Determination of Dissolution Rates of Ag Contained in Metallurgical and Mining Residues in the S2O32−-O2-Cu2+ System: Kinetic Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Characterization
3.2. Mineralogical Characterization
3.3. Physical Characterization
3.4. Dissolution Curves of Ag and Kinetic Model
3.4.1. Effect of Partial Pressure of Oxygen
3.4.2. Particle Size Effect
3.4.3. Thiosulfate Concentration Effect
3.4.4. Cu2+ Concentration Effect
3.4.5. pH Effect
3.4.6. Temperature Effect
3.4.7. Stirring Rate Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez, C. México: Política en Materia Minera. (Diálogo Unión Europea-América Latina Sobre Materias Primas 2014). Available online: europa.eu/geninfo/query/index.do?queryText=plata&query_source=GROWTH&summary=summary&more_options_source=restricted&more_options_date=*&more_options_date_from=&more_options_date_to=&more_options_language=es&more_options_f_formats=*&swlang=en (accessed on 14 July 2018).
- Patiño, F.; Hernández, J.; Flores, M.U.; Reyes, I.A.; Reyes, M.; Juárez, J.C. Characterization and Stoichiometry of the Cyanidation Reaction in NaOH of Argentian Waste Tailings of Pachuca, Hidalgo, México. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2016; Volume 145, pp. 355–362. ISBN 978-1-119-26439-2. [Google Scholar]
- Flores, J.; Hernández, J.; Rivera, I.; Reyes, M.I.; Cerecedo, E.; Reyes, M.; Salinas, E.; Guerrero, M. Study of the Effect of Surface Liquid Flow during Column Flotation of Mining Tailing of the Dos Carlos Dam. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2017; Volume 146, pp. 787–798. [Google Scholar]
- Teja Ruiz, A.M.; Juarez Tapia, J.C.; Reyes Perez, M.; Hernandez Cruz, L.E.; Flores, M.U.; Reyes, I.A.; Perez Labra, M.; Moreno, R. Characterization and Leaching Proposal of Ag(I) from a Zn Concentrate in an S2O32−-O2 Medium. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2017; Volume 146, pp. 567–575. [Google Scholar]
- Hilson, G.; Monhemius, J. Alternatives to cyanide in the gold mining industry: What prospects for the future. J. Clean. Prod. 2015, 14, 1158–1167. [Google Scholar] [CrossRef]
- Hernandez, J.; Patino, F.; Rivera, I.; Reyes, I.A.; Flores, M.U.; Juarez, J.C.; Reyes, M. Leaching kinetics in cyanide media of Ag contained in the industrial mining-metallurgical wastes in the state of Hidalgo, Mexico. Int. J. Min. Sci. Technol. 2014, 24, 689–694. [Google Scholar] [CrossRef]
- Briones, R.; Lapidus, G.T. The leaching of silver sulfide with the thiosulfate–ammonia–cupric ion system. Hydrometallurgy 1998, 50, 243–260. [Google Scholar] [CrossRef]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. Study of silver leaching with the thiosulfate-nitrite-copper alternative system: Effect of thiosulfate concentration and leaching temperature. Miner. Eng. 2016, 86, 140–148. [Google Scholar] [CrossRef]
- Alonso, A.R.; Lapidus, G.T.; González, I. A strategy to determine the potential interval for selective silver electrodeposition from ammoniacal thiosulfate solutions. Hydrometallurgy 2007, 85, 144–153. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Veglio, F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Mohammadi, E.; Pourabdoli, M.; Ghobeiti-Hasab, M.; Heidarpour, A. Ammoniacal thiosulfate leaching of refractory oxide gold ore. Int. J. Miner. Process. 2017, 164, 6–10. [Google Scholar] [CrossRef]
- Ubaldini, S.; Fornari, P.; Massidda, R.; Abbruzzese, C. An innovative thiourea gold leaching process. Hydrometallurgy 1998, 48, 113–124. [Google Scholar] [CrossRef]
- Alonso-Gómez, A.; Lapidus, G. Inhibition of lead solubilization during the leaching of gold and silver in ammoniacal thiosulfate solutions (effect of phosphate addition). Hydrometallurgy 2009, 99, 89–96. [Google Scholar] [CrossRef]
- Deutsch, J.L.; Dreisinger, D.B. Silver sulfide leaching with thiosulfate in the presence of additives part I: Copper–ammonia leaching. Hydrometallurgy 2013, 137, 156–164. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Solis-Marcial, O.J.; Lapidus, G.T. Chalcopyrite leaching in alcoholic acid media. Hydrometallurgy 2014, 147–148, 54–58. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Zipperian, D.; Raghavan, S.; Wilson, J.P. Gold and silver extraction by ammoniacal thiosulfate leaching from a rhyolite ore. Hidrometallurgy 1988, 19, 361–378. [Google Scholar] [CrossRef]
- Patiño, F.; Reyes, I.A.; Flores, M.U.; Pandiyan, T.; Roca, A.; Reyes, M.; Hernández, J. Kinetics modeling and experimental design of the sodium arsenojarosite descomposition in alkaline media: Implications. Hydrometallurgy 2013, 137, 115–125. [Google Scholar] [CrossRef]
- Juárez, J.C.; Rivera, I.; Patiño, F.; Reyes, M. Efecto de la Temperatura y Concentración de Tiosulfatos sobre la Velocidad de Disolución de Plata contenida en Desechos Mineros usando Soluciones S2O32−-O2-Zn2+. Inf. Tecnol. 2012, 18, 1–12. [Google Scholar] [CrossRef]
- Hernández, J.; Rivera, I.; Patiño, F.; Juárez, J.C. Estudio Cinético de la Lixiviación de Plata en el Sistema S2O32−-O2-Cu2+ Contenido en Residuos Minero-Metalúrgicos. Inf. Tecnol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Skoog, A.D.; West, D.M. Analytical chemistry an Introduction, 1st ed.; Reverté: Barcelona, Spain, 2002; pp. 444–445. ISBN 8429175113. [Google Scholar]
- Rivera, I.; Patiño, F.; Roca, A.; Cruells, M. Kinetics of metallic silver leaching in the O2–thiosulfate system. Hydrometallurgy 2015, 156, 63–70. [Google Scholar] [CrossRef]
- Teja-Ruiz, A.M.; Juárez-Tapia, J.C.; Reyes-Domínguez, I.A.; Hernández- Cruz, L.E.; Reyes-Pérez, M.; Patiño-Cardona, F.; Flores-Guerrero, M.U. Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32−-O2-NaOH System. Metals 2017, 7, 411. [Google Scholar] [CrossRef]
- Levenspiel, O. Ingeniería de las Reacciones Químicas, 2nd ed.; Reverté: Barcelona, Spain, 2002; pp. 397–441. ISBN 968-18-5860-3. [Google Scholar]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometallurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Moreno-Tovar, R.; Téllez-Hernández, J.; Monroy-Fernández, M.G. Evaluación geoquímica de residuos mineros (jales o colas) de mineralización de tipo epitermal, Hidalgo, México. Rev. Geol. Am. Cent. 2009, 41, 79–98, ISSN: 0256-7024. [Google Scholar]
- Moreno-Tovar, R.; Monroy, M.G.; Castañeda, E.P. Influencia de los minerales de los jales en la bioaccesibilidad de arsénico, plomo, zinc y cadmio en el distrito minero Zimapán, México. Rev. Geol. Am. Cent. 2012, 28, 3, ISSN: 0188-4999. [Google Scholar]
- Asamoah, R.A.; Skinner, W.; Addai-Mensah, J. Alkaline cyanide leaching of refractory gold flotation concentrates and bio-oxidised products: The effect of process variables. Hydrometallurgy 2018, 179, 79–93. [Google Scholar] [CrossRef]
- Melo, P.; Halmenschlager, P.; Veit, H.M.; Bernardes, A.M. Leaching of gold and silver from printed circuit board of mobile phones. Rev. Esc. Minas 2015, 68, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Sitando, O.; Senanayake, G.; Dai, X.; Nikoloski, A.N.; Breuer, P. A review of factors affecting gold leaching in non-ammoniacal thiosulfate solutions including degradation and in-situ generation of thiosulfate. Hydrometallurgy 2018, 178, 151–175. [Google Scholar] [CrossRef]
- Langhans, J.W., Jr.; Lei, K.P.V.; Carnahan, T.G. Copper-catalyzed thiosulfate leaching of low-grade gold ores. Hydrometallurgy 1992, 29, 191–203. [Google Scholar] [CrossRef]
- Ballester, A.; Felipe Verdeja, L.; Sancho, J. Metalurgia Extractiva Vol. I Fundamentos, 2nd ed.; Síntesis: Barcelona, Spain, 2000; pp. 37–67. ISBN 9788477388029. [Google Scholar]



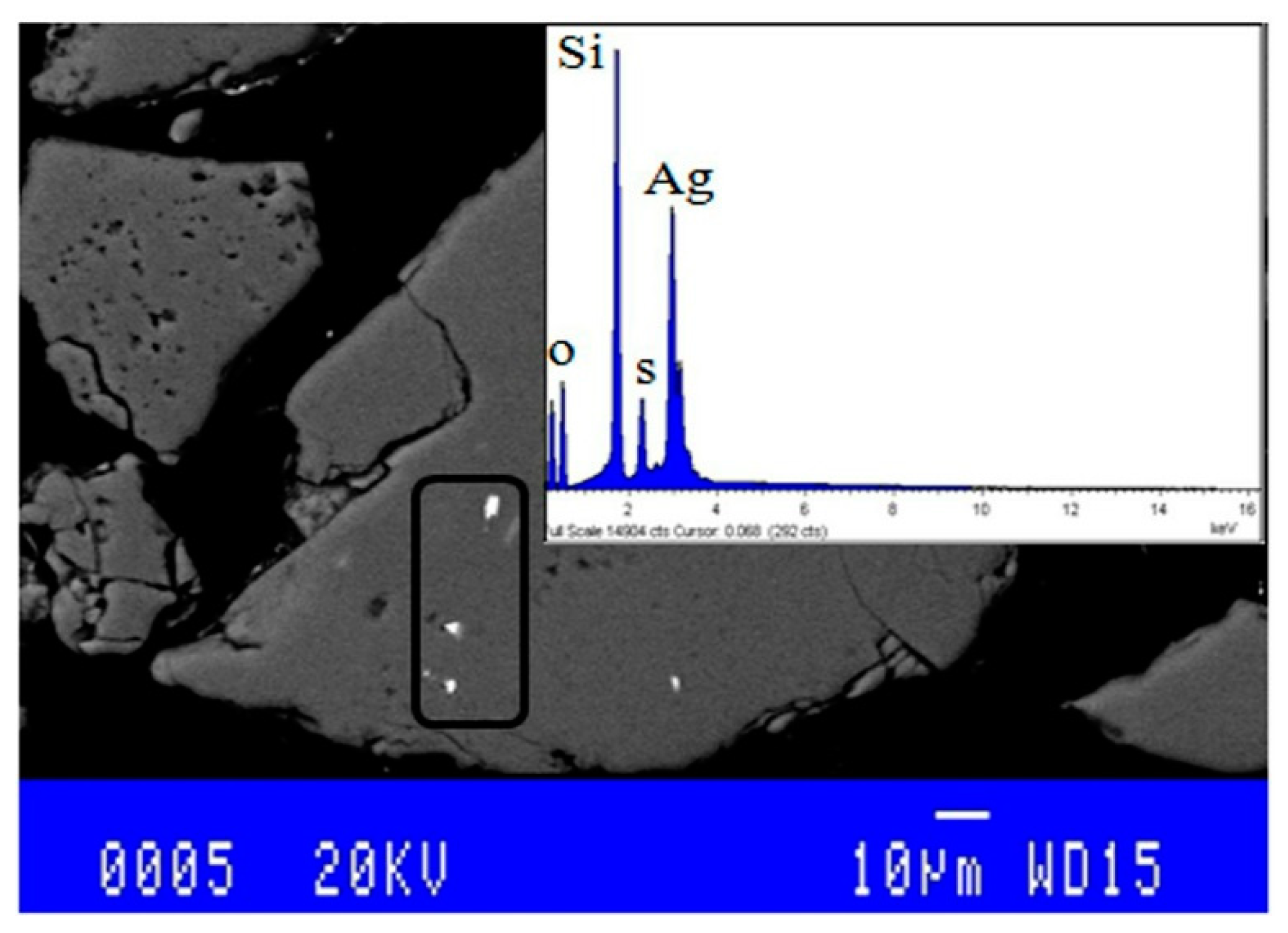





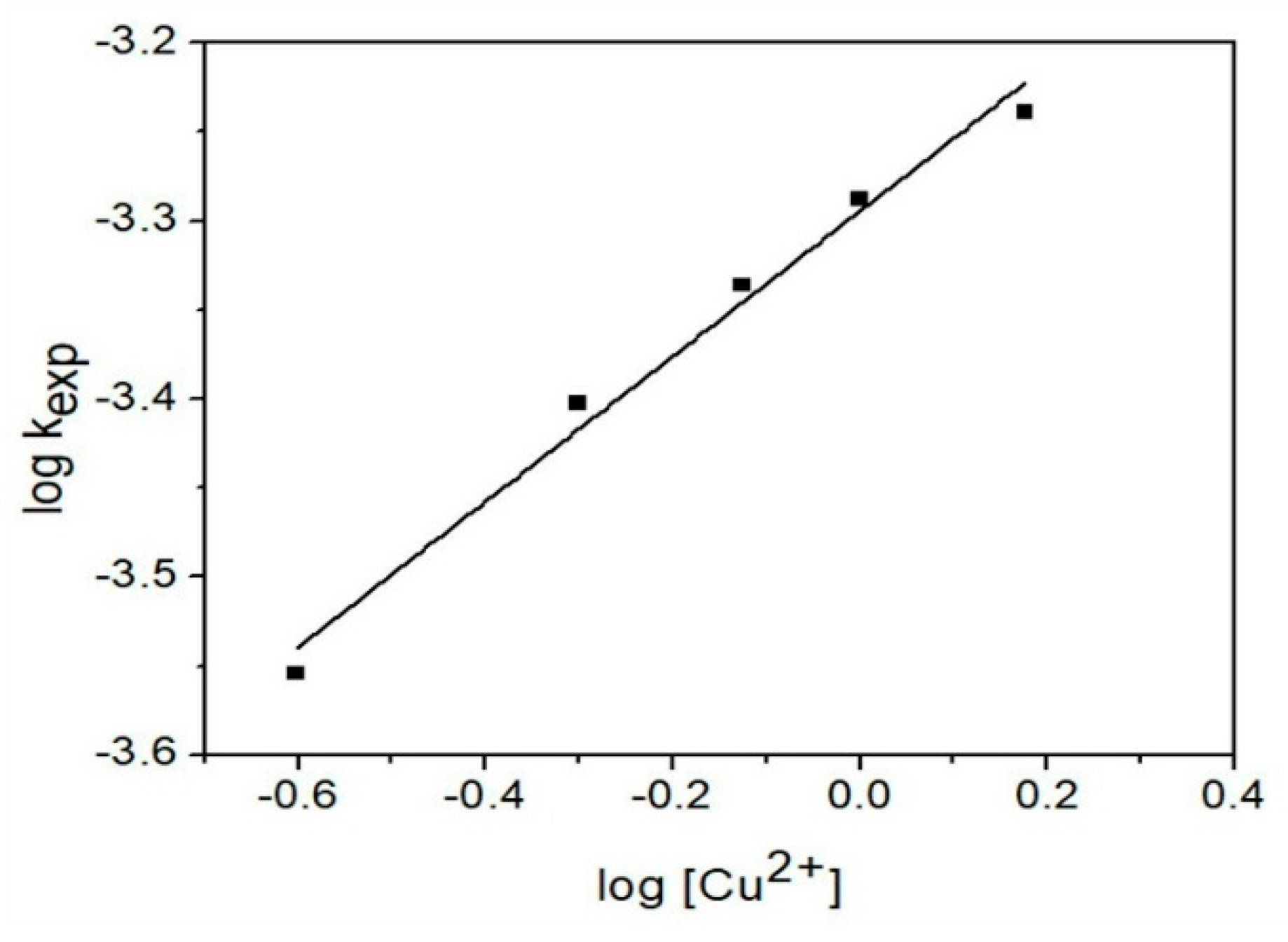



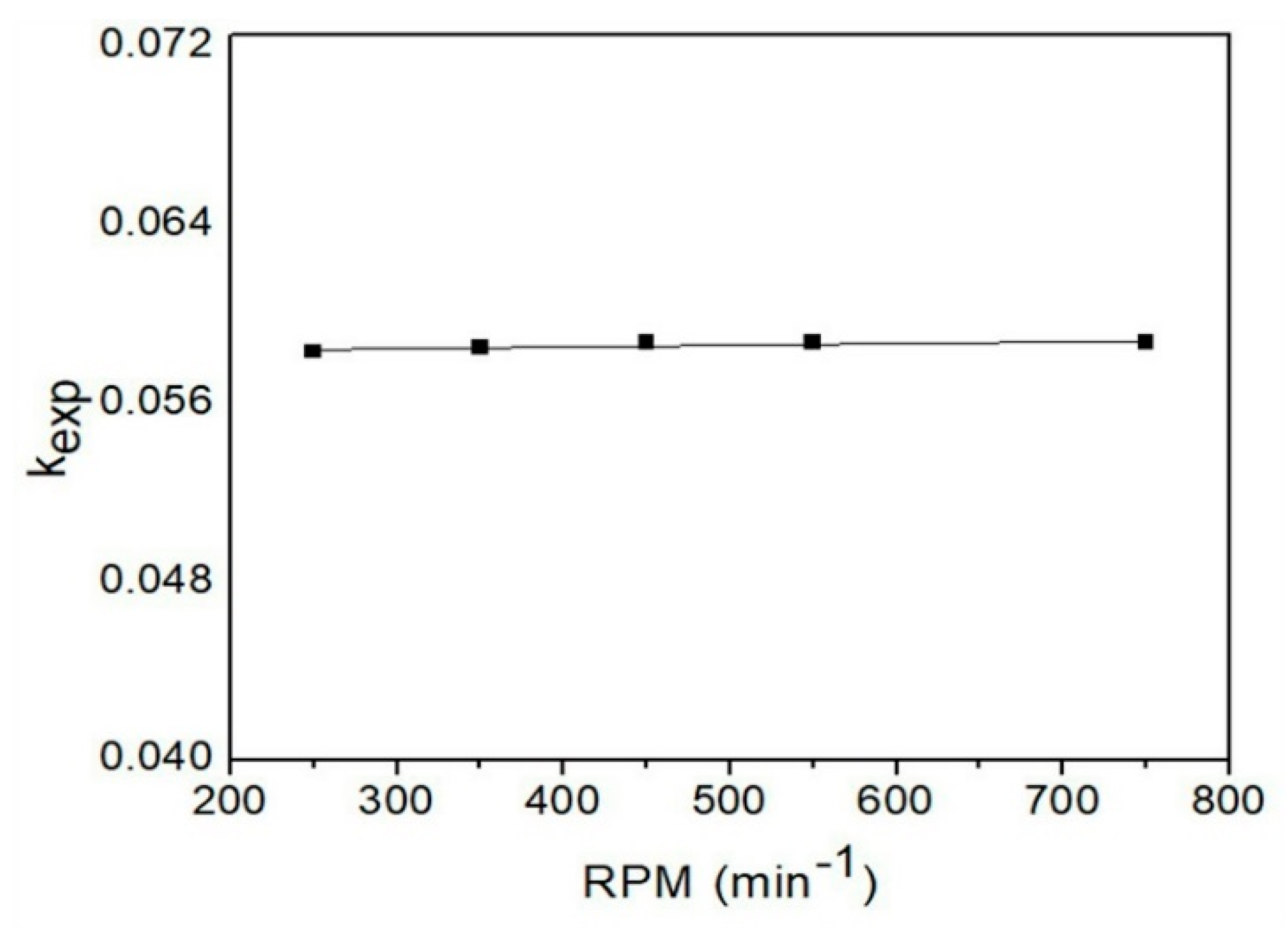
| Parameters | Experimental Conditions |
|---|---|
| d0 (µm) | 149, 106, 75, 56, 44, 37,25 |
| [Cu2+] (mol·L−1) | 0.001, 0.002, 0.003, 0.004, 0.006 |
| [S2O32−] (mol·L−1) | 0.02, 0.04, 0.06, 0.08, 0.16, 0.40 |
| T (K) | 288, 298, 308, 318, 328 |
| RPM (min−1) | 250, 350, 450, 550, 650, 750 |
| PO2 | 1 atm (excess) |
| [OH−] (mol·L−1) | 1 × 10−9, 1 × 10−7, 1 × 10−5,1 × 10−3, 1 × 10−2 |
| Mesh | Opening (µm) | Ag Retained (g/ton−1) | Weight (wt %) | Distribution ofAg (wt %) |
|---|---|---|---|---|
| <100 | 149 | 50.13 | 40 | 43.51 |
| 100–140 | 106 | 24.36 | 53 | 28 |
| 140–200 | 75 | 2.81 | 66 | 4.01 |
| 200–270 | 56 | 1.27 | 63 | 1.73 |
| 270–325 | 44 | 1.18 | 73 | 1.86 |
| 325–400 | 37 | 1.20 | 82 | 2.12 |
| >400 | 25 | 1.65 | 101 | 3.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez Tapia, J.C.; Patiño Cardona, F.; Roca Vallmajor, A.; Teja Ruiz, A.M.; Reyes Domínguez, I.A.; Reyes Pérez, M.; Pérez Labra, M.; Flores Guerrero, M.U. Determination of Dissolution Rates of Ag Contained in Metallurgical and Mining Residues in the S2O32−-O2-Cu2+ System: Kinetic Analysis. Minerals 2018, 8, 309. https://doi.org/10.3390/min8070309
Juárez Tapia JC, Patiño Cardona F, Roca Vallmajor A, Teja Ruiz AM, Reyes Domínguez IA, Reyes Pérez M, Pérez Labra M, Flores Guerrero MU. Determination of Dissolution Rates of Ag Contained in Metallurgical and Mining Residues in the S2O32−-O2-Cu2+ System: Kinetic Analysis. Minerals. 2018; 8(7):309. https://doi.org/10.3390/min8070309
Chicago/Turabian StyleJuárez Tapia, Julio C., Francisco Patiño Cardona, Antonio Roca Vallmajor, Aislinn M. Teja Ruiz, Iván A. Reyes Domínguez, Martín Reyes Pérez, Miguel Pérez Labra, and Mizraim U. Flores Guerrero. 2018. "Determination of Dissolution Rates of Ag Contained in Metallurgical and Mining Residues in the S2O32−-O2-Cu2+ System: Kinetic Analysis" Minerals 8, no. 7: 309. https://doi.org/10.3390/min8070309





