Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland
Abstract
:1. Introduction
2. Materials and Methods
3. Geological and Mineralogical Characteristics of Major REE Deposits and Occurrences in Finland
3.1. REE Deposits in Carbonatites
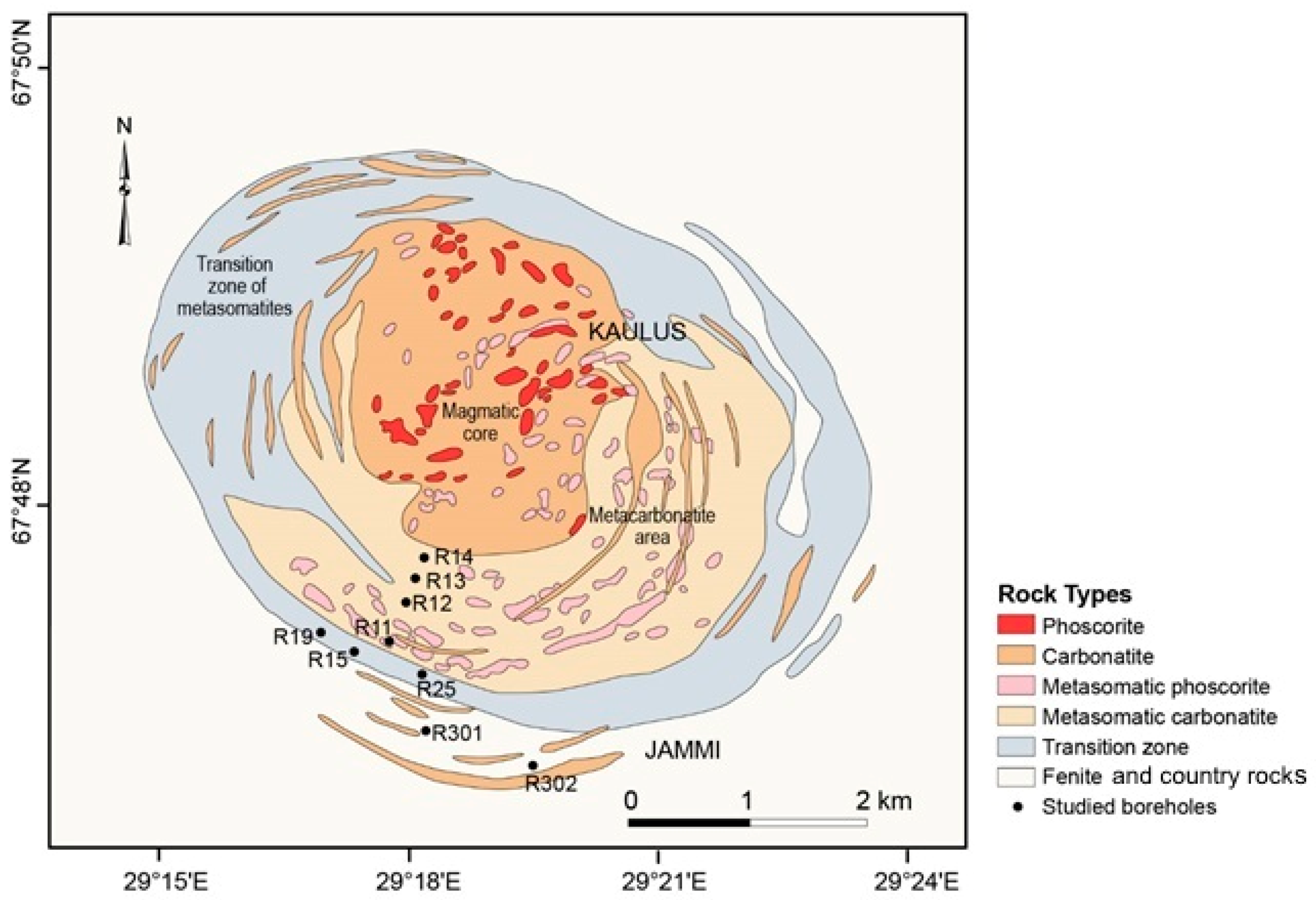
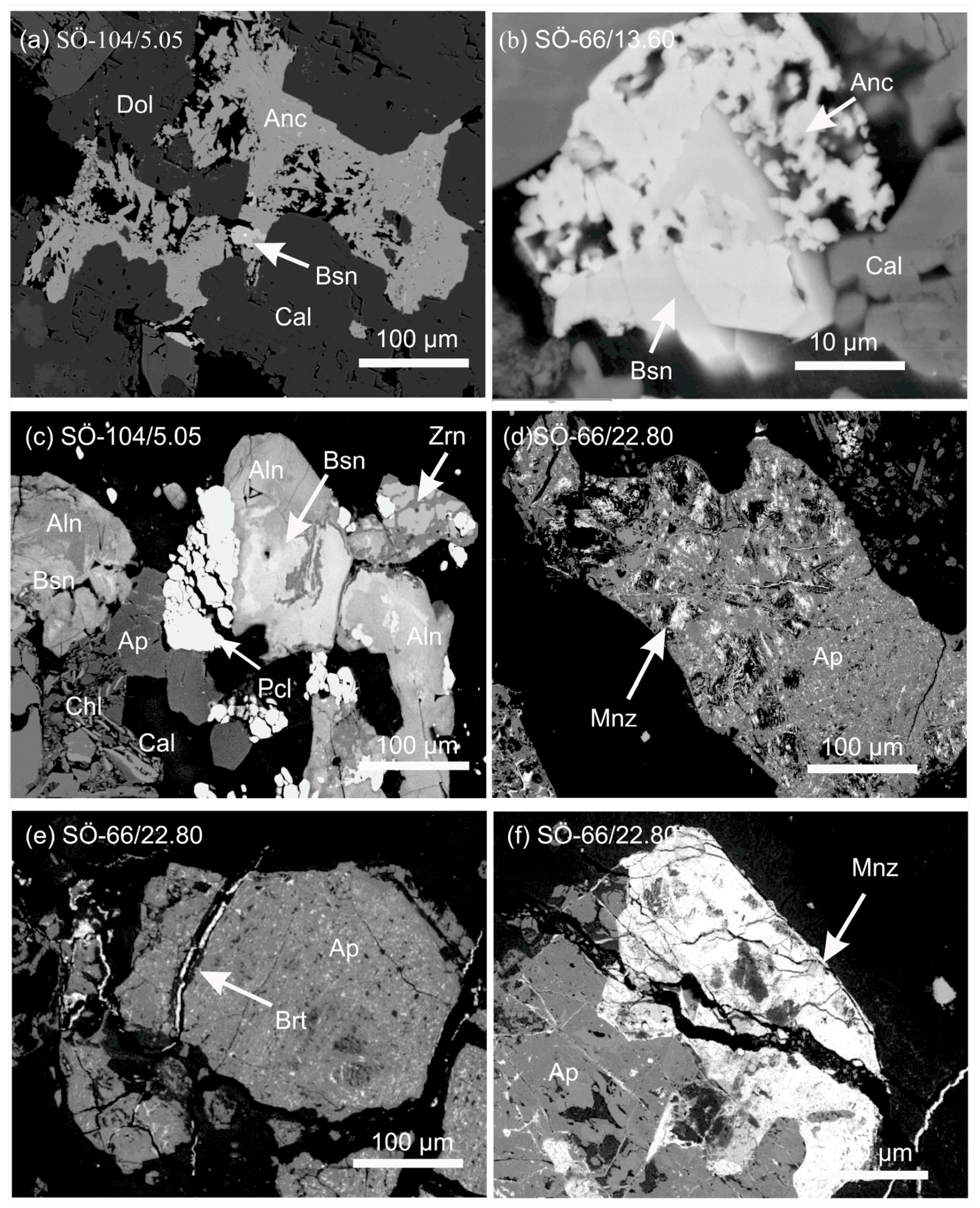
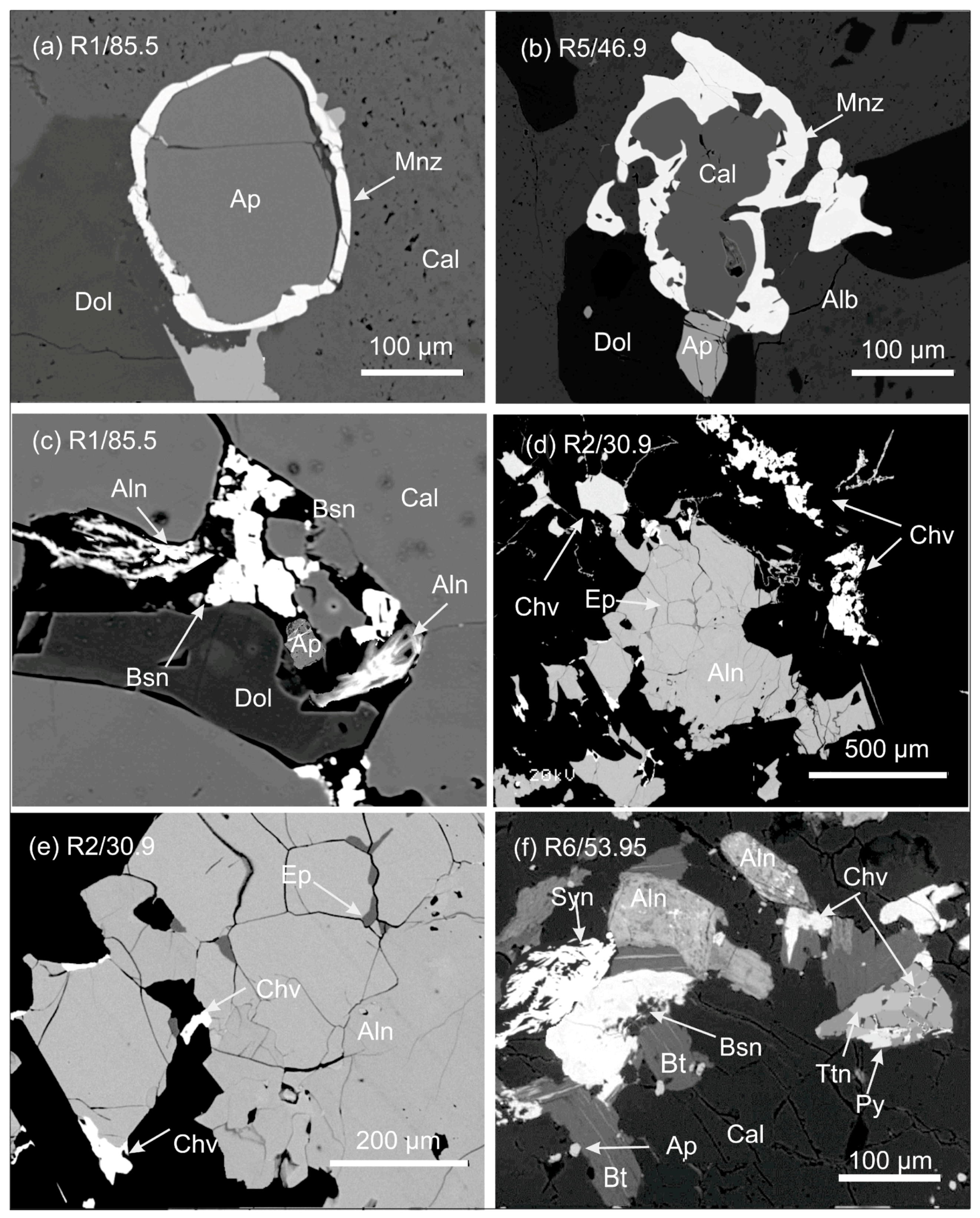
3.1.1. The Jammi and the Kaulus Carbonatite Dikes in the Sokli Complex
3.1.2. Korsnäs Pb-REE Deposit
3.1.3. Kortejärvi and Laivajoki Carbonatites
3.2. REE Deposits Related to Alkaline Intrusions
3.2.1. The Otanmäki Katajakangas Nb-REE Deposit
3.2.2. The Mäkärä Vaulo Area in the Tana Belt
3.3. Hydrothermal REE Deposits
3.3.1. Uuniemi (Kuusamo Belt)
3.3.2. Honkilehto (Kuusamo Belt)
3.4. REE Occurrences in Pegmatite and Greisen (Magmatic-Hydrothermal Systems)
3.4.1. Rapakivi Granite
3.4.2. Kovela Granitoid Complex
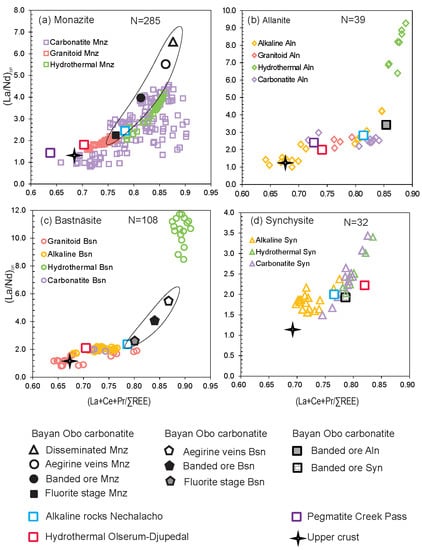
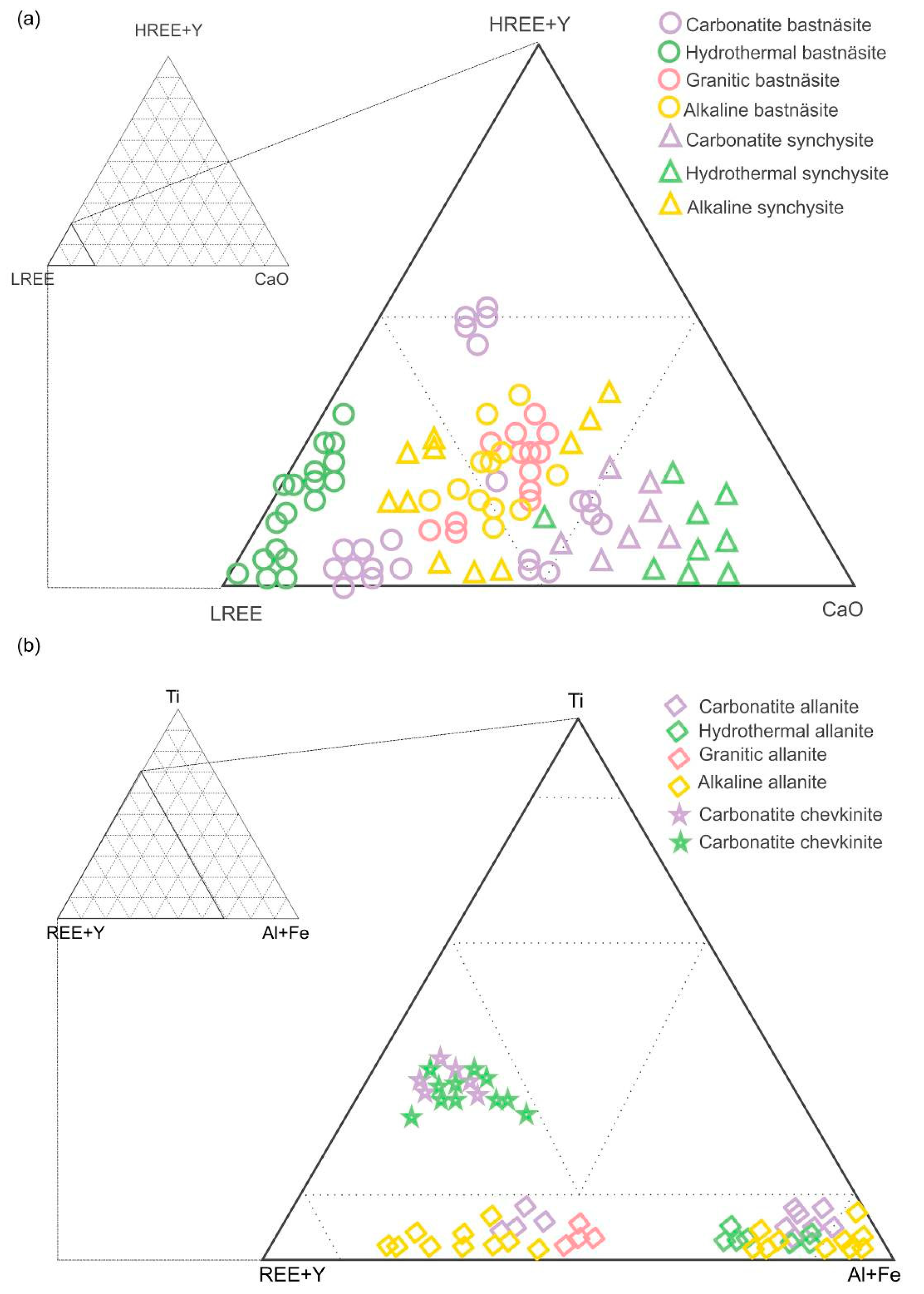
4. Compositions of REE Minerals with Different Origins in Finland
5. Comparison of Results from Finland with Published Compositional Data of Carbonates, REE-Phosphates and Silicates from Other Areas
6. Disscusion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Analytical Methods
References
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Wall, F. Rare earth elements. In Critical Metals Handbook; Gunn, A.G., Ed.; John Wiley & Sons: Stanford, CA, USA, 2014; pp. 312–339. [Google Scholar]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Liu, X.; Wang, K.Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare earth mineralization in igneous rocks: Sources and processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Ling, M.X.; Liu, Y.L.; Williams, I.S.; Teng, F.Z.; Yang, X.Y.; Ding, X.; Wei, G.J.; Xie, L.H.; Deng, W.F.; Sun, W. Formation of the world’s largest REE deposit through protracted fluxing of carbonatite by subduction-derived fluids. Sci. Rep. 2013, 3, 1776. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvige, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Upton, B.G.J.; Emeleus, C.H.; Heaman, L.M.; Goodenough, K.M.; Finch, A.A. Magmatism of the mid-Proterozoic Gardar Province, South Greenland: Chronology, petrogenesis and geological setting. Lithos 2003, 68, 43–65. [Google Scholar] [CrossRef]
- Andreasson, P.G.; Rodhe, A. Geology of the Protogine Zone south of Lake Väittern, southern Sweden: A re-interpretation. Fören. Stockholm Förh 1990, 111, 107–125. [Google Scholar] [CrossRef]
- Wall, F.; Zaitsev, A.N. Phoscorites Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Mineralogical Society of Series; Mineralogical Society: London, UK, 2004; Volume 10, p. 498. [Google Scholar]
- Zaitsev, A.N.; Williams, C.T.; Jeffries, T.E.; Strekopytov, S.; Moutte, J.; Ivashchenkova, O.V.; Spratt, J.; Petrov, S.V.; Wall, F.; Seltmann, R. Reprint of rare earth elements in phoscorites and carbonatites of the Devonian Kola Alkaline Province, Russia: Examples from Kovdor, Khibina, Vuoriyarvi and Turiy Mys complexes. Ore Geol. Rev. 2015, 64, 477–498. [Google Scholar] [CrossRef] [Green Version]
- Sarapää, O.; Al Ani, T.; Lahti, S.I.; Sarala, P.; Torppa, A.; Kontinen, A. Rare earth exploration potential in Finland. J. Geochem. Explor. 2013, 133, 25–41. [Google Scholar] [CrossRef]
- Sarapää, O.; Lauri, L.S.; Ahtola, T.; Al Ani, T.; Grönholm, S.; Kärkkäinen, N.; Lintinen, P.; Torppa, A.; Turunen, P. Discovery Potential of Hi-Tech Metals and Critical Minerals in Finland; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 2015; Volume 219, pp. 1–54.
- Tichomirowa, M.; Grosche, G.; Gotze, J.; Belyatsky, B.V.; Savva, E.V.; Keller, J.; Todt, W. The mineral isotope composition of two Precambrian carbonatite complexes from the kola alkaline province—Alteration versus primary magmatic signatures. Lithos 2006, 91, 229–249. [Google Scholar] [CrossRef]
- Rukhlov, A.S.; Bell, K. Geochronology of carbonatites from the Canadian and Baltic shields, and the Canadian cordillera: Clues to mantle evolution. Miner. Petrol. 2010, 98, 11–54. [Google Scholar] [CrossRef]
- Eilu, P. Mineral Deposits and Metallogeny of Fennoscandia; Special Paper; Geological Survey of Finland: Espoo, Finland, 2012; Volume 53, p. 401.
- Al-Ani, T. Mineralogy and Petrography of Siilinjärvi Carbonatite and Glimmerite Rocks, Eastern Finland; Report of Investigation, 164/2013; Geological Survey of Finland: Espoo, Finland, 2013; 15p. Available online: http://tupa.gtk.fi/raportti/arkisto/164_2013.pdf (accessed on 6 August 2018).
- Korsman, K.; Koistinen, T.; Kohonen, J.; Wennerström, M.; Ekdahl, E.; Honkamo, M.; Idman, H.; Pekkala, Y. Bedrock Map of Finland 1:1,000,000; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 1997.
- Nironen, M.; Kousa, J.; Luukas, J.; Lahtinen, R. Geological Map of Finland—Bedrock. 1:1,000,000; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 2016.
- Vartiainen, H.; Paarma, H. Geological characteristics of the Sokli carbonatite complex, Finland. Econ. Geol. 1979, 74, 1296–1306. [Google Scholar] [CrossRef]
- Kramm, U.; Kogarko, L.N.; Kononova, V.A.; Vartiainen, H. The Kola alkaline province of the CIS and Finland: Precisw Rb-Sr ages define 380–360 Ma age range for all magmatism. Lithos 1993, 30, 33–44. [Google Scholar] [CrossRef]
- Korsakova, M.; Krasotkin, S.; Stromov, V.; Iljina, M.; Lauri, L.; Nilsson, P. Metallogenic areas in Russian part of the Fennoscandian shield. In Mineral Deposits and Metallogeny of Fennoscandia: Geological Survey of Finland; Special Paper; Eilu, P., Ed.; Geological Survey of Finland: Espoo, Finland, 2012; Volume 53, pp. 343–395. [Google Scholar]
- Vartiainen, H. The petrography, mineralogy and petrochemistry of the Sokli carbonatite massif northern Finland. Bull. Geol. Surv. Finl. 1980, 313, 126. [Google Scholar]
- Vartiainen, H.; Vitikka, A. The late stage dikes of the Sokli massif and their tectonic control. Geochem. Int. 1994, 31, 103–106. [Google Scholar]
- Al-Ani, T.; Sarapää, O. Geochemistry and mineral phases of REE in Jammi carbonatite veins and fenites, southern end of the Sokli complex, NE Finland. Geochem. Explor. Environ. Anal. 2013, 13, 217–224. [Google Scholar] [CrossRef]
- Elliotta, H.A.L.; Walla, F.; Chakhmouradianb, A.R.; Siegfriedc, P.R.; Dahlgrend, S.; Weatherleye, S.; Finchf, A.A.; Marksg, M.A.W.; Dowmanh, E.; Deadyi, E. Fenites associated with carbonatite complexes: A review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, M.I.; Kujala, H.; Kärkkäinen, N.; Lehtonen, A.; Mäkitie, H.; Mänttäri, I.; Virransalo, P.; Vuokko, J. The Bedrocks of Southern Ostrobothnian Schist Belt; Report of Investigatio; Geological Survey of Finland: Espoo, Finland, 2005; p. 158.
- Al-Ani, T.; Konnunaho, K.; Sarapää, O. Microprobe Analysis on REE-Minerals of Enontekiö (Palkiskuru and Palovaara) and Kuusamo (Honkilehto) Hydrothermal Mineralization and Kortejärvi-Laivajoki Carbonatites, Northern Finland; Report M42/2010/66; Geological Survey of Finland: Espoo, Finland, 2010; p. 20.
- Papunen, H.; Gorbunov, G. Nickel Copper Deposits of the Baltic Shield and Scandinavian Caledonides; Geological Survey of Finland: Espoo, Finland, 1985; Volume 333, p. 394.
- Huhma, H.; Eero Hanski, E.; Vuollo, J. Sm-Nd isotopes in Paleoproterozoic mafic rocks in Finland—Range of ages, mantle sources and crust-mantle interaction. Bull. Geol. Surv. Finl. 2006, 1, 54. [Google Scholar]
- Nykänen, J.; Laajoki, K.; Karhu, J. Geology and geochemistry of the early Proterozoic Kortejärvi and Laivajoki carbonatites, central Fennoscandian Shield, Finland. Bull. Geol. Soc. Finl. 1997, 69, 5–30. [Google Scholar] [CrossRef]
- Huhma, H.; Eero Hanski Kontinen, A.; Vuollo, J.; Mänttäri, I.; Lahaye, Y. Sm–Nd and U–Pb Isotope Geochemistry of the Palaeoproterozoic Mafic Magmatism in Eastern and Northern Finland; Geological Survey of Finland: Espoo, Finland, 2018; Volume 405, 150p.
- Lindholm, O.; Anttonen, R. Geology of the Otanmäki mine. In Precambrian Ores of Finland, 26th International Geological Congress, Guide to Excursions 078A+C, Part 2 (Finland); Häkli, T.A., Ed.; Geological Survey of Finland: Espoo, Finland, 1980; pp. 25–33. [Google Scholar]
- Talvitie, J.; Paarma, H. Precambrian basic magmatism and the Ti-Fe ore formation in central and northern Finland. Bull. Geol. Surv. Finl. 1980, 307, 98–107. [Google Scholar]
- Puustinen, K. The Finnish Mining Industry and Production of Mineral Raw Materials in the Period 1530–2001, A Historical Overview of Production; Archive Report; Geological Survey of Finland: Espoo, Finland, 2003; p. 578.
- Lahtinen, R. Main Geological Features of Fennoscandia; Special Paper; Geological Survey of Finland: Espoo, Finland, 2012; Volume 53, pp. 13–18.
- Hugg, R.; Heiskanen, V. Exploration of Nb-La Deposits in Otanmäki Area; Report OU 28/85; Rautaruukki CO. Ore Exploration: Oulu, Finland, 1986; p. 14. [Google Scholar]
- Salminen, R.; Tarvainen, T. Geochemical mapping and databases in Finland. In Geochemical exploration 1993. J. Geochem. Explor. 1995, 55, 321–327. [Google Scholar] [CrossRef]
- Rasilainen, K.; Lahtinen, R.; Bornhorst, T.J. Chemical Characteristics of Finnish Bedrock—1:1,000,000 Scale Bedrock Map Units; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 2008; Volume 171, p. 94.
- Sarapää, O.; Sarala, P. Rare earth element and gold exploration in glaciated terrain: Example from the Mäkärä area, Northern Finland. Geochem. Explor. Environ. Anal. 2013, 13, 131–143. [Google Scholar] [CrossRef]
- Salmirinne, H.; Turunen, P.; Sarapää, O. On Prospecting for REE in the Tana Belt, Northern Finland; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 2013; Volume 198, pp. 152–155.
- Tuisku, P.; Huhma, H. Evolution of migmatitic granulite complexes: Implications from Lapland Granulite Belt, part II: Isotopic dating. Bull. Geol. Soc. Finl. 2006, 78, 143–175. [Google Scholar] [CrossRef]
- Cagnard, F.; Barbey, P.; Garpais, D. Transition between “Archaean-type” and “modern-type” tectonics: Insights from the Finnish Lapland Granulite Belt. Precambrian Res. 2011, 187, 127–142. [Google Scholar] [CrossRef]
- Al-Ani, T.; Sarapää, O. REE-Mineralogy of Arkosic Gneisses from Mäkärä-Vaulo Area, Tana Belt, Northern Finland; Investigation Report 74/2012; Geological Survey of Finland: Espoo, Finland, 2012; p. 36.
- Heilimo, E.; Halla, J.; Lauri, L.S.; Rämö, O.T.; Huhma, H.; Kurhila, M.I.; Front, K. The Paleoproterozoic Nattanen-type granites in northern Finland and vicinity—A post collisional oxidized A-type suite. Bull. Geol. Soc. Finl. 2009, 81, 7–38. [Google Scholar] [CrossRef]
- Berger, A.; Gnos, E.; Janots, E.; Fernandez, A.; Giese, J. Formation and composition of rhabdophane, bastnäsite and hydrated thorium minerals during alteration: Implications for geochronology and low-temperature processes. Chem. Geol. 2008, 254, 238–248. [Google Scholar] [CrossRef]
- Buda, G.; Nagy, G.; Pál-Molnár, E. Allanite and monazite occurrences in Variscan granitoids of Tisza Mega-Unit (South Hungary). Carpathian J. Earth Environ. Sci. 2014, 9, 57–68. [Google Scholar]
- Budzyń, B.; Hetherington, C.J.; Williams, M.L.; Jercinovic, M.J.; Michalik, M. Fluid mineral interactions and constraints on monazite alteration during metamorphism. Miner. Mag. 2010, 74, 659–681. [Google Scholar] [CrossRef]
- Förster, H.J. Cerite-(Ce) and thorian synchysite-(Ce) from the Niederbobritzsch granite, Erzgebirge, Germany: Implications for the differential mobility of the LREE and Th during alteration. Can. Miner. 2000, 38, 67–79. [Google Scholar] [CrossRef]
- Hirtopanu, P.; Andersen, J.C.; Fairhurst, R.J.; Jakab, G. Allanite-(Ce) and its associations, from the Ditrau alkaline intrusive massif, East Carpathians, Romania. Proc. Romanian Acad. Ser. 2013, 15, 59–74. [Google Scholar]
- Buda, G.; Nagy, G. Some REE-bearing accessory minerals in two types of Variscan granitoids, Hungary. Geol. Carpathica 1995, 46, 67–78. [Google Scholar]
- Meintzer, R.E.; Mitchell, R.S. The epigene alteration of allanite. Can. Miner. 1988, 26, 945–955. [Google Scholar]
- Wing, B.A.; Ferry, J.M.; Harrison, T.M. Prograde destruction and formation of monazite and allanite during contact and regional metamorphism of pelites: Petrology and geochronology. Contrib. Miner. Pet. 2003, 145, 228–250. [Google Scholar] [CrossRef]
- Hanson, S.L.; Falster, A.U.; Simmons, W.B.; Brown, T.J.A. Allanite-(Nd) from the Kingman feldspar mine, Mojave pegmatite district, Northwester Arizona, USA. Can. Miner. 2012, 50, 815–824. [Google Scholar] [CrossRef]
- Pankka, H.S.; Vanhanen, E.J. Early Proterozoic Au-Co-U mineralization in the Kuusamo district, Northeastern Finland. Precambrian Res. 1992, 58, 387–400. [Google Scholar] [CrossRef]
- Frietsch, R.; Tuisku, P.; Martinsson, O.; Perdahl, J.-A. Early Proterozoic Cu-(Au) and Fe ore deposits associated with regional Na-Cl metasomatism in northern Fennoscandia. Ore Geol. Rev. 1997, 12, 1–34. [Google Scholar] [CrossRef]
- Vanhanen, E. Geology, mineralogy and geochemistry of the Fe-Co-Au-(U) deposits in the Paleoproterozoic Kuusamo Schist Belt, Northeastern Finland. Bull. Geol. Surv. Finl. 2001, 399, 229. [Google Scholar]
- Mutanen, T. Alkaline and Appintic Rocks. Report on the Project Magmatism and Ore formation 2002–2005; Report 9/2011, (Finnish with English abstract); Geological Survey of Finland: Espoo, Finland, 2011; p. 627.
- Pankka, H. Geology and Mineralogy of Au-Co-U Deposits in the Proterozoic Kuusamo. Volcano Sedimentary Belt, Northeastern Finland. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 1992; p. 233. [Google Scholar]
- Rämö, O.T.; Haapala, I. Rapakivi granites. Precambrian Geology of Finland, Key to the Evolution of the Fennoscandian Shield. Dev. Precambrian Geol. 2005, 14, 533–562. [Google Scholar] [CrossRef]
- Haapala, I. Petrography and geochemistry of the Eurajoki stock, a rapakivi-granite complex with greisen-type mineralization in Southwestern Finland. Bull. Geol. Surv. Finl. 1977, 286, 128. [Google Scholar]
- Al-Ani, T.; Ahtola, T.; Kuusela, J. Critical Metals Mineralization in the Late-Stage Intrusions of Vyborg Batholith, Southern Finland; Report of Investigation 21/2017; Geological Survey of Finland: Espoo, Finland, 2017; p. 39.
- Al-Ani, T.; Ahtola, T.; Kuusela, J.; Al-Ansari, N. Mineralogical and petrographic characteristics of indium and REE-bearing accessory phases in the Kymi Granite Stock, Southern Finland. Nat. Resour. 2018, 9, 23–41. [Google Scholar] [CrossRef]
- Lukkari, S.; Thomas, R.; Haapala, I. Crystallization of the Kymi topaz granite stock within the Vyborg rapakivi granite batholith, Finland: Evidence from melt inclusions. Can. Miner. 2009, 47, 1359–1374. [Google Scholar] [CrossRef]
- Al-Ani, T. REE-bearing minerals in the Rapakivi Granite, Southern Finland. Open Geosci. 2015, 7, 646–662. [Google Scholar] [CrossRef]
- Hölttä, P.; Huhma, H.; Mänttäri, I.; Paavola, J. P-T-t development of Archaean granulites in Varpaisjärvi, central Finland. II. Dating of high-grade metamorphism with the U-Pb and Sm-Nd methods. Lithos 2000, 50, 121–136. [Google Scholar] [CrossRef]
- Pabst, A.; Hutton, C.O. Huttonite, a new monoclinic thorium silicate, with an account on its occurrence, analysis, and properties. Am. Miner. 1951, 36, 60–65. [Google Scholar]
- Linthout, K. Tripartite division of the system 2REEPO4-CaTh (PO4)2-2ThSiO4, discreditation of brabantite, and recognition of cheralite as the name for members dominated by CaTh (PO4)2. Can. Miner. 2007, 45, 503–508. [Google Scholar] [CrossRef]
- Förster, H.J.; Harlov, D.E. Monazite-(Ce) huttonite solid solutions in granulite-facies metabasites from the Ivrea-Verbano Zone, Italy. Miner. Mag. 1999, 63, 587–594. [Google Scholar] [CrossRef]
- Overstreet, W.C. The geological occurrence of monazite. Geol. Surv. Prog. Pap. 1967, 530, 327. [Google Scholar]
- Mohr, D.W. Zoned porphyroblasts of metamorphic monazite in the Anakeesta Formation, Great Smoky Mountains, North Carolina. Am. Miner. 1984, 69, 98–103. [Google Scholar]
- Kelly, N.M.; Harley, S.L.; Möller, A. Complexity in the behaviour and recrystallization of monazite during high-T metamorphism and fluid infiltration. Chem. Geol. 2012, 322–323, 192–208. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Hughes, J.M.; Mariano, A.N. The crystal chemistry of monazite and xenotime. Am. Miner. 1995, 80, 21–26. [Google Scholar]
- Haas, J.R.; Shock, E.L.; Sassani, D.C. Rare earth elements in hydrothermal systems: Estimates of standard partial modal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta 1995, 59, 4329–4350. [Google Scholar] [CrossRef]
- Bagiński, B.; Macdonald, R.; Dzierżanowski, P.; Zozulya, D.; Kartashov, P.M. Hydrothermal alteration of chevkinite-group minerals: Products and mechanisms. Part 1. Hydration of chevkinite-(Ce). Miner. Mag. 2015, 79, 1039–1059. [Google Scholar] [CrossRef]
- Macdonald, R.; Bagiński, B.; Kartashov, P.M.; Zozulya, D.; Dzierżanowski, P. Hydrothermal alteration of chevkinite-group minerals. Part 2. Metasomatite from the Keivy massif, Kola Peninsula, Russia. Miner. Mag. 2015, 79, 1019–1037. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Mercer, W.; Gunning, C.; Prout, S. Quantitative characterization of the REE minerals by QEMSCAN from the Nechalacho Heavy Rare Earth Deposit, Thor Lake Project, NWT, Canada. SGS Miner. Serv. Tech. Pap. 2011, 7, 1–11. [Google Scholar]
- Andersson, S.S.; Wagner, T.; Jonsson, E.; Michallik, R.M. Mineralogy, paragenesis and mineral chemistry of REEs in the Olserum-Djupedal REE-phosphate mineralization, SE Sweden. Am. Miner. 2018, 103, 125–142. [Google Scholar] [CrossRef]
- Hanson, S.L.; Simmons, W.B.; Webber, K.L.; Falster, A.U. Rare-earth-element mineralogy of granitic pegmatites in the Trout Creek Pass District, Chaffee County, Colorado. Can. Miner. 1992, 30, 673–686. [Google Scholar]
- Papoutsa, A.; Pe-Piper, G. The relationship between REE-Y-Nb-Th minerals and the evolution of an A-type granite, Wentworth Pluton, Nova Scotia. Am. Miner. 2013, 98, 444–462. [Google Scholar] [CrossRef]
- Holtstam, D.; Andersson, U.B. The REE minerals of the Bastnäs-type deposits, South-Central Sweden. Can. Miner. 2007, 45, 1073–1114. [Google Scholar] [CrossRef]







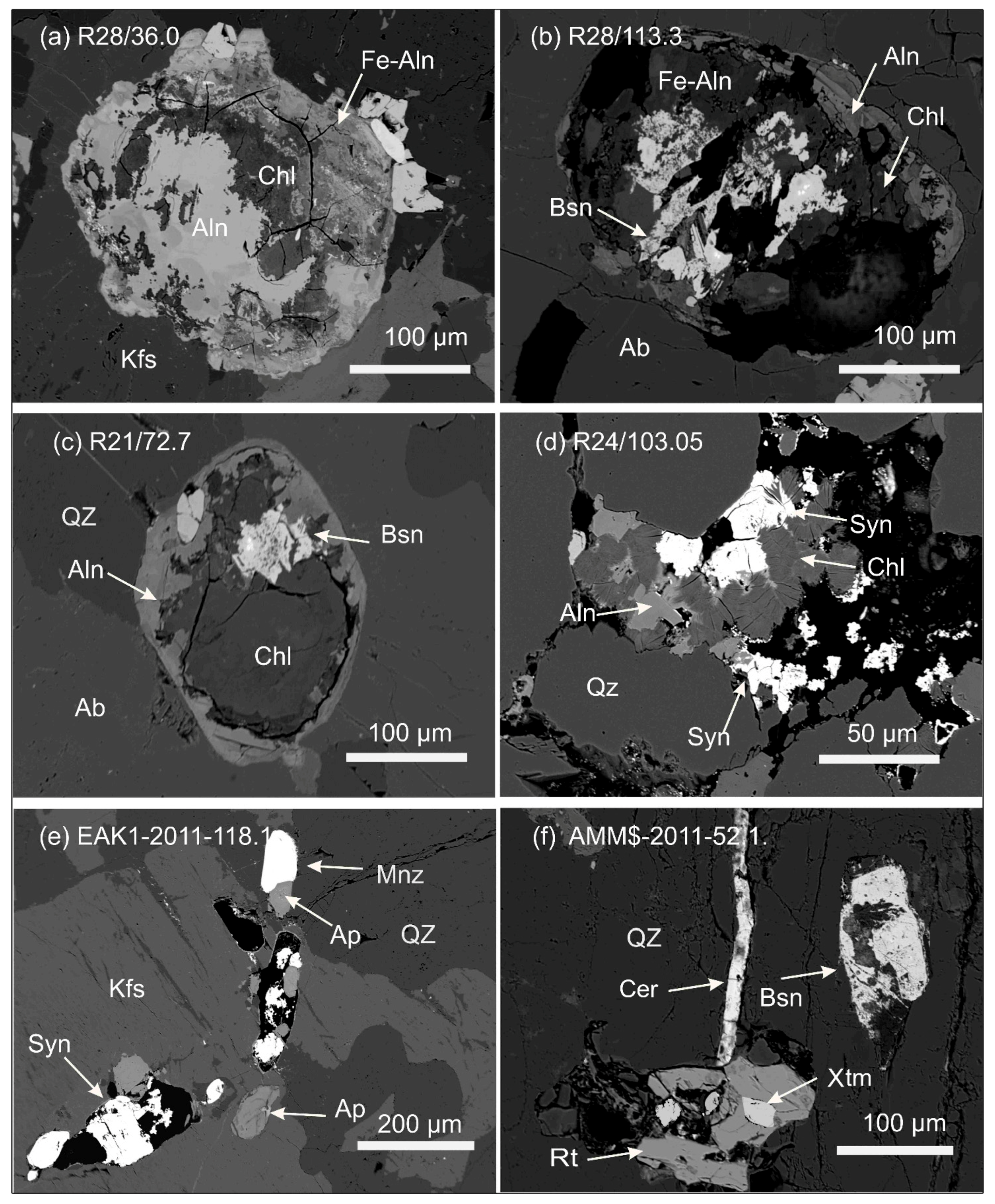
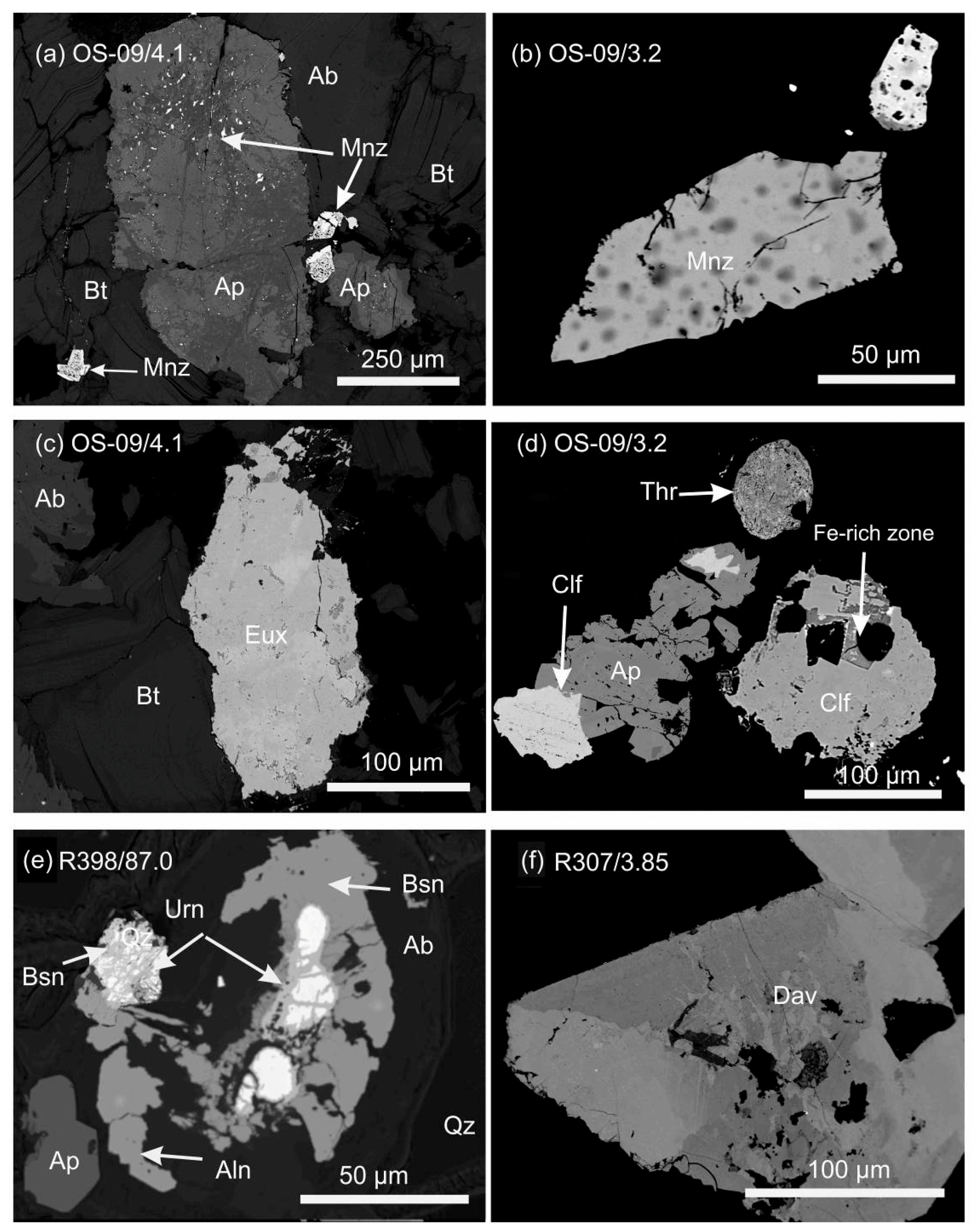






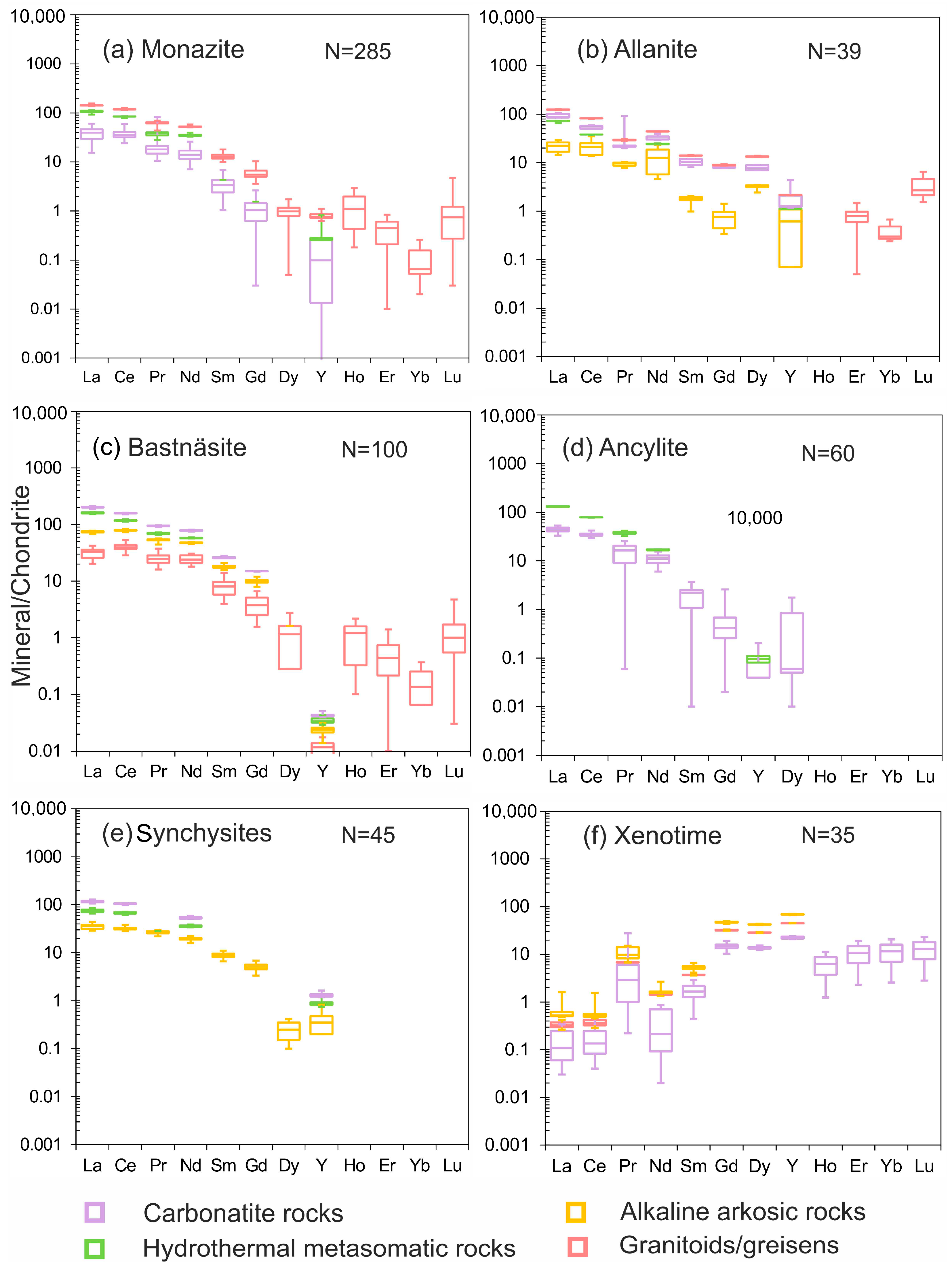
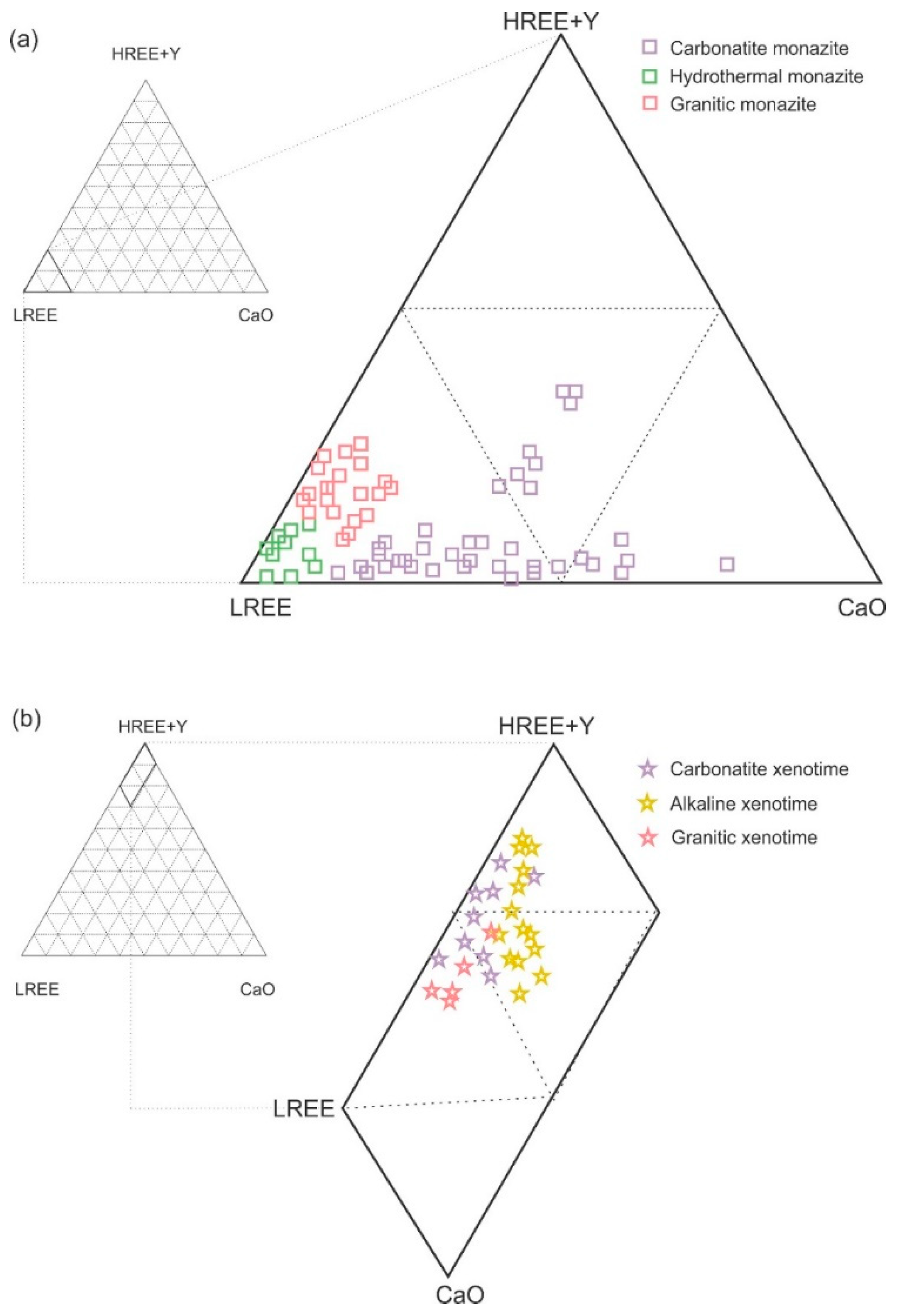

| Deposit Examples | Ore-Bearing Rocks | Age(Ga) | Major REE Bearing Minerals |
|---|---|---|---|
| Carbonatite rocks | |||
| Sokli Jammi/ Kaulus | Carbonatites | 0.38–0.36 | Ancylite-(Ce), monazite, bastnäsite, allanite, xenotime-(Y), Sr-apatite, strontianite, barite |
| Korsnäs | Calcsilicate rocks (skarn) next to carbonatite | 1.90–1.83 | Apatite, monazite, Ca-ancylite, bastnäsite, barite, calcite |
| Kortejärvi-Laivajoki | Carbonatites | 1.88–1.85 | Apatite, allanite, chevkinite-(Ce) monazite, bastnäsite, columbite |
| Alkaline rocks | |||
| Otanmäki and Katajakangas | Alkaline gneiss | 2.30–1.90 | Fergusonite(Y), fergusonite (U), allanite, Ca-REE fluorocarbonates (bastnäsite and synchysite), columbite |
| Tana Belt (Vaulo and Mäkära) | Arkose gneiss | 3.10–2.60 | Euxenite, columbite, zircon |
| Hydrothermal alteration zones | |||
| Kuusamo_Uuniniemi | Epigenetic hydrothermal | 1.90–1.80 | Apatite, monazite, allanite euxenite, Fe-columbite, thorite |
| Kuusamo_Honkilehto | Albitite | 2.45–2.44 | Apatite, bastnäsite, ancylite, allanite, chevkinite-(Ce) davidite, uraninite |
| Granitoids/greisens | |||
| Kovela | Pegmatite in monzogranite | 1.85–1.79 | Monazite, huttonite/thorite, xenotime |
| Kymi granite | Greisen in Rapakivi Granite | 1.64–1.63 | Apatite, monazite, bastnäsite, allanite, xenotime, thorite |
| Mineral | (La + Ce + Pr)/(ΣREE) | (La/Nd)cn | Mineral | (La + Ce + Pr)/(ΣREE) | (La/Nd)cn |
|---|---|---|---|---|---|
| Monazite | Allanite | ||||
| Carbonatite rocks | 0.80 | 0.80–4.55 | Carbonatite rocks | 0.75 | 2.17–2.98 |
| Hydrothermal metasomatic rocks | 0.85 | 2.05–4.17 | Hydrothermal metasomatic rocks | 0.87 | 6.87–9.26 |
| Granitoids/greisens | 0.75 | 1.57–2.27 | Granitoids/greisens | 0.77 | 2.09–2.71 |
| Alkaline rocks Nechalacho | 0.77 | 2.03–2.26 | Alkaline arkosic rocks | 0.71 | 1.05–4.23 |
| Hydrothermal ore, Olserum-Djupedal | 0.70 | 0.60–2.50 | Alkaline rocks, Nechalacho | 0.83 | 2.8–2.9 |
| Carbonatite, Bayan Obo | 0.85 | 4.50–6.20 | Carbonatite, Bayan Obo | 0.86 | 3.8 |
| Pegmatite, Creek Pass | 0.63 | 1.48–1.51 | Pegmatite Creek Pass | 0.72 | 1.70–3.40 |
| Hydrothermal ore, Olserum-Djupedal | 0.74 | 1.67–2.48 | |||
| Upper Crust | 0.68 | 1.2 | |||
| Bastnäsite | Synchysite | ||||
| Carbonatite rocks | 0.78 | 0.50–3.66 | Carbonatite rocks | 0.78 | 1.49–3.43 |
| Hydrothermal metasomatic rocks | 0.89 | 8.50–12.84 | Hydrothermal metasomatic rocks | 0.80 | 1.94–3.43 |
| Granitoids/greisens | 0.70 | 0.80–2.00 | Alkaline arkosic rocks | 0.71 | 1.56–2.37 |
| Alkaline arkosic rocks | 0.73 | 1.71–2.21 | Alkaline rocks, Nechalacho | 0.76 | 1.73–2.26 |
| Alkaline rocks Nechalacho | 0.79 | 2.22–2.28 | Carbonatite, Bayan Obo | 0.79 | 0.60–2.20 |
| Hydrothermal ore, Olserum-Djupedal | 0.72 | 1.70–2.38 | Hydrothermal ore, Olserum-Djupedal | 0.84 | 2.0–2.3 |
| Upper Crust | 0.68 | 1.2 | Upper Crust | 0.68 | 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ani, T.; Molnár, F.; Lintinen, P.; Leinonen, S. Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland. Minerals 2018, 8, 356. https://doi.org/10.3390/min8080356
Al-Ani T, Molnár F, Lintinen P, Leinonen S. Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland. Minerals. 2018; 8(8):356. https://doi.org/10.3390/min8080356
Chicago/Turabian StyleAl-Ani, Thair, Ferenc Molnár, Panu Lintinen, and Seppo Leinonen. 2018. "Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland" Minerals 8, no. 8: 356. https://doi.org/10.3390/min8080356