Experimental Simulation of the Self-Trapping Mechanism for CO2 Sequestration into Marine Sediments
Abstract
:1. Introduction
2. Experimental Program
2.1. Soil Used
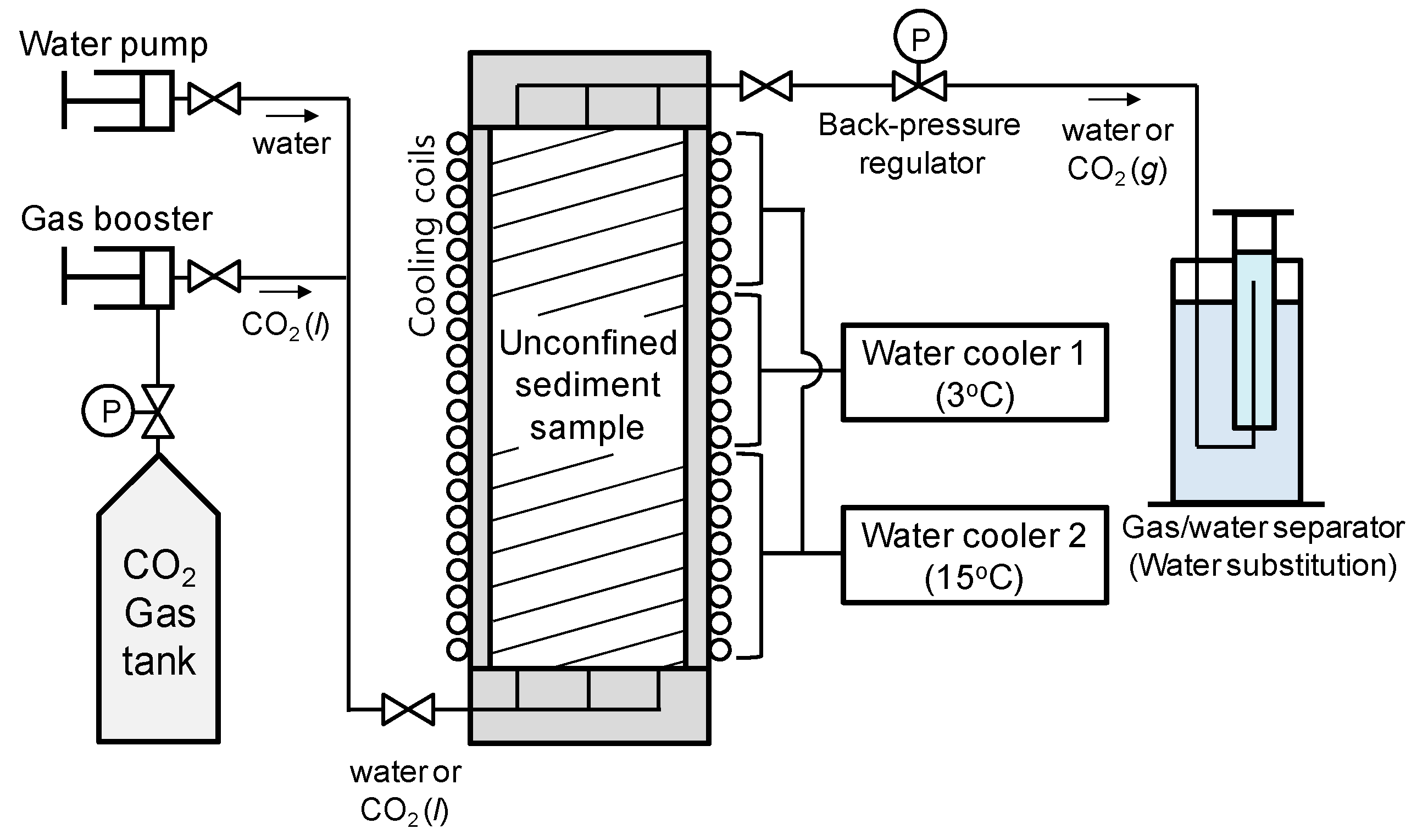
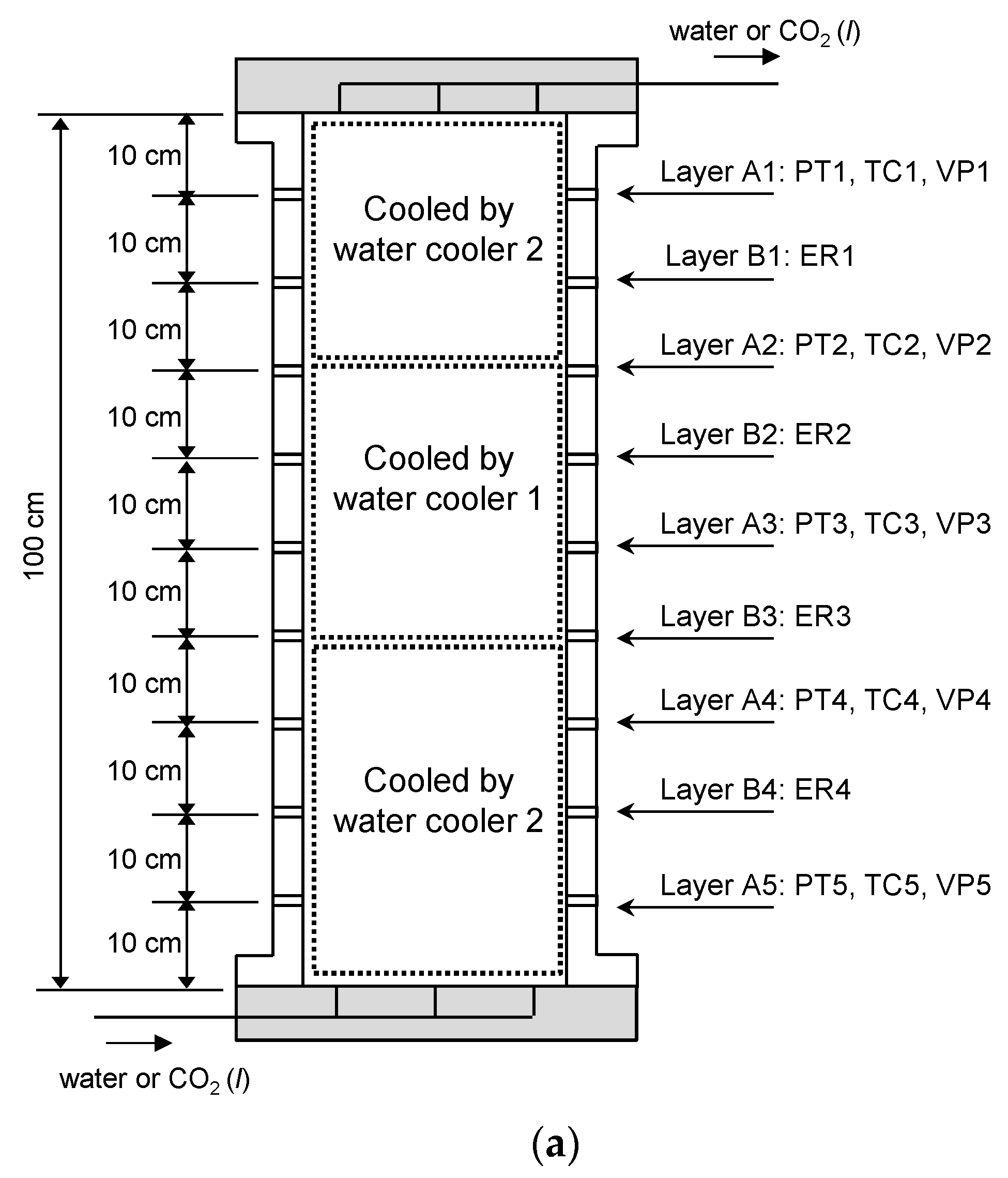
2.2. Experiment Setup
2.3. Experimental Procedure
2.3.1. Water-Saturated Sample Preparation
2.3.2. Injection of CO2 Liquid
2.3.3. Depressurization
3. Experimental Results
3.1. Liquid CO2 Injection Process
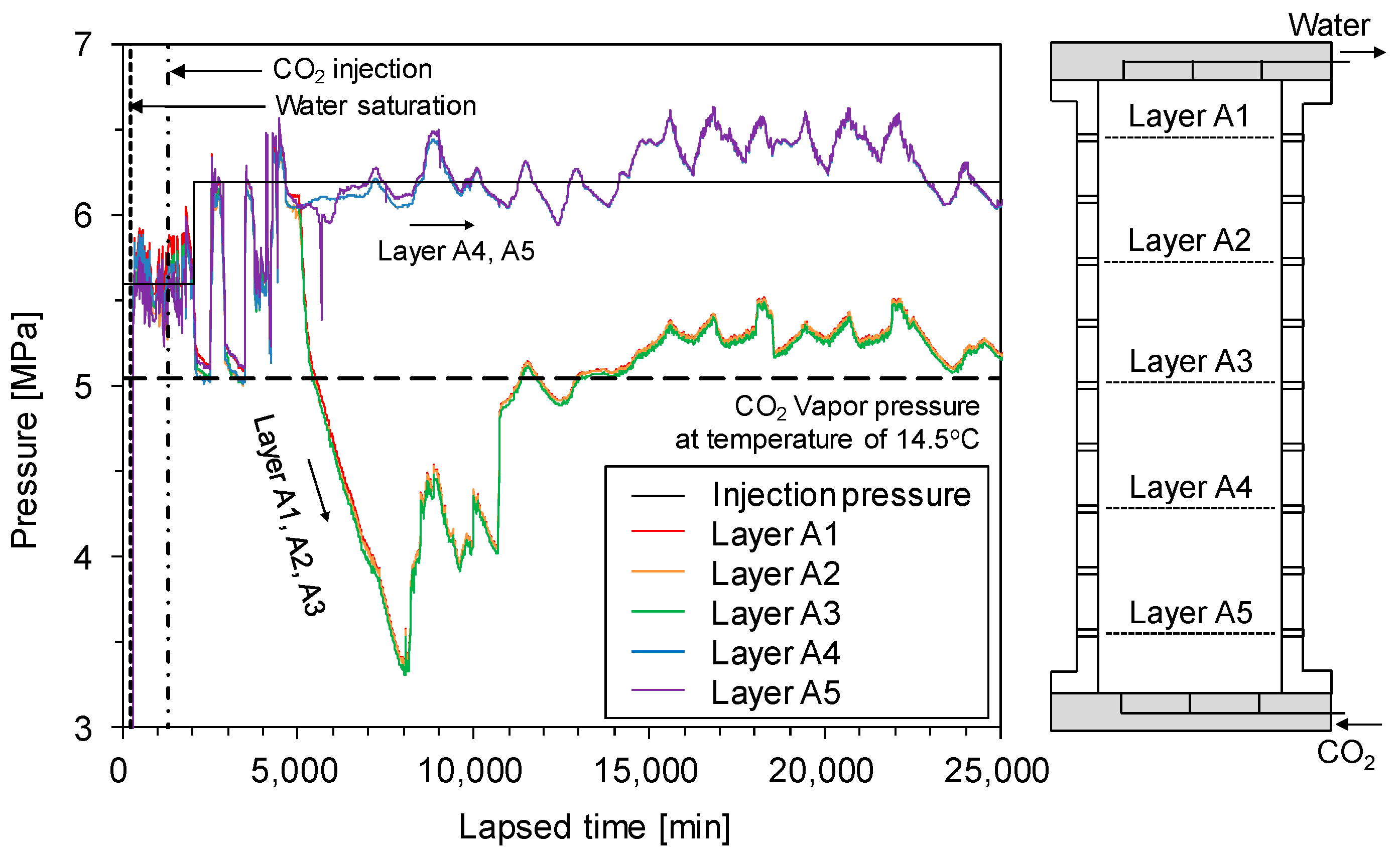
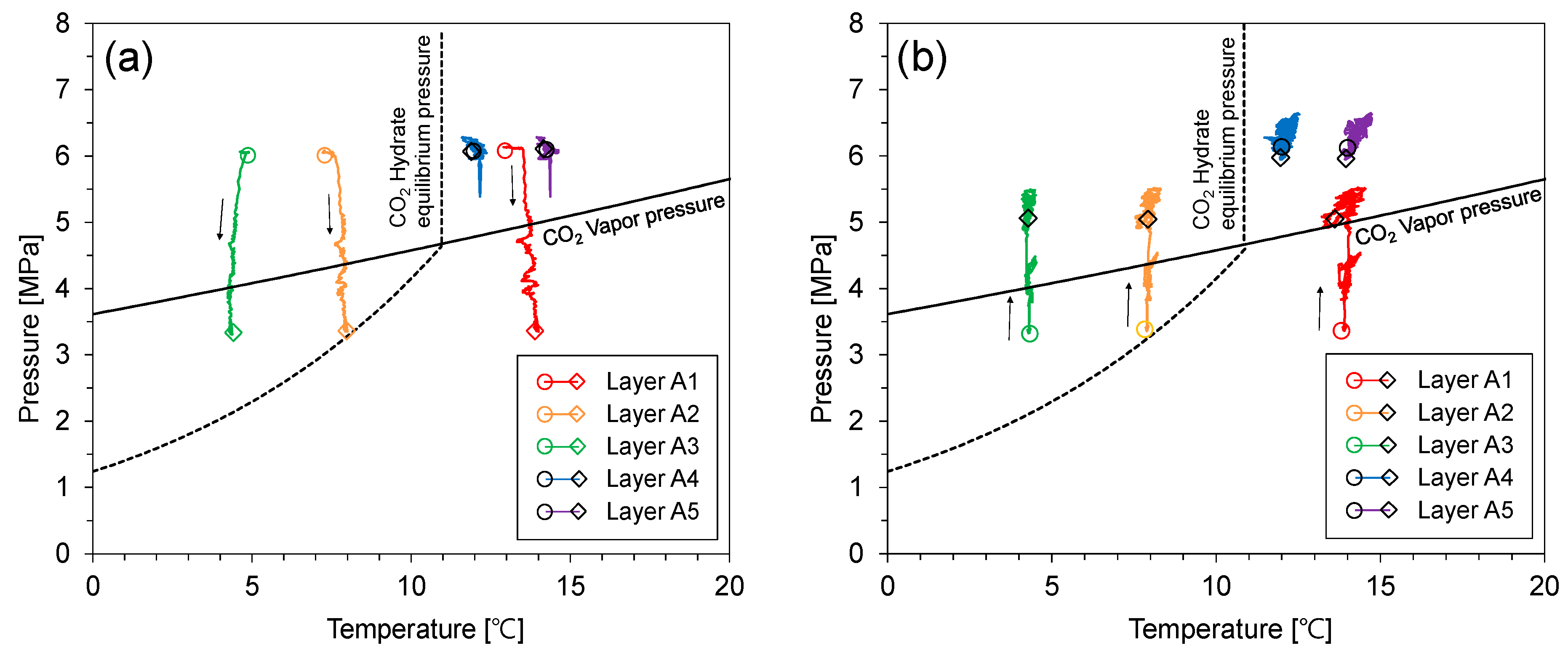
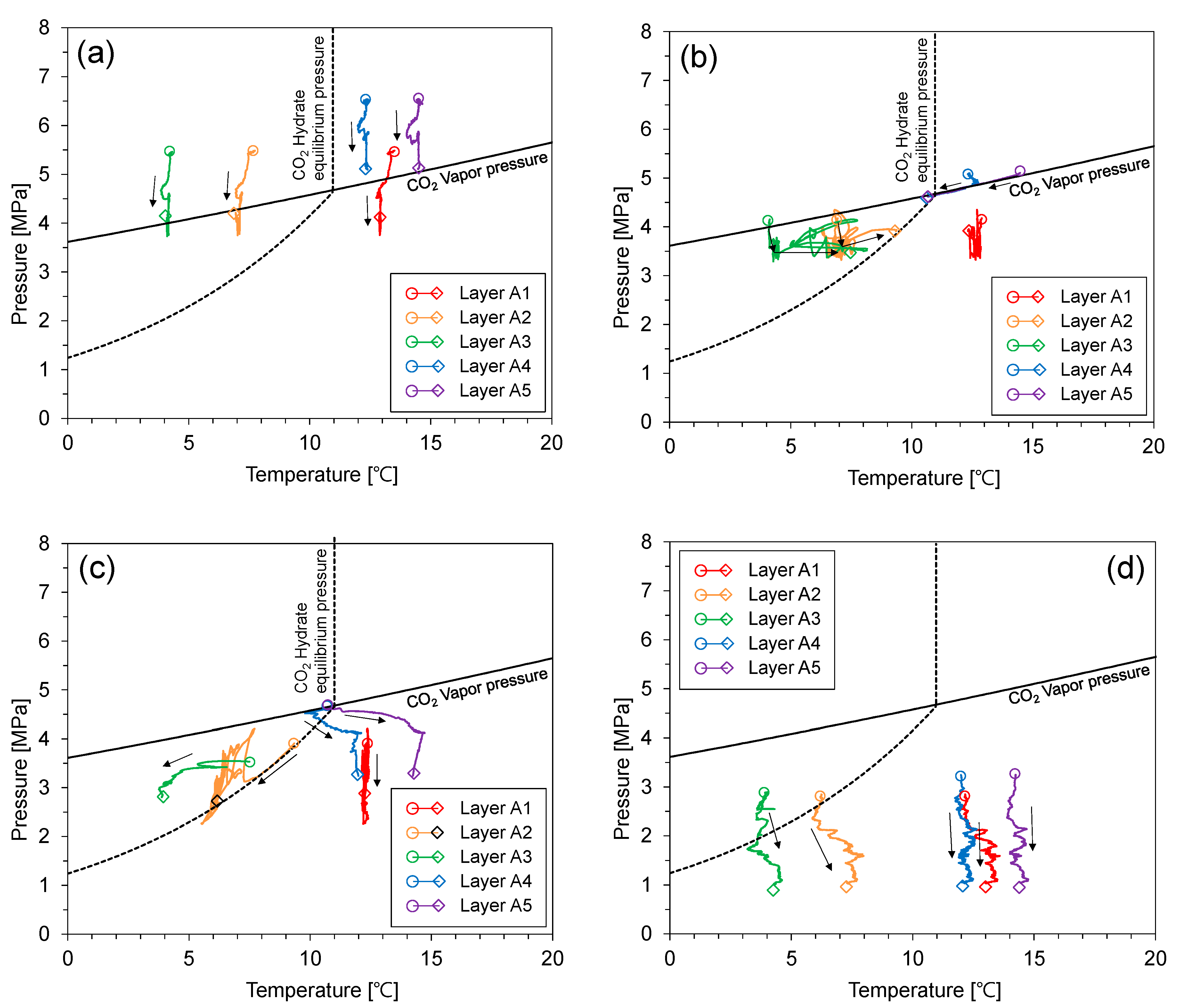
3.1.1. Temperature and Pressure
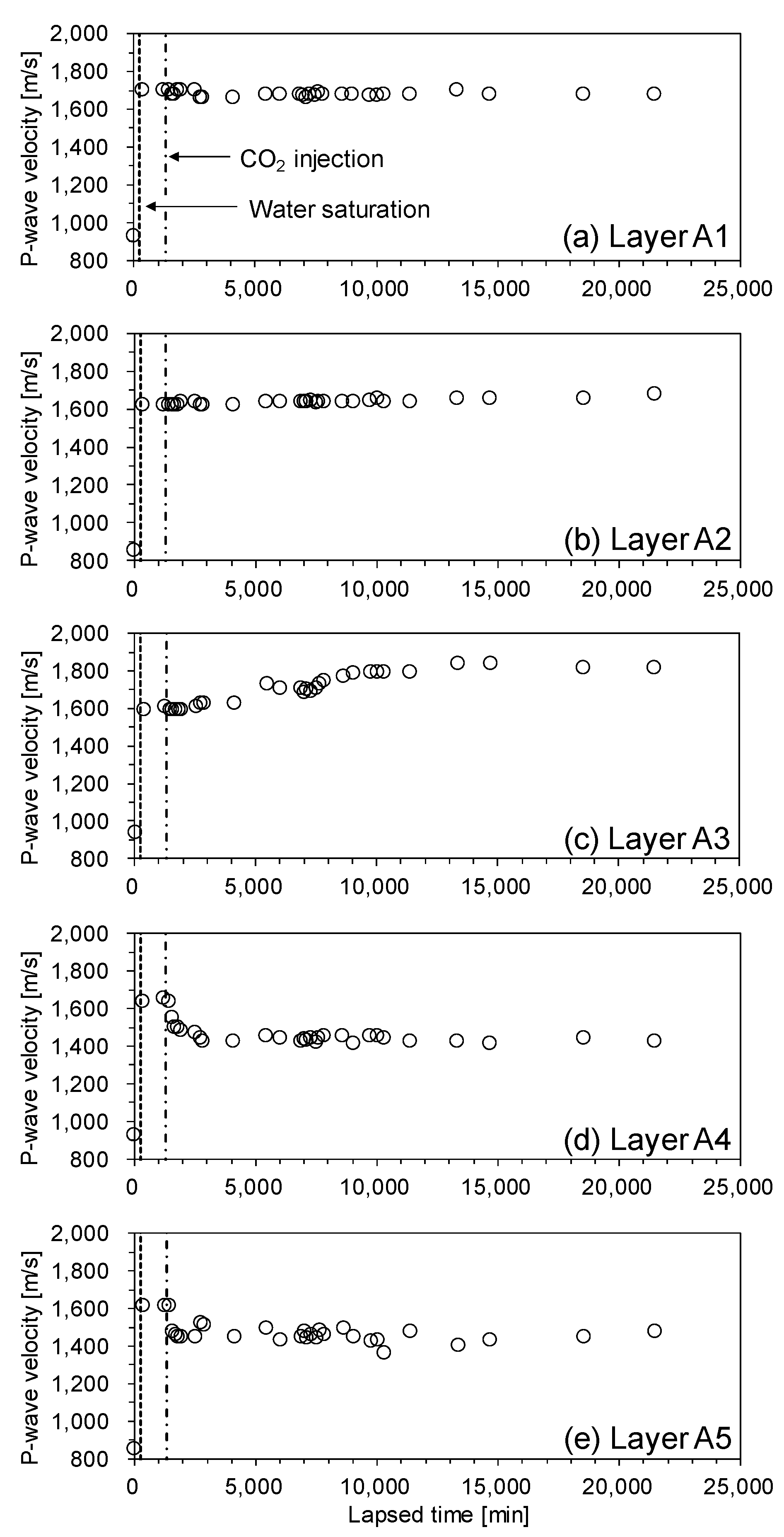
3.1.2. P-Wave Velocity
3.1.3. Electrical Resistance
3.2. Depressurization Process
3.2.1. Temperature and Pressure
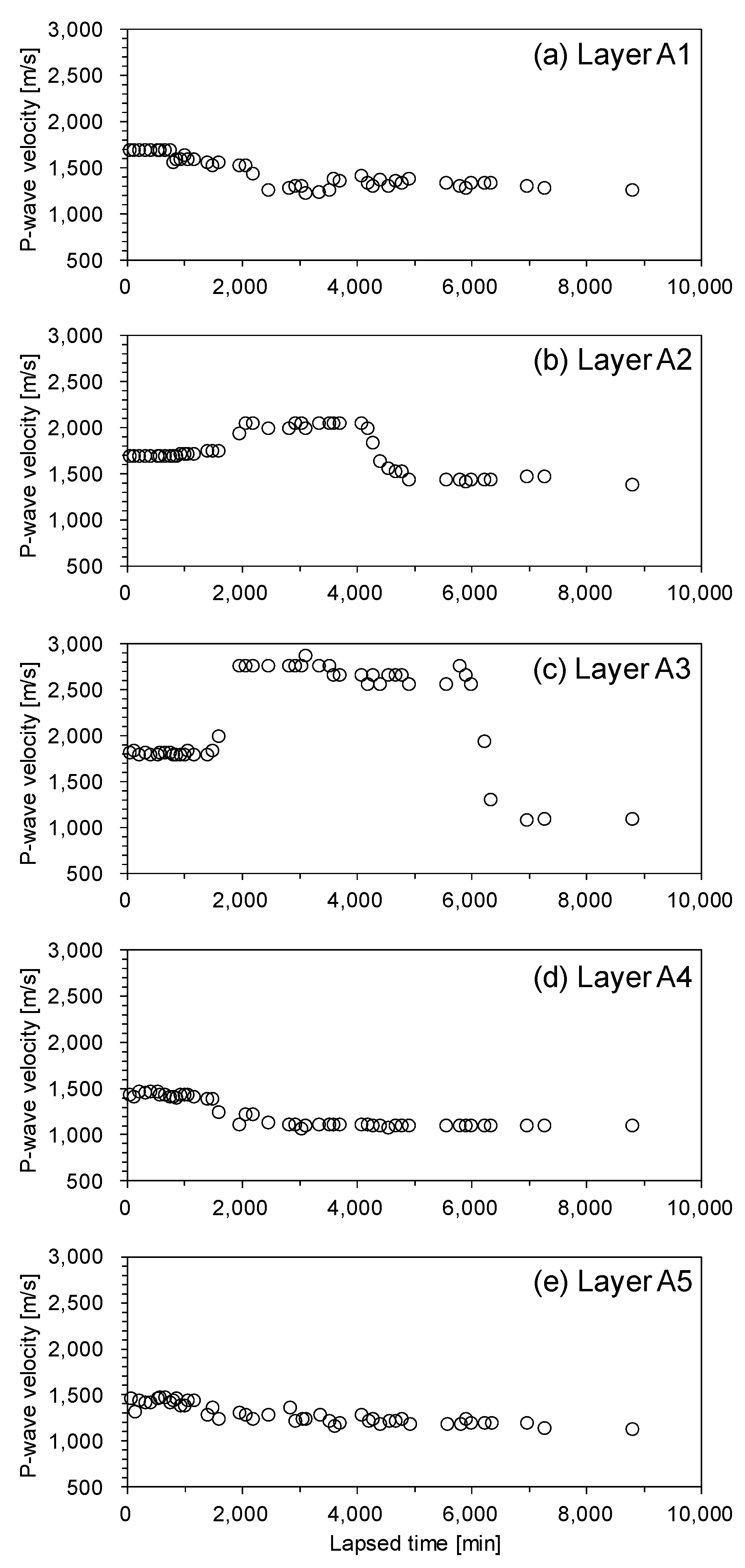
3.2.2. P-Wave Velocity
3.2.3. Electrical Resistance
4. Discussion: Simple Analysis on the Sealing Capacity of CO2 Hydrate-Bearing Sediments
4.1. Capillary Sealing Capacity
4.2. Permeability Sealing Capacity
4.3. Comparison with Other Materials
5. Conclusions
- CO2 hydrates formed in the CO2 hydrate stability zone of the cell during the CO2 liquid injection process. The CO2 hydrate-bearing sediment layer prevented any upward flow of CO2. This self-trapping effect was confirmed by monitoring pressure, P-wave velocity, and electrical resistance.
- The original CO2 hydrates partially dissociated during the depressurization process, and additional CO2 hydrates instantly formed in the upper layer, which was in the CO2 hydrate stability zone. When CO2 hydrates dissociated, CO2 hydrates could re-form in the upper layer (i.e., cooler layer for marine sediments) instantly. This behavior is a positive characteristic of CO2 hydrates for use as cap-rock in CGS applications.
- The CO2 hydrate-bearing sediment layer maintained a pressure of 0.71 MPa during the experiment. Simple analyses revealed that the capillary and permeability sealing capacity of CO2 hydrate-bearing sediments are considerably high.
Author Contributions
Funding
Conflicts of Interest
References
- Hepple, R.; Benson, S. Implications of Surface Seepage on the Effectiveness of Geologic Storage of Carbon Dioxide as a Climate Change Mitigation Strategy. In Proceedings of the Greenhouse Gas Control Technologies—6th International Conference, Kyoto, Japan, 1–4 October 2002. [Google Scholar]
- Shaffer, G. Long-term effectiveness and consequences of carbon dioxide sequestration. Nat. Geosci. 2010, 3, 464. [Google Scholar] [CrossRef]
- Sohrabi, M.; Riazi, M.; Jamiolahmady, M.; Kechut, N.I.; Ireland, S.; Robertson, G. Carbonated water injection (CWI)—A productive way of using CO2 for oil recovery and CO2 storage. Energy Procedia 2011, 4, 2192–2199. [Google Scholar] [CrossRef]
- Sohrabi, M.; Kechut, N.I.; Riazi, M.; Jamiolahmady, M.; Ireland, S.; Robertson, G. Coreflooding Studies to Investigate the Potential of Carbonated Water Injection as an Injection Strategy for Improved Oil Recovery and CO2 Storage. Transp. Porous Media 2012, 91, 101–121. [Google Scholar] [CrossRef]
- Bagalkot, N.; Hamouda, A.A. Diffusion coefficient of CO2 into light hydrocarbons and interfacial tension of carbonated water–hydrocarbon system. J. Geophys. Eng. 2018, 15, 2516–2529. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Bagalkot, N. Effect of salts on interfacial tension and CO2 mass transfer in carbonated water injection. Energies 2019, 12, 748. [Google Scholar] [CrossRef]
- Brewer, P.G. Direct Experiments on the Ocean Disposal of Fossil Fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Kuypers, M.M.M.; Tsunogai, U.; Ishibashi, J.; Nakamura, K.; Treude, T.; Ohkubo, S.; Nakaseama, M.; Gena, K.; Chiba, H.; et al. Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system. Proc. Natl. Acad. Sci. USA 2006, 103, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Nimblett, J.; Ruppel, C. Permeability evolution during the formation of gas hydrates in marine sediments: Gas hydrate and permeability changes. J. Geophys. Res. Solid Earth 2003, 108, 2420. [Google Scholar] [CrossRef]
- Koide, H.; Takahashi, M.; Tsukamoto, H.; Shindo, Y. Self-trapping mechanisms of carbon dioxide in the aquifer disposal. Energy Convers. Manag. 1995, 36, 505–508. [Google Scholar] [CrossRef]
- Tohidi, B.; Yang, J.; Salehabadi, M.; Anderson, R.; Chapoy, A. CO 2 Hydrates Could Provide Secondary Safety Factor in Subsurface Sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Cho, G.C.; Santamarina, J.C. Gas hydrate dissociation in sediments: Pressure-temperature evolution: Gas hydrate dissociation in sediments. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Anomalous Preservation of Pure Methane Hydrate at 1 atm. J. Phys. Chem. B 2001, 105, 1756–1762. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Koide, H.; Takahashi, M.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Hydrate formation in sediments in the sub-seabed disposal of CO2. Energy 1997, 22, 279–283. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochelle, C.A.; Camps, A.P.; Long, D.; Milodowski, A.; Bateman, K.; Gunn, D.; Jackson, P.; Lovell, M.A.; Rees, J. Can CO 2 hydrate assist in the underground storage of carbon dioxide? Geol. Soc. Lond. Spec. Publ. 2009, 319, 171–183. [Google Scholar] [CrossRef]
- Massah, M.; Sun, D.; Sharifi, H.; Englezos, P. Demonstration of gas-hydrate assisted carbon dioxide storage through horizontal injection in lab-scale reservoir. J. Chem. Thermodyn. 2018, 117, 106–112. [Google Scholar] [CrossRef]
- Gauteplass, J.; Almenningen, S.; Ersland, G.; Barth, T. Hydrate seal formation during laboratory CO2 injection in a cold aquifer. Int. J. Greenh. Gas Control 2018, 78, 21–26. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kwon, T.H.; Oh, T.M.; Choo, Y.W.; Lee, C.H.; Lee, K.R.; Cho, G.C. Geomechanical and thermal responses of hydrate-bearing sediments subjected to thermal stimulation: Physical modeling using a geotechnical centrifuge. Energy Fuels 2013, 27, 4507–4522. [Google Scholar] [CrossRef]
- Sloan, E.D. Gas Hydrates: Review of Physical/Chemical Properties. Energy Fuels 1998, 12, 191–196. [Google Scholar] [CrossRef]
- Dai, S.; Santamarina, J.C.; Waite, W.F.; Kneafsey, T.J. Hydrate morphology: Physical properties of sands with patchy hydrate saturation: Patchy hydrate saturation. J. Geophys. Res. Solid Earth 2012, 117. [Google Scholar] [CrossRef]
- Kim, H.S.; Oh, T.M.; Cho, G.C. P-wave velocity estimation of unconsolidated sediments containing CO2. Int. J. Greenh. Gas Control 2015, 33, 18–26. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Börner, J.H.; Herdegen, V.; Repke, J.U.; Spitzer, K. The impact of CO2 on the electrical properties of water bearing porous media—Laboratory experiments with respect to carbon capture and storage. Geophys. Prospect. 2013, 61, 446–460. [Google Scholar] [CrossRef]
- Fleury, M.; Deschamps, H. Electrical conductivity and viscosity of aqueous NaCl solutions with dissolved CO2. J. Chem. Eng. Data 2008, 53, 2505–2509. [Google Scholar] [CrossRef]
- Zhou, X.; Lakkaraju, V.R.; Apple, M.; Dobeck, L.M.; Gullickson, K.; Shaw, J.A.; Cunningham, A.B.; Wielopolski, L.; Spangler, L.H. Experimental observation of signature changes in bulk soil electrical conductivity in response to engineered surface CO2 leakage. Int. J. Greenh. Gas Control 2012, 7, 20–29. [Google Scholar] [CrossRef]
- Buffett, B.A.; Zatsepina, O.Y. Formation of gas hydrate from dissolved gas in natural porous media. Mar. Geol. 2000, 164, 69–77. [Google Scholar] [CrossRef]
- Santamarina, J.C.; Ruppel, C.D. The impact of hydrate saturation on the mechanical, electrical, and thermal properties of hydrate-bearing sand, silts, and clay. In Proceedings of the 6th International Conference on Gas. Hydrates (ICGH 2008), Vancouver, BC, Canada, 6–10 July 2008. [Google Scholar]
- Falenty, A.; Kuhs, W.F. “Self-Preservation” of CO2 gas hydrates-surface microstructure and ice perfection. J. Phys. Chem. B 2009, 113, 15975–15988. [Google Scholar] [CrossRef]
- Berg, R.R. Capillary Pressures in Stratigraphic Traps. AAPG Bull. 1975, 59, 939–956. [Google Scholar]
- Schowalter, T.T. Mechanics of secondary hydrocarbon migration and entrapment. AAPG Bull. 1979, 63, 720–760. [Google Scholar]
- Chun, B.S.; Wilkinson, G.T. Interfacial tension in high-pressure carbon dioxide mixtures. Ind. Eng. Chem. Res. 1995, 34, 4371–4377. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Santamarina, J.C. Water-CO2-mineral systems: Interfacial tension, contact angle, and diffusion-Implications to CO2 geological storage. Water Resour. Res. 2010, 46, W07537. [Google Scholar] [CrossRef]
- Nielsen, L.C.; Bourg, I.C.; Sposito, G. Predicting CO2–water interfacial tension under pressure and temperature conditions of geologic CO2 storage. Geochim. Cosmochim. Acta 2012, 81, 28–38. [Google Scholar] [CrossRef]
- Washburn, E.W. Note on a Method of Determining the Distribution of Pore Sizes in a Porous Material. Proc. Natl. Acad. Sci. USA 1921, 7, 115–116. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Dong, M.; Li, Z.; Huang, S.; Qing, H.; Nickel, E. Gas breakthrough pressure for hydrocarbon reservoir seal rocks: Implications for the security of long-term CO2 storage in the Weyburn field: Gas breakthrough pressure for different gas/liquid systems. Geofluids 2005, 5, 326–334. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology (NIST Chemistry WebBook): Gaithersburg, MD, USA, 1998.
- Taylor, D.W. Fundamentals of Soil Mechanics; Wiley: New York, NY, USA, 1948. [Google Scholar]
- Lambe, T.W.; Whitman, R.V. Soil Mechanics; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Espinoza, D.N.; Santamarina, J.C. CO2 breakthrough—Caprock sealing efficiency and integrity for carbon geological storage. Int. J. Greenh. Gas Control 2017, 66, 218–229. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Q. CO2 breakthrough pressure and permeability for unsaturated low-permeability sandstone of the Ordos Basin. J. Hydrol. 2017, 550, 331–342. [Google Scholar] [CrossRef]
- Angeli, M.; Soldal, M.; Skurtveit, E.; Aker, E. Experimental percolation of supercritical CO2 through a caprock. Energy Procedia 2009, 1, 3351–3358. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Cho, G.-C. Experimental Simulation of the Self-Trapping Mechanism for CO2 Sequestration into Marine Sediments. Minerals 2019, 9, 579. https://doi.org/10.3390/min9100579
Kim H-S, Cho G-C. Experimental Simulation of the Self-Trapping Mechanism for CO2 Sequestration into Marine Sediments. Minerals. 2019; 9(10):579. https://doi.org/10.3390/min9100579
Chicago/Turabian StyleKim, Hak-Sung, and Gye-Chun Cho. 2019. "Experimental Simulation of the Self-Trapping Mechanism for CO2 Sequestration into Marine Sediments" Minerals 9, no. 10: 579. https://doi.org/10.3390/min9100579





