.54 | 2.92 | 2.94 | 2.93 | 3.55 | 3.45 | 2.78 | 2.88 | 2.25 | 2.36 | 2.37 | 2.24 | 2.07 | 2.03 | 1.23 | 1.09 | 0.95 | 1.72 | 770 |
| 4 | 7.71 | 5.44 | 6.64 | 6.43 | 5.02 | 5.47 | 5.21 | 3.23 | 2.70 | 1.92 | 2.03 | 1.87 | 1.73 | 1.70 | 1.63 | 1.23 | 0.76 | 0.95 | 4.55 | 1304 |
| 5 | 7.41 | 5.28 | 6.68 | 6.52 | 5.00 | 5.59 | 5.04 | 3.11 | 2.55 | 1.72 | 1.85 | 1.70 | 1.61 | 1.55 | 1.50 | 1.28 | 0.75 | 0.93 | 4.79 | 1270 |
| 6 | 6.80 | 5.13 | 6.15 | 6.19 | 5.04 | 5.57 | 4.74 | 2.92 | 2.41 | 1.61 | 1.75 | 1.64 | 1.55 | 1.51 | 1.47 | 1.28 | 0.79 | 0.92 | 4.49 | 1206 |
| 7 | 4.71 | 3.44 | 4.46 | 4.50 | 3.69 | 3.80 | 3.27 | 1.95 | 1.54 | 0.95 | 1.09 | 1.00 | 0.91 | 0.90 | 0.84 | 1.22 | 0.75 | 0.87 | 5.21 | 829 |
| 8 | 6.66 | 4.69 | 6.46 | 6.55 | 5.38 | 5.55 | 4.76 | 2.91 | 2.31 | 1.40 | 1.64 | 1.53 | 1.39 | 1.40 | 1.34 | 1.22 | 0.72 | 0.86 | 4.75 | 1170 |
| 9 | 7.05 | 4.30 | 6.47 | 6.40 | 5.37 | 5.72 | 4.76 | 2.91 | 2.24 | 1.37 | 1.59 | 1.43 | 1.27 | 1.24 | 1.19 | 1.25 | 0.64 | 0.86 | 5.68 | 1143 |
| 10 | 6.78 | 4.38 | 6.55 | 6.59 | 5.64 | 5.82 | 4.79 | 2.77 | 2.10 | 1.19 | 1.41 | 1.24 | 1.12 | 1.09 | 1.03 | 1.24 | 0.66 | 0.84 | 6.21 | 1141 |
| 11 | 7.81 | 4.89 | 7.56 | 7.73 | 6.54 | 6.52 | 5.36 | 3.14 | 2.41 | 1.42 | 1.69 | 1.57 | 1.45 | 1.43 | 1.34 | 1.20 | 0.64 | 0.84 | 5.45 | 1308 |
| 12 | 7.17 | 4.87 | 6.88 | 6.95 | 5.81 | 5.64 | 4.68 | 2.67 | 1.99 | 1.15 | 1.39 | 1.28 | 1.21 | 1.16 | 1.16 | 1.18 | 0.69 | 0.83 | 6.16 | 1219 |
| 13 | 6.79 | 4.48 | 6.64 | 6.52 | 5.32 | 5.14 | 4.27 | 2.47 | 1.86 | 1.00 | 1.28 | 1.18 | 1.12 | 1.10 | 1.06 | 1.17 | 0.67 | 0.79 | 6.17 | 1136 |
| 14 | 4.99 | 3.59 | 4.82 | 4.90 | 4.03 | 3.76 | 3.25 | 1.98 | 1.58 | 0.91 | 1.14 | 1.10 | 1.03 | 1.02 | 1.00 | 1.12 | 0.73 | 0.80 | 4.90 | 876 |
| 15 | 4.28 | 3.50 | 3.85 | 3.86 | 3.40 | 3.65 | 3.42 | 2.48 | 2.46 | 1.66 | 1.96 | 1.98 | 2.00 | 2.15 | 2.09 | 1.18 | 0.86 | 0.85 | 1.99 | 821 |
| 16 | 4.62 | 3.94 | 4.11 | 4.02 | 3.52 | 3.73 | 3.61 | 2.70 | 2.63 | 1.86 | 2.09 | 2.13 | 2.12 | 2.23 | 2.13 | 1.15 | 0.90 | 0.89 | 2.07 | 895 |
| 17 | 4.07 | 3.29 | 3.55 | 3.49 | 2.98 | 3.19 | 3.11 | 2.38 | 2.36 | 1.60 | 1.88 | 1.90 | 1.94 | 2.09 | 1.97 | 1.15 | 0.86 | 0.85 | 1.95 | 768 |
| 18 | 4.48 | 3.65 | 3.87 | 3.79 | 3.26 | 3.45 | 3.34 | 2.36 | 2.20 | 1.34 | 1.66 | 1.68 | 1.76 | 1.86 | 1.75 | 1.16 | 0.87 | 0.81 | 2.41 | 826 |
| 19 | 3.18 | 2.54 | 2.56 | 2.41 | 1.98 | 2.16 | 2.19 | 1.66 | 1.66 | 1.09 | 1.33 | 1.37 | 1.45 | 1.53 | 1.50 | 1.15 | 0.89 | 0.82 | 2.08 | 572 |
| 20 | 2.75 | 2.33 | 2.27 | 2.16 | 1.83 | 2.01 | 1.99 | 1.50 | 1.53 | 1.14 | 1.25 | 1.30 | 1.33 | 1.45 | 1.38 | 1.17 | 0.93 | 0.91 | 1.91 | 520 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhu, Q.; Jia, S.; Zou, J.; Nechaev, V.; Dai, S. Origin of Minerals and Critical Metals in an Argillized Tuff from the Huayingshan Coalfield, Southwestern China. Minerals 2017, 7, 92. https://doi.org/10.3390/min7060092
Zhao L, Zhu Q, Jia S, Zou J, Nechaev V, Dai S. Origin of Minerals and Critical Metals in an Argillized Tuff from the Huayingshan Coalfield, Southwestern China. Minerals. 2017; 7(6):92. https://doi.org/10.3390/min7060092
Chicago/Turabian StyleZhao, Lei, Qin Zhu, Shaohui Jia, Jianhua Zou, Victor Nechaev, and Shifeng Dai. 2017. "Origin of Minerals and Critical Metals in an Argillized Tuff from the Huayingshan Coalfield, Southwestern China" Minerals 7, no. 6: 92. https://doi.org/10.3390/min7060092
APA StyleZhao, L., Zhu, Q., Jia, S., Zou, J., Nechaev, V., & Dai, S. (2017). Origin of Minerals and Critical Metals in an Argillized Tuff from the Huayingshan Coalfield, Southwestern China. Minerals, 7(6), 92. https://doi.org/10.3390/min7060092






