Reprocessing of a Southern Chilean Zn Tailing by Flotation—A Case Study
Abstract
:1. Introduction
1.1. Valorization of Tailings
1.2. Material Characterization
1.3. Procsssing Options for Sphalerite Tailings in a Froth Flotation Context
1.4. Scope of Work
2. Materials and Methods
2.1. General Sample Preparation for Analytical Methods
2.1.1. X-ray Powder Diffraction
2.1.2. Polarization Microscopy
2.1.3. Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX) and EPMA
2.1.4. Mineral Liberation Analyzer (MLA)
2.1.5. Handheld X-ray Fluorescence Spectroscopy (h-XRF)
2.2. Chemicals
2.3. Material Preparation
2.4. Flotation Test Work
3. Results
3.1. Mineralogical Characterization
3.1.1. Determination of the Mineral Content by Polarization Microscopy and SEM-EDX or EPMA-EDX
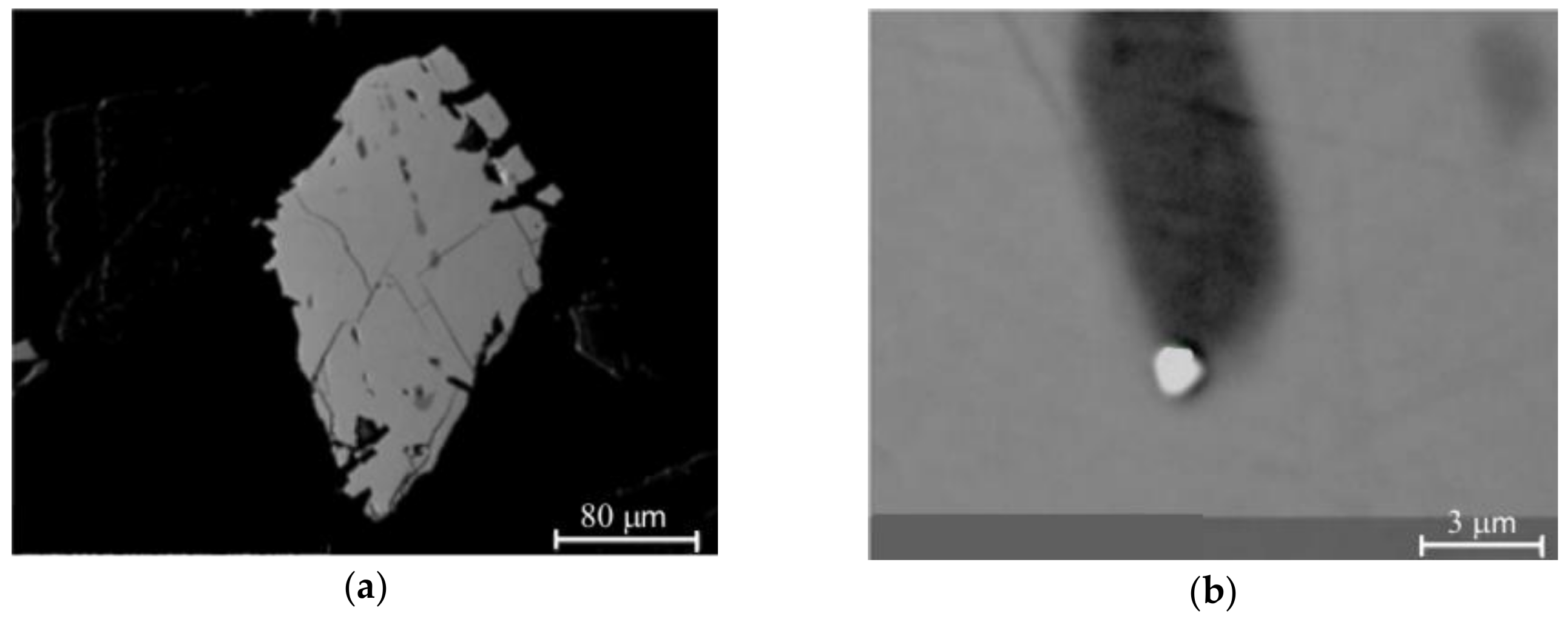
3.1.2. Detailed Study of Individual Sphalerites
3.1.3. MLA
3.2. Flotation Test Work
MLA Based Performance Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Lottermoser, B. Mine Wastes: Characterization, Treatment and Environmental Impacts; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Wang, C.; Harbottle, D.; Liu, Q.; Xu, Z. Current state of fine mineral tailings treatment: A critical review on theory and practice. Miner. Eng. 2014, 58, 113–131. [Google Scholar] [CrossRef] [Green Version]
- Kausch, P.; Bertau, M.; Gutzmer, J.; Matschullat, J. Strategische Rohstoffe—Risikovorsorge; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Gu, Y. Automated scanning electron microscope based mineral liberation analysis an introduction to JKMRC/FEI mineral liberation analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Kohmuench, J.N.; Mankosa, M.J.; Thanasekaran, H.; Hobert, A. Improving coarse particle flotation using the HydroFloat™ (raising the trunk of the elephant curve). Miner. Eng. 2018, 121, 137–145. [Google Scholar] [CrossRef]
- Mankosa, M.J.; Kohmuench, J.N.; Christodoulou, L.; Yan, E.S. Improving fine particle flotation using the StackCell™ (raising the tail of the elephant curve). Miner. Eng. 2018, 121, 83–89. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chehreh Chelgani, S.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96, 94–98. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Miner. Eng. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Videla, A.R.; Morales, R.; Saint-Jean, T.; Gaete, L.; Vargas, Y.; Miller, J.D. Ultrasound treatment on tailings to enhance copper flotation recovery. Miner. Eng. 2016, 99, 89–95. [Google Scholar] [CrossRef]
- Bryan, C.G.; Hallberg, K.B.; Johnson, D.B. Mobilisation of metals in mineral tailings at the abandoned São Domingos copper mine (Portugal) by indigenous acidophilic bacteria. Hydrometallurgy 2006, 83, 184–194. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, M.; Akbar Abdollahzadeh, A.; Askari, M. Bioleaching of copper, nickel and cobalt from the low grade Sulfidic tailing of Golgohar iron mine, Iran. Hydrometallurgy 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Okrusch, M.; Matthes, S. Mineralogie: Eine Einführung in die spezielle Mineralogie, Petrologie und Lagerstättenkunde; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lottermoser, B.G. Recycling, reuse and rehabilitation of mine wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Büttner, P.; Osbahr, I.; Zimmermann, R.; Leißner, T.; Satge, L.; Gutzmer, J. Recovery potential of flotation tailings assessed by spatial modelling of automated mineralogy data. Miner. Eng. 2018, 116, 143–151. [Google Scholar] [CrossRef]
- Mackay, I.; Mendez, E.; Molina, I.; Videla, A.R.; Cilliers, J.J.; Brito-Parada, P.R. Dynamic froth stability of copper flotation tailings. Miner. Eng. 2018, 124, 103–107. [Google Scholar] [CrossRef]
- Clemente, D.; Newling, P.; Botelho de Sousa, A.; LeJeune, G.; Barber, S.P.; Tucker, P. Reprocessing slimes tailings from a tungsten mine. Miner. Eng. 1993, 6, 831–839. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Alcalde, J.; Kelm, U.; Vergara, D. Historical assessment of metal recovery potential from old mine tailings: A study case for porphyry copper tailings, Chile. Miner. Eng. 2018. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Qiu, T. Recovery of zinc from cyanide tailings by flotation. Miner. Eng. 2015, 84, 100–105. [Google Scholar] [CrossRef]
- Bussey, S.D.; Kakarieka, A.; Meinert, L.D. Skarn, Porphyry, Vein, and Replacement Mineralization in the Toqui District, Southern Chile. In The Challenge of Finding New Mineral Resources: Global Metallogeny, Innovative Exploration, and New Discoveries; Goldfarb, R.J., Marsh, E.E., Monecke, T., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2010. [Google Scholar]
- Bulatovic, S.M. 14—Flotation of Lead–Zinc Ores. In Handbook of Flotation Reagents; Elsevier: Amsterdam, The Netherlands, 2007; pp. 323–366. [Google Scholar]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, N.P. The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1997, 52, 81–120. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Effect of iron content in sphalerite on flotation. Miner. Eng. 2005, 18, 1120–1122. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Critical copper concentration in sphalerite flotation: Effect of temperature and collector. Int. J. Miner. Process. 2016, 146, 15–22. [Google Scholar] [CrossRef]
- Tong, X.; Song, S.; He, J.; Rao, F.; Lopez-Valdivieso, A. Activation of high-iron marmatite in froth flotation by ammoniacal copper(II) solution. Miner. Eng. 2007, 20, 259–263. [Google Scholar] [CrossRef]
- Clarke, P.; Fornasiero, D.; Ralston, J.; Smart, R.S.C. A study of the removal of oxidation products from sulfide mineral surfaces. Miner. Eng. 1995, 8, 1347–1357. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Depression of iron sulphide flotation in zinc roughers. Miner. Eng. 2001, 14, 1067–1079. [Google Scholar] [CrossRef]
- Sun, W.; Liu, R.-Q.; Cao, X.-F.; Hu, Y.-H. Flotation separation of marmatite from pyrrhotite using DMPS as depressant. Trans. Nonferr. Met. Soc. China 2006, 16, 671–675. [Google Scholar] [CrossRef]
- He, M.-F.; Qin, W.-Q.; Li, W.-Z.; Zeng, K. Pyrite depression in marmatite flotation by sodium glycerine-xanthate. Trans. Nonferr. Met. Soc. China 2011, 21, 1161–1165. [Google Scholar] [CrossRef]
- Sinclair, R.J. The Extractive Metallurgy of Zinc; AusIMM: Carlton, Australia, 2005. [Google Scholar]
- Zhang, G.; Germaine, J.T.; Martin, R.T.; Whittle, A.J. A simple sample-mounting method for random powder X-ray diffraction. Clays Clay Miner. 2003, 51, 218–225. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.J.; Johnson, N.W.; Manlapig, E.V.; Thorne, C.G. Mineral and Coal Flotation Circuits—Their Simulation and Control, Developments in Mineral Processing Series; Elsevier Scientific Publishing Company: New York, NY, USA, 1981. [Google Scholar]
- Şahbaz, O.; Uçar, A.; Öteyaka, B. Velocity gradient and maximum floatable particle size in the Jameson cell. Miner. Eng. 2013, 41, 79–85. [Google Scholar] [CrossRef]








| Element | Composition Range |
|---|---|
| Zn | 47 to 56% |
| Fe | <10% |
| Pb | <3% |
| Cu | <2% |
| S | 30 to 32% |
| Sample | pH | CuSO4 g/t | MiBC in g/t | Danafloat 507-B g/t | KCN g/t |
|---|---|---|---|---|---|
| B1_TP_1 | 11 | 250 | 25 | 25 | 0 |
| B1_TP_2 | 12 | 100 | 25 | 25 | 0 |
| B1_TP_3 | 12 | 0 | 25 | 25 | 0 |
| B1_TP_4 | 11 | 100 | 25 | 25 | 0 |
| B1_TP_6 | 11.5 | 500 | 35 | 40 | 0 |
| B1_TP_7 | 10.5 | 500 | 50 | 50 | 100 |
| B1_TP_8 | 11 | 600 | 50 | 50 | 200 |
| Mineral Phases | A1F-Bulk | A1F-SM | B1-H | B1-J |
|---|---|---|---|---|
| Actinolite | 5.1 ± 1.1 | 5.7 ± 1.2 | - | - |
| Garnet (And) | 1.1 ± 0.4 | 2.1 ± 0.5 | - | 0.4 ± 0.1 |
| Calcite | 10.8 ± 0.9 | 7.0 ± 0.9 | 11.5 ± 0.3 | 9.9 ± 0.3 |
| Chlorite | 7.9 ± 1.0 | 10.7 ± 1.3 | 8.9 ± 0.4 | 17.1± 0.8 |
| Diopside | 2.8 ± 1.2 | 5.8 ± 1.1 | 2.7 ± 0.2 | 2.4 ± 0.3 |
| Epidote | 5.2 ± 1.2 | 9.2 ± 1.8 | 2.8 ± 0.2 | 2.1 ± 0.3 |
| Magnetite | 0.8 ± 0.3 | 1.8 ± 0.5 | - | - |
| K-Felspar (Microcline) | 1.9 ± 0.9 | - | - | 1.6 ± 0.3 |
| Muscovite | 1.1 ± 0.6 | - | - | 3.3 ± 0.4 |
| Plagioclase | 1.2 ± 0.7 | - | - | 1.4 ± 0.3 |
| Pyrite | 3.4 ± 0.3 | 8.2 ± 0.5 | 2.0 ± 0.1 | 1.0 ± 0.1 |
| Pyrrhotite | 7.0 ± 0.4 | 10.4 ± 0.6 | 3.2 ± 0.1 | 8.4 ± 0.2 |
| Quartz | 28.6 ± 1.2 | 8.3 ± 0.5 | 21.7 ± 0.4 | 23.1 ± 0.5 |
| Saponite | 10.2 ± 2.2 | 18.2 ± 3.0 | 4.9 ± 0.6 | 2.7 ± 0.4 |
| Sphalerite | 0.9 ± 0.2 | 1.9 ± 0.2 | 0.2 ± 0.03 | 0.9 ± 0.04 |
| Arsenopyrite | - | - | 0.3 ± 0.07 | 1.0 ± 0.1 |
| Hornblende | - | - | 2.4 ± 0.2 | - |
| Gypsum | - | - | 2.2 ± 0.1 | 1.8 ± 0.1 |
| Enstatite | - | - | - | 2.5 ± 0.3 |
| Amorphous and others | 12.1 ± 4.5 | 8.8 ± 5.7 | 37.0 ± 1.2 | 20.9 ± 1.8 |
| Mineral Phases | A1F-SM | B1-J |
|---|---|---|
| Amphibole | 17.1 | - |
| Apatite | 0.3 | 0.3 |
| Biotite | 0.6 | 1.2 |
| Ca-Mg-Fe-Carbonate | 0.5 | 0.6 |
| Chlorite | 1.0 | 1.8 |
| Fe-Gedrite | 13.3 | 16.7 |
| Hornblende | 0.1 | 0.1 |
| Jacobsite | 0.1 | - |
| Muscovite | 1.2 | 2.8 |
| Orthoclase | 0.3 | 1.6 |
| Pyrite | 28.5 | 26.3 |
| Rutile | 0.1 | 0.1 |
| Sphalerite | 2.9 | - |
| Ca-Mg-Siderite | 0.2 | 0.3 |
| Epidote | 5.0 | 0.7 |
| Fe-Oxide | 8.5 | 1.5 |
| Ilmenite | 0.1 | - |
| Jarosite | 1.4 | 1.3 |
| Olivine | 0.1 | - |
| Plagioclase | 0.4 | 0.6 |
| Quartz | 5.6 | 22.3 |
| Siderite | 0.6 | - |
| Arsenopyrite | - | 0.5 |
| Titanite | 0.9 | 0.1 |
| Anhydrite | - | 1.7 |
| Mn-Fe-Mg-Calcite | - | 1.7 |
| Fe-Mn-Oxide | - | 0.1 |
| Anorthite | - | 1.1 |
| Element | AN | Series | unn. C (wt %) | norm. C (wt %) | Atom. C (at %) | Error (wt %) |
|---|---|---|---|---|---|---|
| Sphalerite | ||||||
| C | 6 | K | 13.3 | 13.02 | 37.21 | 2.6 |
| S | 16 | K | 29.9 | 29.27 | 31.34 | 1.1 |
| Mn | 25 | K | 0.8 | 0.78 | 0.49 | 0.1 |
| Fe | 26 | K | 12.33 | 12.07 | 7.42 | 0.4 |
| Zn | 30 | K | 45.83 | 44.86 | 23.55 | 1.3 |
| Σ | 102.15 | 100 | 100 | |||
| Pyrrhotite inclusion | ||||||
| C | 6 | K | 15.17 | 14.4 | 37.99 | 2.9 |
| S | 16 | K | 33.68 | 31.97 | 31.58 | 1.2 |
| Fe | 26 | K | 56.5 | 53.63 | 30.43 | 1.6 |
| Σ | 105.36 | 100 | 100 | |||
| Element | AN | Series | unn. C (wt %) | norm. C (wt %) | Atom. C (at %) | Error (wt %) |
|---|---|---|---|---|---|---|
| S | 16 | K | 18.7 | 19.2 | 40.1 | 0.7 |
| Fe | 26 | K | 6.8 | 6.9 | 8.3 | 0.3 |
| Zn | 30 | K | 29.8 | 30.6 | 31.4 | 1.1 |
| Ag | 47 | K | 18.7 | 19.2 | 12 | 0.8 |
| Au | 79 | K | 23.4 | 24.1 | 8.2 | 0.9 |
| Σ | 97.4 | 100 | 100 |
| Element | AN | Series | unn. C (wt %) | norm. C (wt %) | Atom. C (at %) | Error (wt %) |
|---|---|---|---|---|---|---|
| S | 16 | K | 18.7 | 19.2 | 40.1 | 0.7 |
| Fe | 26 | K | 6.8 | 6.9 | 8.3 | 0.3 |
| Zn | 30 | K | 29.8 | 30.6 | 31.4 | 1.1 |
| Ag | 47 | K | 18.7 | 19.2 | 12 | 0.8 |
| Au | 79 | K | 23.4 | 24.1 | 8.2 | 0.9 |
| Σ | 97.4 | 100 | 100 |
| Grading (Sphalerite) in % | Liberation Class/Degree in % |
|---|---|
| 71.7 | 100 |
| 21.1 | 95–100 |
| 3.8 | 90–95 |
| Rest of 3.4 | <90 |
| Sample | Zn Grade in % | Fe Grade in % | S Grade in % |
|---|---|---|---|
| TP_1 | 13 | 28 | 32 |
| TP_2 | 8 | 32 | 30 |
| TP_3 | 2 | 19 | 18 |
| TP_4 | 6 | 34 | 29 |
| TP_6 | 6 | 32 | 32 |
| TP_7 | 10 | 28 | 34 |
| TP_8 | 6 | 35 | 28 |
| Minerals/Grouping | Concentrate Grade in wt % | Recoveries in % | Enrichment Factor |
|---|---|---|---|
| low iron sphalerite | 28.0 | 58.8 | 20.1 |
| high iron sphalerite | 1.5 | 40 | 13.7 |
| sulfidic gangue | 39.5 | 7.7 | 2.7 |
| gangue | 31.0 | 1.1 | 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babel, B.; Penz, M.; Schach, E.; Boehme, S.; Rudolph, M. Reprocessing of a Southern Chilean Zn Tailing by Flotation—A Case Study. Minerals 2018, 8, 295. https://doi.org/10.3390/min8070295
Babel B, Penz M, Schach E, Boehme S, Rudolph M. Reprocessing of a Southern Chilean Zn Tailing by Flotation—A Case Study. Minerals. 2018; 8(7):295. https://doi.org/10.3390/min8070295
Chicago/Turabian StyleBabel, Bent, Maike Penz, Edgar Schach, Stefanie Boehme, and Martin Rudolph. 2018. "Reprocessing of a Southern Chilean Zn Tailing by Flotation—A Case Study" Minerals 8, no. 7: 295. https://doi.org/10.3390/min8070295





