Estimates of scCO2 Storage and Sealing Capacity of the Janggi Basin in Korea Based on Laboratory Scale Experiments
Abstract
:1. Introduction
2. Materials and Methods
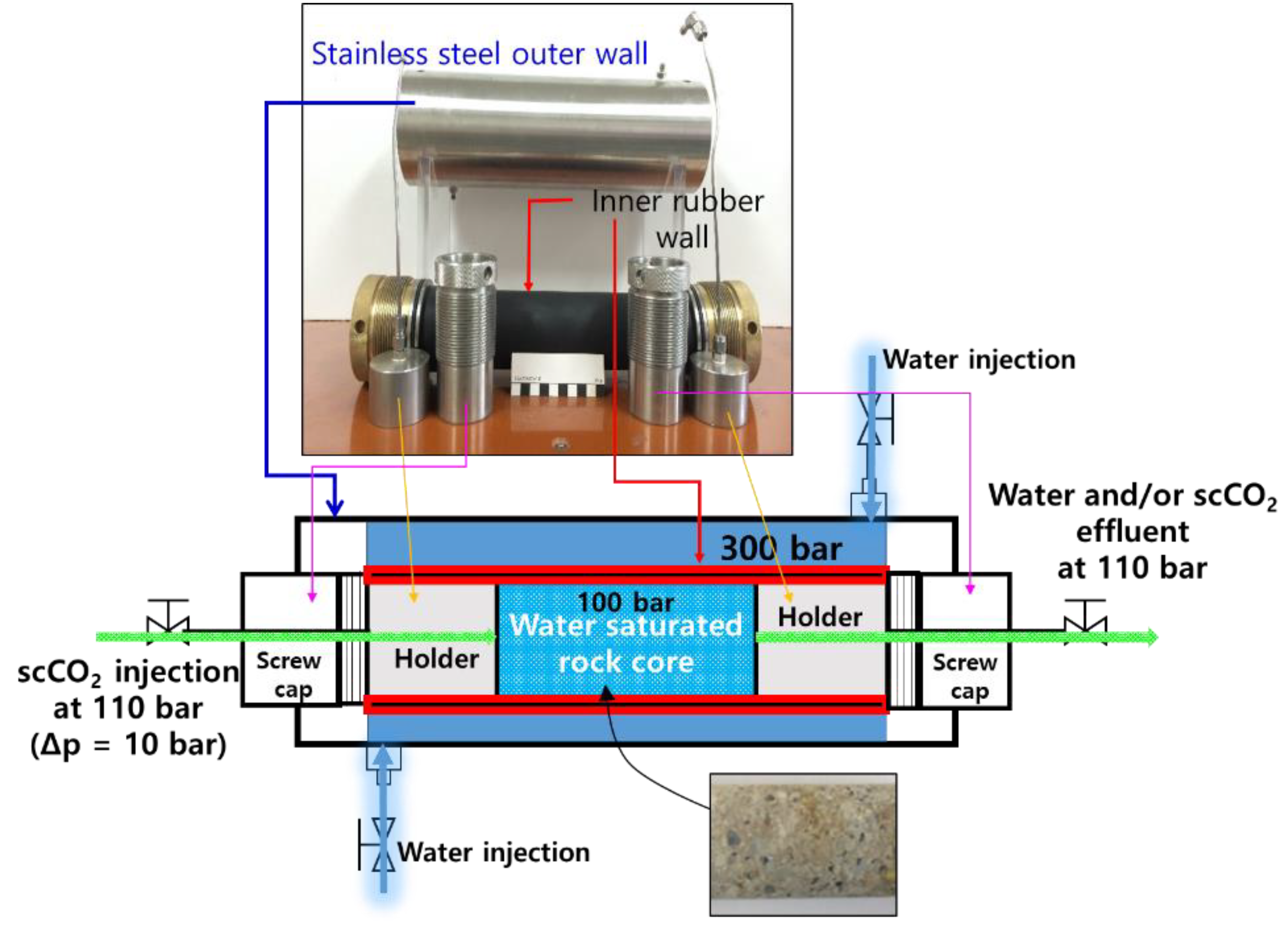
2.1. Preparation of the scCO2 Reservoir and Capping Rock Cores
2.2. Measurement of the scCO2 Storage Ratio for the Conglomerate and Sandstone Cores
2.3. Measurement of the Initial scCO2 Capillary Entry Pressure for Mudstone and Dacitic Tuff
3. Results and Discussion
3.1. Measurement of the scCO2 Storage Ratio for the Conglomerate and Sandstone Cores
3.2. Measurement of the Initial scCO2 Capillary Entry Pressure for Mudstone and Dacitic Tuff
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S. Review of CO2 storage efficiency in deep saline aquifers. Int. J. Greenh. Gas Control 2015, 40, 188–202. [Google Scholar] [CrossRef]
- IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Incheon, Korea, 2018.
- Kim, M.-C.; Kim, J.-S.; Jung, S.; Son, M.; Sohn, Y.K. Bimodal volcanism and classification of the Miocene basin fill in the northern area of the Janggi-myeon, Pohang, Southeast Korea. J. Geol. Soc. Korea 2011, 47, 585–612. [Google Scholar]
- Song, C.W. A study on potential geologic facility sites for carbon dioxide storage in the Miocene Pohang Basin, SE Korea. J. Geol. Soc. Korea 2015, 51, 53–66. [Google Scholar] [CrossRef]
- Kim, M.-C.; Gihm, Y.S.; Son, E.-Y.; Son, M.; Hwang, I.G.; Shinn, Y.J.; Choi, H. Assessment of the potential for geological storage of CO2 based on its structural and sedimentologic characteristics in the Miocene Janggi Basin, SE Korea. J. Geol. Soc. Korea 2015, 51, 253–271. [Google Scholar] [CrossRef]
- Gu, H.-C.; Hwang, I.G. Depositional history of the Janggi Conglomerate controlled by tectonic subsidence, during the early stage of Janggi Basin evolution. J. Geol. Soc. Korea 2017, 53, 221–240. [Google Scholar] [CrossRef]
- Gu, H.-C.; Gim, J.-H.; Hwang, I.G. Variation in depositional environments controlled by tectonics and volcanic activities in the lower part of the Seongdongri Formation, Janggi Basin. J. Geol. Soc. Korea 2018, 54, 21–46. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Lindeberg, E.; Vuillaume, J.F.; Ghaderi, A. Determination of the CO2 storage capacity of the Utsira formation. Energy Procedia 2009, 1, 2777–2784. [Google Scholar] [CrossRef]
- Kopp, A.; Class, H.; Helmig, R. Investigations on CO2 storage capacity in saline aquifers. Part 1. Dimensional analysis of flow processes and reservoir characteristics. Int. J. Greenh. Gas Control 2009, 3, 263–276. [Google Scholar] [CrossRef]
- Pingping, S.; Xinwei, L.; Qiujie, L. Methodology for estimation of CO2 storage capacity in reservoirs. Pet. Explor. Dev. 2009, 36, 216–220. [Google Scholar] [CrossRef]
- Goodman, A.; Hakala, A.; Bromhal, G.; Deel, D.; Rodosta, T.; Frailey, S.; Small, M.; Allen, D.; Romanov, V.; Fazio, J.; et al. U.S. DOE methodology for the development of geologic storage potential for carbon dioxide at the national and regional scale. Int. J. Greenh. Gas Control 2011, 5, 952–965. [Google Scholar] [CrossRef]
- Knopf, S.; May, F. Comparing methods for the estimation of CO2 storage capacity in saline aquifers in Germany: Regional aquifer based vs. structural trap based assessments. Energy Procedia 2017, 114, 4710–4721. [Google Scholar] [CrossRef]
- Elenius, M.; Skurtveit, E.; Yarushina, V.; Baig, I.; Sundal, A.; Wangen, M.; Landschulze, K.; Kaufmann, R.; Choi, J.C.; Hellevang, H.; et al. Assessment of CO2 storage capacity based on sparse data: Skade Formation. Int. J. Greenh. Gas Control 2018, 79, 252–271. [Google Scholar] [CrossRef]
- Doughty, C.; Pruess, K.; Benson, S.; Hovorka, S.D.; Knox, P.R.; Green, C.P. Capacity investigation of brine-bearing sands of the Frio formation for geologic sequestration of CO2. Lawrence Berkeley Natl. Lab. 2001. Available online: http://hdl.handle.net/2152/64418 (accessed on 26 August 2019).
- Zhou, Q.; Birkholzer, J.T.; Tsang, C.F.; Rutqvist, J. A method for quick assessment of CO2 storage capacity in closed and semi-closed saline formations. Int. J. Greenh. Gas Control 2008, 2, 626–639. [Google Scholar] [CrossRef]
- Wang, S.; Kim, J.; Lee, M. Measurement of the scCO2 storage ratio for the CO2 reservoir rocks in Korea. Energy Procedia 2016, 97, 342–347. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, M.; Wang, S. Evaluation of the CO2 storage capacity by the measurement of the scCO2 displacement efficiency for the sandstone and the conglomerate in Janggi Basin. Econ. Environ. Geol. 2016, 49, 469–477. [Google Scholar] [CrossRef]
- Annual Report 2008: NETL (National Energy Technology Laboratory) Carbon Sequestration ATLAS of the United States and Canada; DOE: Washington, DC, USA, 2008.
- Tasianas, A.; Koukouzas, N. CO2 storage capacity estimate in the lithology of the mesohellenic trough, Greece. Energy Procedia 2016, 86, 334–341. [Google Scholar] [CrossRef]
- An, J.; Lee, M.; Wang, S. Evaluation of the sealing capacity of the supercritical CO2 by the measurement of its injection pressure into the tuff and the mudstone in the Janggi Basin. Econ. Environ. Geol. 2017, 50, 303–311. [Google Scholar]
- Iglauer, S. Optimum storage depths for strucural CO2 trapping. Int. J. Greenh. Gas Control 2018, 77, 82–87. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Park, J.; Baek, K.; Lee, M.; Wang, S. Physical property changes of sandstones in Korea derived from the supercritical CO2–sandstone–groundwater geochemical reaction under CO2 sequestration condition. Geosci. J. 2015, 19, 313–324. [Google Scholar] [CrossRef]
- Jun, Y.S.; Giammar, D.E.; Werth, C.J. Impacts of geochemical reactions on geologic carbon sequestration. Environ. Sci. Technol. 2013, 47, 3–8. [Google Scholar] [CrossRef]
- Park, J.; Baek, K.; Lee, M.; Chung, C.-W.; Wang, S. The use of the surface roughness value to quantify the extent of supercritical CO2 involved geochemical reaction at a CO2 sequestration site. Appl. Sci. 2017, 7, 572. [Google Scholar] [CrossRef]
- IPCC Special Report on Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Lodon, UK, 2005. [Google Scholar]
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Wigand, M.; Carey, J.W.; Schütt, H.; Spangenberg, E.; Erzinger, J. Geochemical effects of CO2 sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Appl. Geochem. 2008, 23, 2735–2745. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, W.; Zhang, F.; Lu, C.; Du, J.; Zhu, R.; Sun, L. Evaluation of CO2 solubility-trapping and mineral-trapping in microbial-mediated CO2–brine–sandstone interaction. Mar. Pollut. Bull. 2014, 85, 78–85. [Google Scholar] [CrossRef]
- Dawson, G.K.W.; Pearce, J.K.; Biddle, D.; Golding, S.D. Experimental mineral dissolution in Berea Sandstone reacted with CO2 or SO2–CO2 in NaCl brine under CO2 sequestration conditions. Chem. Geol. 2015, 399, 87–97. [Google Scholar] [CrossRef]
- Mediato, J.F.; García-Crespo, J.; Izquierdo, E.; García-Lobón, J.L.; Ayala, C.; Pueyo, E.L.; Molinero, R. Three-dimensional reconstruction of the caspe geological structure (Spain) for evaluation as a potential CO2 storage site. Energy Procedia 2017, 114, 4486–4493. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. CO2–H2O mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12–100 °C and up to 600 bar. Geochim. Cosmochim. Acta 2005, 69, 3309–3320. [Google Scholar] [CrossRef]
- Sendula, E.; Páles, M.; Szabó, B.P.; Udvardi, B.; Kovács, I.; Kónya, P.; Freiler, Á.; Besnyi, A.; Király, C.; Székely, E.; et al. Experimental study of CO2-saturated water-illite/kaolinite/montmorillonite system at 70–80 °C, 100–105 bar. Energy Procedia 2017, 114, 4934–4947. [Google Scholar] [CrossRef]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale alteration after exposure to supercritical CO2. Int. J. Greenh. Gas Control 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Luo, X.; Ren, X.; Wang, S. Supercritical CO2–water–shale interactions under supercritical CO2 simulation conditions. Energy Procedia 2018, 144, 182–185. [Google Scholar] [CrossRef]
 ) in the Janggi Basin, Korea, photographs of rock cores used for the experiment and the stratigraphic columnar section (from left to right) used for the experiment (modified from [7,8]).
) in the Janggi Basin, Korea, photographs of rock cores used for the experiment and the stratigraphic columnar section (from left to right) used for the experiment (modified from [7,8]).



| Rock Type | Mineral Portion (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartz | K-Feldspar | Plagioclase | Rock Fragment | Fine Matrix and Clay | Calcite | Micas | Others | |
| JG1-C1 | 7.8 | 1.8 | 1.2 | 80.6 | 8.0 | 0.2 | 0.0 | 0.4 |
| JG1-C2 | 17.8 | 6.6 | 4.8 | 57.4 | 10.8 | 0.4 | 1.2 | 1.0 |
| JG1-C3 | 6.4 | 1.8 | 1.0 | 85.4 | 3.8 | 0.4 | 0.4 | 0.8 |
| Average ± standard deviation | 10.7 ± 6.2 | 3.4 ± 2.8 | 2.3 ± 2.1 | 74.5 ± 15.0 | 7.5 ± 3.5 | 0.3 ± 0.1 | 0.5 ± 0.6 | 0.7 ± 0.3 |
| JG1-S1 | 33.6 | 8.8 | 12.0 | 24.8 | 16.0 | 2.2 | 1.2 | 1.4 |
| JG1-S2 | 31.6 | 11.6 | 8.2 | 24.2 | 18.4 | 0.6 | 4.4 | 1.0 |
| JG1-S3 | 32.2 | 10.4 | 7.0 | 22.4 | 20.4 | 4.6 | 1.8 | 1.2 |
| Average ± standard deviation | 32.5 ± 1.0 | 10.3 ± 1.4 | 9.1 ± 2.6 | 23.8 ± 1.2 | 18.3 ± 2.2 | 2.5 ± 2.0 | 2.5 ± 1.7 | 1.2 ± 0.2 |
| JG1-M1 | 40.8 | 3.0 | 1.4 | 0.4 | 47.4 | 1.2 | 2.6 | 3.2 |
| JG1-M2 | 43.8 | 2.8 | 0.6 | 0.0 | 47.4 | 0.4 | 2.4 | 2.6 |
| JG1-M3 | 33.6 | 2.4 | 0.4 | 0.2 | 58.6 | 0.2 | 2.2 | 2.4 |
| Average ± standard deviation | 39.4 ± 5.2 | 2.7 ± 0.3 | 0.8 ± 0.5 | 0.2 ± 0.2 | 51.1 ± 6.5 | 0.6 ± 0.5 | 2.4 ± 0.2 | 2.7 ± 0.4 |
| JG1-T1 | 26.0 | 12.2 | 11.6 | 18.6 | 19.6 | 1.4 | 3.8 | 6.8 |
| JG1-T2 | 19.4 | 10.4 | 14.8 | 11.6 | 32.4 | 1.2 | 4.2 | 6.0 |
| JG1-T3 | 24.5 | 9.7 | 13.2 | 16.7 | 22.0 | 2.4 | 3.1 | 8.4 |
| Average ± standard deviation | 23.3 ± 3.5 | 10.8 ± 1.3 | 13.2 ± 1.6 | 15.6 ± 3.6 | 24.7 ± 6.8 | 1.7 ± 0.6 | 3.7 ± 0.6 | 7.1 ± 1.2 |
| Core Number | Rock Type | Porosity (%) | The scCO2 Storage Ratio (%) |
|---|---|---|---|
| JG1-C1 | Conglomerate | 19.23 | 23.56 |
| JG1-C2 | 18.92 | 35.41 | |
| JG1-C3 | 15.12 | 31.20 | |
| Average | 17.76 | 30.72 | |
| JG1-S1 | Rudaceous sandstone | 10.54 | 13.12 |
| JG1-S2 | 16.44 | 16.28 | |
| JG1-S3 | 17.26 | 9.64 | |
| Average | 14.75 | 13.01 |
| Rock Type | The Calculated Amount of scCO2 Storage for A Conglomerate and Rudaceous Formation (metric ton) |
|---|---|
| Conglomerate | V(50 m × 250 m × 1000 m) × φ(0.1776) × ρ*(400 kg/m3) × ε(0.3072) = 272,793.6 |
| Rudaceous sandstone | V(50 m × 250 m × 1000 m) × φ(0.1475) × ρ*(400 kg/m3) × ε(0.1301) = 95,948.8 |
| Composition | Ratio (wt. %) | |||||
|---|---|---|---|---|---|---|
| JG1-M1 | JG1-M2 | JG1-T1 | ||||
| Before | After 90-day Reaction | Before | After 90-day Reaction | Before | After 90-day Reaction | |
| SiO2 | 57.20 | 56.51 | 56.36 | 55.65 | 57.01 | 56.61 |
| Al2O3 | 20.84 | 20.89 | 17.88 | 17.94 | 18.21 | 18.33 |
| TiO2 | 0.78 | 0.78 | 0.62 | 0.62 | 0.76 | 0.76 |
| Fe2O3 | 5.32 | 5.29 | 4.99 | 5.06 | 7.04 | 7.10 |
| MnO | 0.09 | 0.08 | 0.12 | 0.12 | 0.15 | 0.15 |
| MgO | 0.79 | 0.92 | 0.71 | 0.81 | 1.95 | 2.04 |
| CaO | 1.14 | 1.08 | 1.16 | 1.09 | 5.03 | 4.64 |
| Na2O | 1.63 | 1.73 | 1.32 | 1.36 | 2.55 | 2.60 |
| K2O | 2.30 | 2.40 | 2.04 | 2.09 | 0.95 | 0.94 |
| P2O5 | 0.09 | 0.09 | 0.15 | 0.15 | 0.05 | 0.05 |
| LOI | 9.61 | 10.07 | 14.44 | 14.96 | 6.12 | 6.58 |
| Total | 99.80 | 99.85 | 99.79 | 99.85 | 99.82 | 99.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yang, M.; Kim, S.; Lee, M.; Wang, S. Estimates of scCO2 Storage and Sealing Capacity of the Janggi Basin in Korea Based on Laboratory Scale Experiments. Minerals 2019, 9, 515. https://doi.org/10.3390/min9090515
Park J, Yang M, Kim S, Lee M, Wang S. Estimates of scCO2 Storage and Sealing Capacity of the Janggi Basin in Korea Based on Laboratory Scale Experiments. Minerals. 2019; 9(9):515. https://doi.org/10.3390/min9090515
Chicago/Turabian StylePark, Jinyoung, Minjune Yang, Seyoon Kim, Minhee Lee, and Sookyun Wang. 2019. "Estimates of scCO2 Storage and Sealing Capacity of the Janggi Basin in Korea Based on Laboratory Scale Experiments" Minerals 9, no. 9: 515. https://doi.org/10.3390/min9090515





