New Insights into the Configurations of Lead(II)-Benzohydroxamic Acid Coordination Compounds in Aqueous Solution: A Combined Experimental and Computational Study
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Details
2.1.1. Materials
2.1.2. UV-Vis Tests
2.1.3. ESI-MS Tests
2.2. Computational Methods
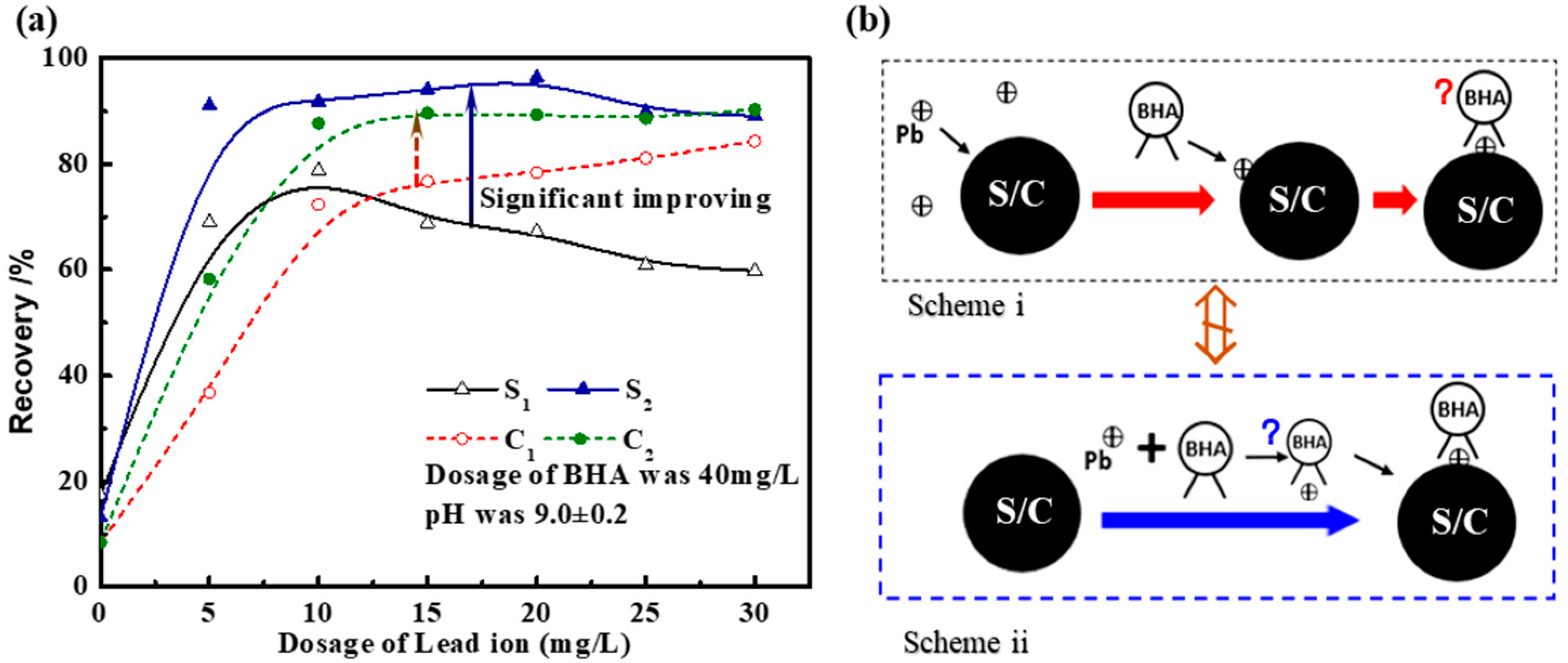
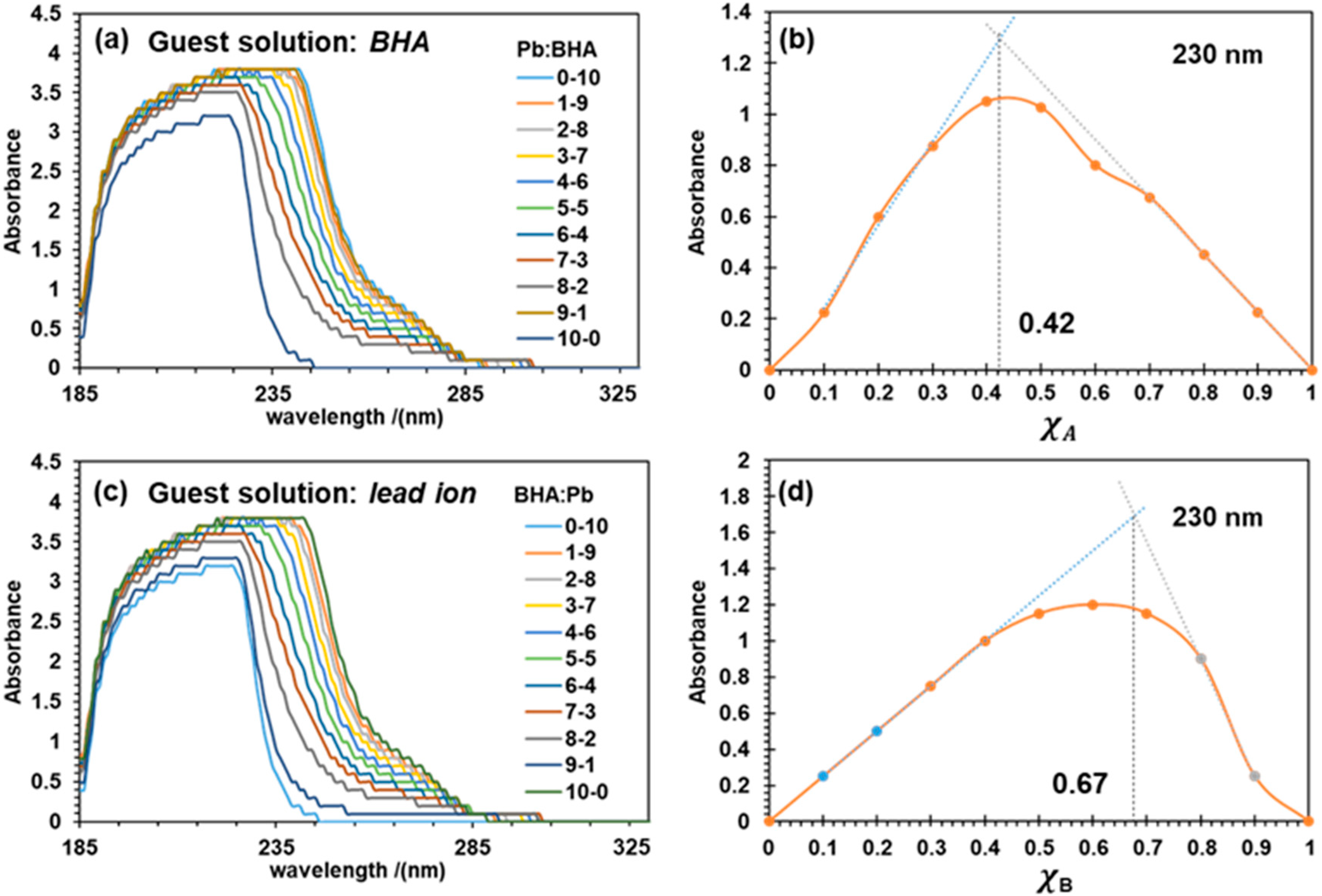
3. Results and Discussion
3.1. Experimental
3.1.1. UV-Vis Results
3.1.2. ESI-MS Results
3.2. Theoretical Prediction
3.2.1. Prediction of Stable BHA Isomers and Pb(II)–BHA Complexes
3.2.2. Single Pb–BHA in Aqueous Solution
3.2.3. High Coordination Complexes
3.2.4. Frontier Molecular Orbital Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, G.L.; Chen, B.; Transue, W.J.; Yang, Z.; Grutzmacher, H.; Driess, M.; Cummins, C.C.; Borden, W.T.; Wang, X.B. Spectroscopic characterization, computational investigation, and comparisons of ecx- (e = as, p, and n; x = s and o) anions. J. Am. Chem. Soc. 2017, 139, 8922–8930. [Google Scholar] [CrossRef] [PubMed]
- Kuta, J.; Clark, A.E. Trends in aqueous hydration across the 4f period assessed by reliable computational methods. Inorg. Chem. 2010, 49, 7808–7817. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, E.; Moro, A.J.; Gavara, R.; Alfonso, I.; Perez, Y.; Zaccaria, F.; Guerra, C.F.; Malfois, M.; Baucells, C.; Ferrer, M.; et al. Reversible self-assembly of water-soluble gold(i) complexes. Inorg. Chem. 2017, 57, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Akturk, E.S.; Scappaticci, S.J.; Seals, R.N.; Marshak, M.P. Bulky beta-diketones enabling new lewis acidic ligand platforms. Inorg. Chem. 2017, 56, 11466–11469. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, S.R.; Mondal, B.; Vijaykumar, G.; Thakur, A. Ict-isomerization-induced turn-on fluorescence probe with a large emission shift for mercury ion: Application in combinational molecular logic. Inorg. Chem. 2017, 56, 11577–11590. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.D.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-enhanced photocatalytic CO2 conversion within metal–organic frameworks under visible light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Jalilehvand, F.; Sisombath, N.S.; Schell, A.C.; Facey, G.A. Lead(II) complex formation with l-Cysteine in aqueous solution. Inorg. Chem. 2015, 54, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Xu, M.; Liu, W.; Sandraz, E.; Lan, H.; Wang, J.; Cohen, S.M. Metal–organic frameworks as micromotors with tunable engines and brakes. J. Am. Chem. Soc. 2017, 139, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Chen, K.; Zhu, Y.; Nguyen, A.V.; Tian, M.; Wang, L.; Yue, T.; et al. Novel catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Tian, M.; Hu, Y.; Sun, W.; Liu, R. Study on the mechanism and application of a novel collector-complexes in cassiterite flotation. Colloid Surf. A Physicochem. Eng. Aspects 2017, 522, 635–641. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus bha flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloid Surf. A: Physicochem. Eng. Aspects 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Sun, L.; Hu, Y.; Sun, W. Effect and mechanism of octanol in cassiterite flotation using benzohydroxamic acid as collector. Trans. Nonferrous Met. Soc. China 2016, 26, 3253–3257. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Han, H.-S.; Liu, W.-L.; Hu, Y.-H.; Sun, W.; Li, X.-D. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb–BHA complexes in shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, G.; Miller, J.D. Hydrodynamic interactions between particles in aggregation and flotation. Int. J. Miner. Process. 2003, 70, 157–170. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Zhong, H. Study on the activation of scheelite and wolframite by lead nitrate. Minerals 2015, 5, 247–258. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral ph. Miner. Eng. 2014, 66, 135–144. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Lu, J.; Jin, J.; Min, F. The effects of calcium ions on the flotation of sillimanite using dodecylammonium chloride. Minerals 2017, 7, 28. [Google Scholar] [CrossRef]
- Materna, K.L.; Crabtree, R.H.; Brudvig, G.W. Anchoring groups for photocatalytic water oxidation on metal oxide surfaces. Chem. Soc. Rev. 2017, 46, 6099–6110. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interf. Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved flotation separation of cassiterite from calcite using a mixture of lead(II) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Schraml, J. Derivatives of hydroxamic acids. Appl. Organomet. Chem. 2000, 14, 604–610. [Google Scholar] [CrossRef]
- Codd, R. Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord. Chem. Rev. 2008, 252, 1387–1408. [Google Scholar] [CrossRef]
- Flipo, M.; Charton, J.; Hocine, A.; Dassonneville, S.; Deprez, B.; Deprez-Poulain, R. Hydroxamates: Relationships between structure and plasma stability. J. Med. Chem. 2009, 52, 6790–6802. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, A.A. Conformational analysis and vibrational assignments of benzohydroxamic acid and benzohydrazide. J. Mol. Struct. 2012, 1023, 115–122. [Google Scholar] [CrossRef]
- Lynch, A.J.; Watt, J.S.; Fich, J.A.; Harbort, G.J. History of flotation technology. In Forth Flotation: A Century of Innovation; SME: Littleton, CO, USA, 2007; pp. 65–91. [Google Scholar]
- Lei, X.L.; Pan, B.C. The geometries and proton transfer of hydrated divalent lead ion clusters [Pb(H2O)n]2+(n = 1–17). J. Theor. Comput. Chem. 2012, 11, 1149–1164. [Google Scholar] [CrossRef]
- Wander, M.C.F.; Rustad, J.R.; Casey, W.H. Influence of explicit hydration waters in calculating the hydrolysis constants for geochemically relevant metals. J. Phys. Chem. A 2010, 114, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- García, B.; Ibeas, S.; Leal, J.M.; Secco, F.; Venturini, M.; Senent, M.L.; Niño, A.; Muñoz, C. Conformations, protonation sites, and metal complexation of benzohydroxamic acid. A theoretical and experimental study. Inorg. Chem. 2005, 44, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, E.; Yilmaz, F.; Emirik, M.; Ozil, M. Synthesis and characterization of two new hydroxamic acids derivatives and their metal complexes. An investigation on the keto/enol, E/Z and hydroxamate/hydroximate forms. J. Mol. Struct. 2017, 1127, 403–412. [Google Scholar] [CrossRef]
- Caudle, M.T.; Crumbliss, A.L. Dissociation kinetics of (N-methylacetohydroxamato) iron(III) complexes: A model for probing electronic and structural effects in the dissociation of siderophore complexes. Inorg. Chem. 1994, 33, 4077–4085. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Farkas, E.; Gil, M.J.; Fitzgerald, D.; Castineras, A.; Nolan, K.B. Metal complexes of salicylhydroxamic acid (H2Sha), anthranilic hydroxamic acid and benzohydroxamic acid. Crystal and molecular structure of [Cu(phen)2(Cl)]Cl × H2Sha, a model for a peroxidase-inhibitor complex. J. Inorg. Biochem. 2000, 79, 47–51. [Google Scholar] [CrossRef]
- Bodwin, J.J.; Cutland, A.D.; Malkani, R.G.; Pecoraro, V.L. Cheminform abstract: The development of chiral metallacrowns into anion recognition agents and porous materials. Coord. Chem. Rev. 2001, 216, 489–512. [Google Scholar] [CrossRef]
- Pereira, C.F.; Howarth, A.J.; Vermeulen, N.A.; Almeida Paz, F.A.; Tomé, J.P.C.; Hupp, J.T.; Farha, O.K. Towards hydroxamic acid linked zirconium metal–organic frameworks. Mater. Chem. Front. 2017, 1, 1194–1199. [Google Scholar] [CrossRef]
- He, J.; Han, H.; Zhang, C.; Xu, Z.; Yuan, D.; Chen, P.; Sun, W.; Hu, Y. Novel insights into the surface microstructures of lead(ii) benzohydroxamic on oxide mineral. Appl. Surf. Sci. 2018, 458, 405–412. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Nagata, C. Theory of substitution in conjugated molecules. Bull. Chem. Soc. Jpn. 1954, 27, 423–427. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural bond orbital methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Perera, W.N.; Hefter, G.A.; Sipos, P.M. An investigation of the lead(II)−hydroxide system. Inorg. Chem. 2001, 40, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of continuous variations: Applications of job plots to the study of molecular associations in organometallic chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Becke’s three parameter hybrid method using the lyp correlation functional. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yu, H.S.; Li, S.L.; Truhlar, D.G. Perspective: Kohn-sham density functional theory descending a staircase. J. Chem. Phys. 2016, 145, 130901. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Ertem, M.Z. Calculating free energy changes in continuum solvation models. J. Phys. Chem. B 2016, 120, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Malekghassemi, M. An Exploration of Molecular Mechanics and Quantum Chemical Methods. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.567.4830&rep=rep1&type=pdf (accessed on 18 August 2018).
- Lee, Y.S.; Ermler, W.C.; Pitzer, K.S. Ab initio effective core potentials including relativistic effects. V. SCF calculations with ω–ω coupling including results for Au2+, Tlh, Pbs, and PbSe. J. Chem. Phys. 1993, 73, 159–165. [Google Scholar] [CrossRef]
- Wander, M.C.F.; Clark, A.E. Hydration properties of aqueous Pb(II) ion. Inorg. Chem. 2008, 47, 8233–8241. [Google Scholar] [CrossRef] [PubMed]
- Ochterski, J.W. Vibrational Analysis in Gaussian. Available online: https://www.lindsayengineering.com/img/resources/vib.pdf (accessed on 18 August 2018).
- Arora, R.; Issar, U.; Kakkar, R. Theoretical study of the molecular structure and intramolecular proton transfer in benzohydroxamic acid. Comput. Theor. Chem. 2017, 1105, 18–26. [Google Scholar] [CrossRef]
- Steinberg, G.M.; Swidler, R. The benzohydroxamate anion. J. Org. Chem. 1965, 30, 2362–2365. [Google Scholar] [CrossRef]
- Ochterski, J.W. Thermochemistry in Gaussian. Available online: http://www.lct.jussieu.fr/manuels/Gaussian03/g_whitepap/thermo/thermo.pdf (accessed on 18 August 2018).
- Dennington, R.; Keith, T.; Millam, J. Gaussview, Version 5. Available online: https://gaussview.soft112.com (accessed on 18 August 2018).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wave function analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.; Inoue, Y.; Tobey, S.; Metzger, A.; Anslyn, E. Ion-pairing molecular recognition in water: Aggregation at low concentrations that is entropy-driven. J. Am. Chem. Soc. 2002, 124, 14959–14967. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, E.; Lavabre, D.; Levy, G.; Micheau, J.C. Quantitative analysis of continuous-variation plots with a comparison of several methods: Spectrophotometric study of organic and inorganic 1:1 stoichiometry complexes. J. Chem. Educ. 1992, 69, 833. [Google Scholar] [CrossRef]
- Wise, W.M.; Brandt, W.W. Spectrophotometric determination of vandium(v) with benzohydroxamic acid and 1-hexanol. Anal. Chem. 1955, 27, 1392–1395. [Google Scholar] [CrossRef]
- Leon-Pimentel, C.I.; Amaro-Estrada, J.I.; Saint-Martin, H.; Ramirez-Solis, A. Born-Oppenheimer molecular dynamics studies of Pb(II) micro hydrated gas phase clusters. J. Chem. Phys. 2017, 146, 084307. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Ebrahimi, A. The X−···benzohydrazide complexes: The interplay between anion-π and H-bond interactions. Struct. Chem. 2017, 28, 687–695. [Google Scholar] [CrossRef]
- Moncomble, A.; Cornard, J.P.; Meyer, M. A quantum chemistry evaluation of the stereochemical activity of the lone pair in PbII complexes with sequestering ligands. J. Mol. Model. 2017, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Calculation of molecular orbital composition. Acta Chim. Sin. 2011, 69, 2393–2406. [Google Scholar]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb–BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]







| CN of Water Molecules (m) | ΔG/(Kcal/mol) | Pb–O Mean Distance (Å) | ||
|---|---|---|---|---|
| (Pb–BHA)+(H2O)m | Pb(H2O)m2+ | (Pb–BHA)+(H2O)m | Pb(H2O)m2+ | |
| 1 | 1.41 | −50.48 | 2.53 | 2.34 |
| 2 | 1.38 | −36.75 | 2.64 | 2.38 |
| 3 | 7.40 | −27.34 | 2.69 | 2.42 |
| 4 | 4.64 | −17.65 | 2.77 | 2.49 |
| 5 | 8.35 | −14.56 | 2.83 | 2.55 |
| CN | Reaction | ΔG(Kcal/mol) | Pb–O (Å) | εgap (eV) |
|---|---|---|---|---|
| 1 | Pb2+ + BHA- | −356.55 | 2.26 | 4.17 |
| 2 | Pb(BHA)+ + BHA- | −27.20 | 2.40 | 4.50 |
| 3 | Pb(BHA)2 + BHA- | +0.03 | 2.58 | 4.17 |
| Pb–BHA | Orbital Composition Assigned to Pb atom/% | |||
|---|---|---|---|---|
| HOMO−1 | HOMO | LUMO | LUMO+1 | |
| Pb(BHA)+ | 0.03 | 3.55 | 91.93 | 77.54 |
| Pb(BHA)2 | 1.14 | 1.28 | 1.14 | 0.02 |
| Pb(BHA) | 1.01 | 0.75 | 0.13 | 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Han, H.; Zhang, C.; Hu, Y.; Yuan, D.; Tian, M.; Chen, D.; Sun, W. New Insights into the Configurations of Lead(II)-Benzohydroxamic Acid Coordination Compounds in Aqueous Solution: A Combined Experimental and Computational Study. Minerals 2018, 8, 368. https://doi.org/10.3390/min8090368
He J, Han H, Zhang C, Hu Y, Yuan D, Tian M, Chen D, Sun W. New Insights into the Configurations of Lead(II)-Benzohydroxamic Acid Coordination Compounds in Aqueous Solution: A Combined Experimental and Computational Study. Minerals. 2018; 8(9):368. https://doi.org/10.3390/min8090368
Chicago/Turabian StyleHe, Jianyong, Haisheng Han, Chenyang Zhang, Yuehua Hu, Dandan Yuan, Mengjie Tian, Daixiong Chen, and Wei Sun. 2018. "New Insights into the Configurations of Lead(II)-Benzohydroxamic Acid Coordination Compounds in Aqueous Solution: A Combined Experimental and Computational Study" Minerals 8, no. 9: 368. https://doi.org/10.3390/min8090368
APA StyleHe, J., Han, H., Zhang, C., Hu, Y., Yuan, D., Tian, M., Chen, D., & Sun, W. (2018). New Insights into the Configurations of Lead(II)-Benzohydroxamic Acid Coordination Compounds in Aqueous Solution: A Combined Experimental and Computational Study. Minerals, 8(9), 368. https://doi.org/10.3390/min8090368








