Latticed Gold Nanoparticle Conjugation via Monomeric Streptavidin in Lateral Flow Assay for Detection of Autoantibody to Interferon-Gamma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval
2.3. Type of Cells
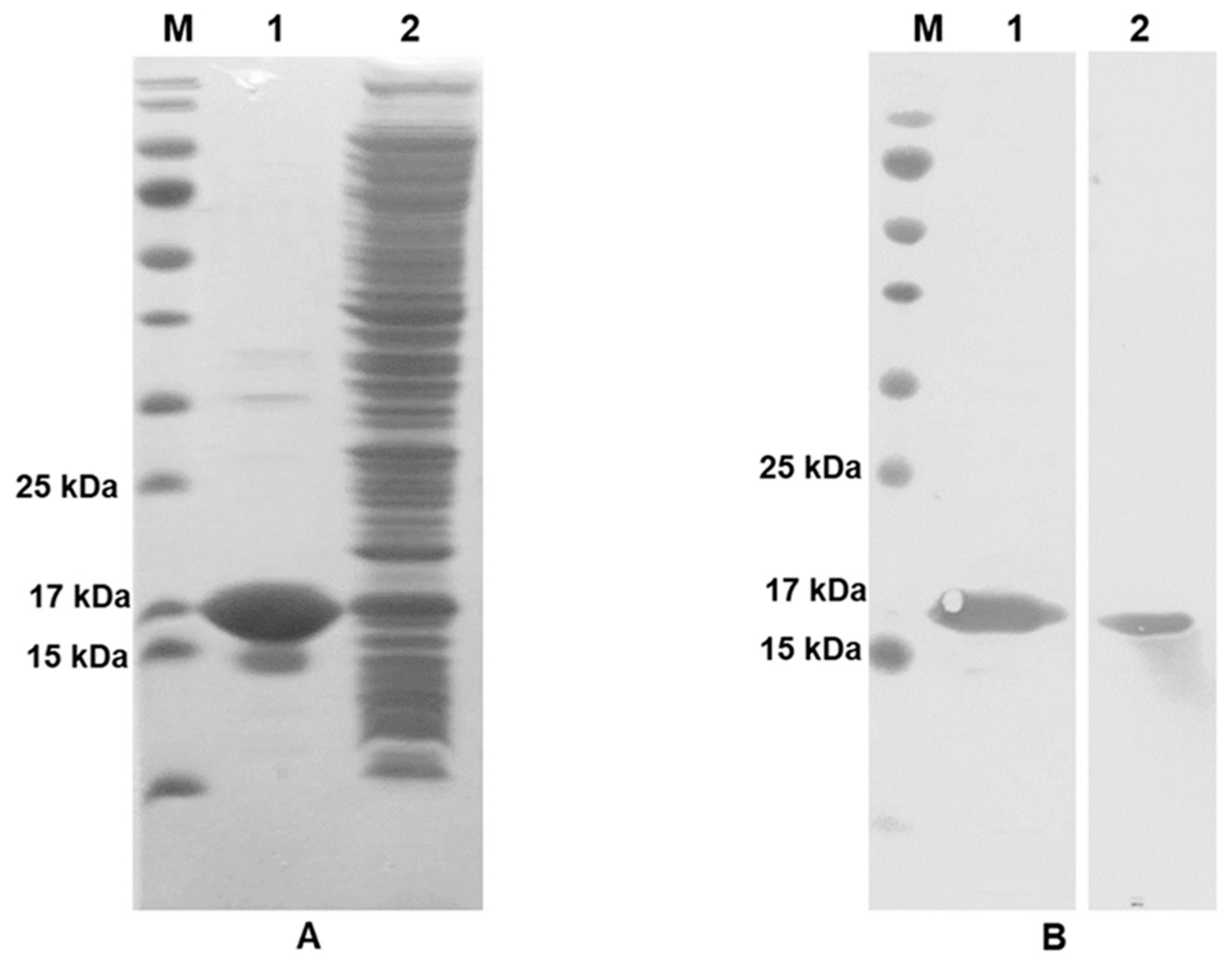
2.4. Production of IFN-γ Protein
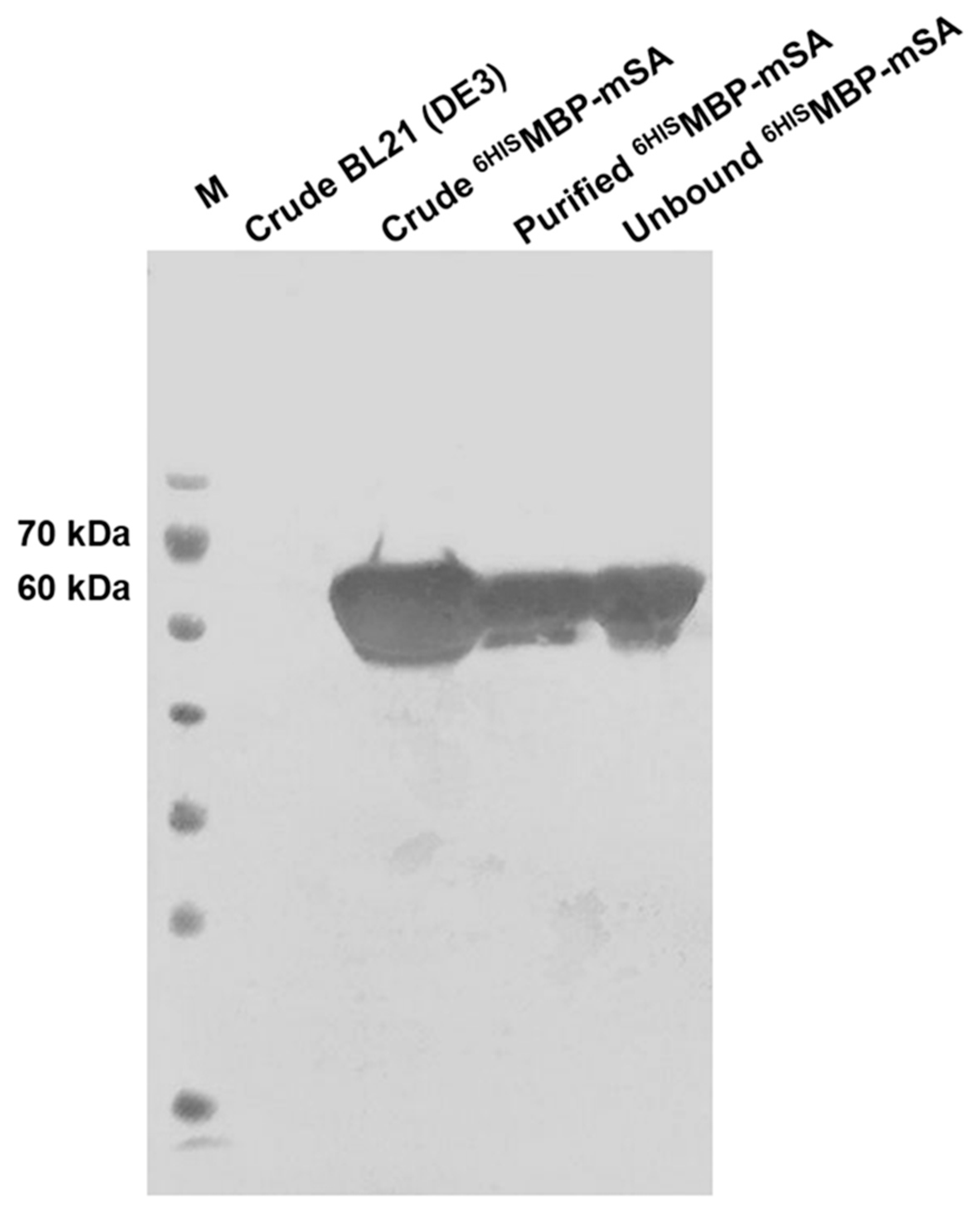
2.5. Expression of 6HISMBP-mSA
2.6. Detection of Anti-IFN-γ AutoAbs Using Conventional Indirect ELISA Assay
2.7. Biotinylation of Recombinant IFN-γ
2.8. Preparation of Colloidal Gold Nanoparticles
2.9. Synthesis of mSA-Colloidal Gold Conjugates
2.10. Binding Activity of the Conjugation Material
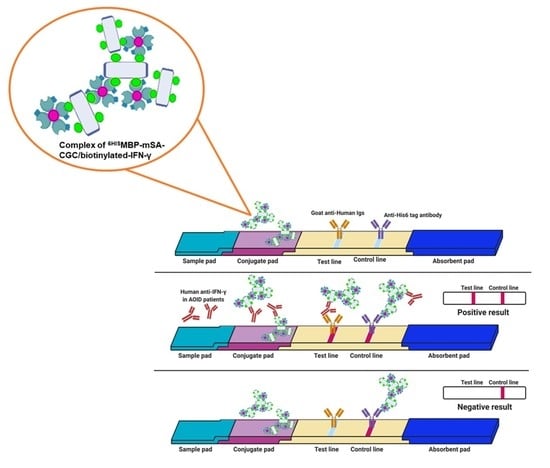
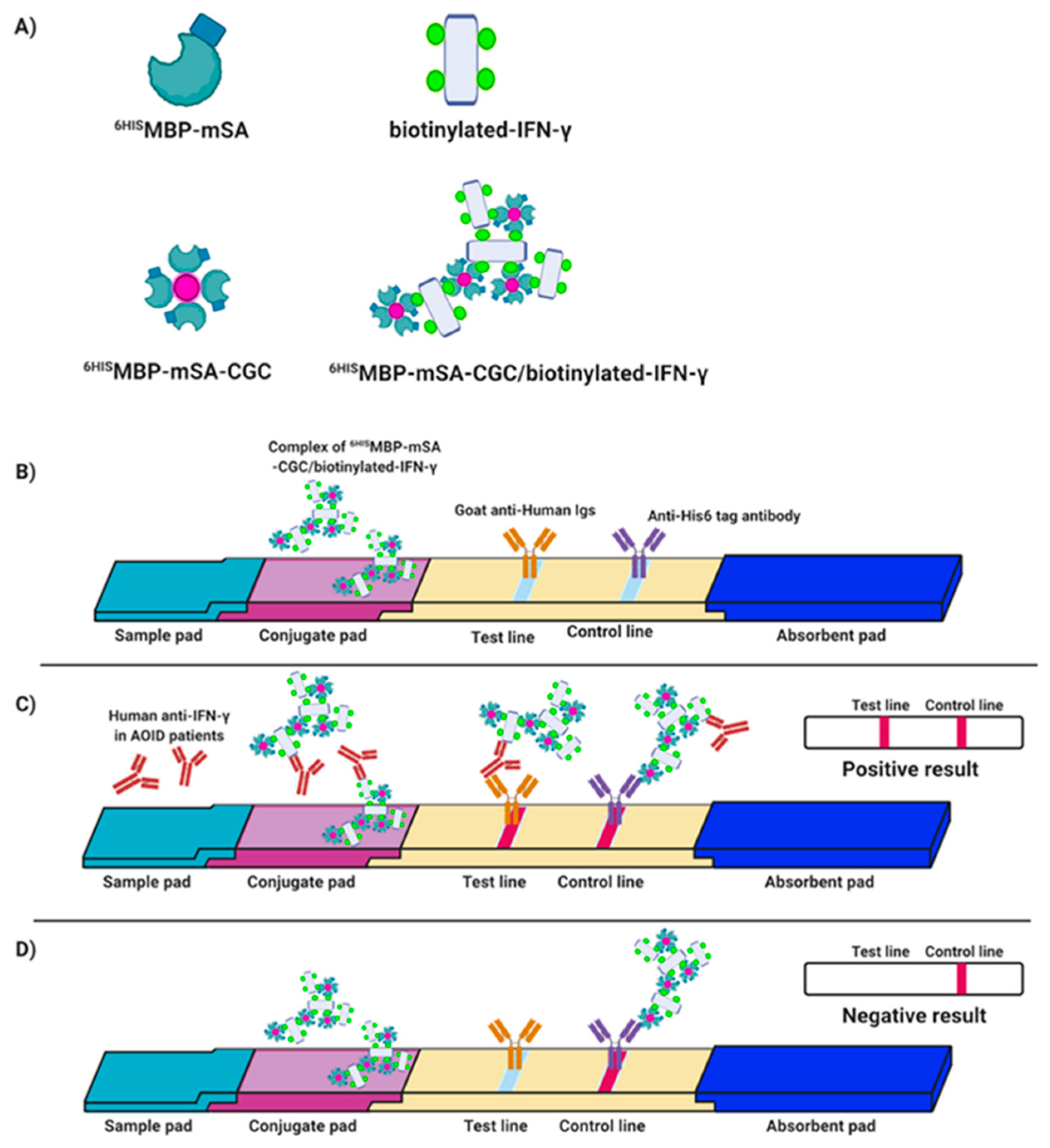
2.11. Evaluation of Indirect IC Strip for Anti-IFN-γ mAb Detection
2.12. Preparation of the IC Strip Test
2.13. Statistical Analysis
3. Results
3.1. Validation of Recombinant IFN-γ Expression
3.2. Determination of 6HISMBP-mSA Expression
3.3. Detection of AutoAbs against IFN-γ Using Conventional Indirect ELISA
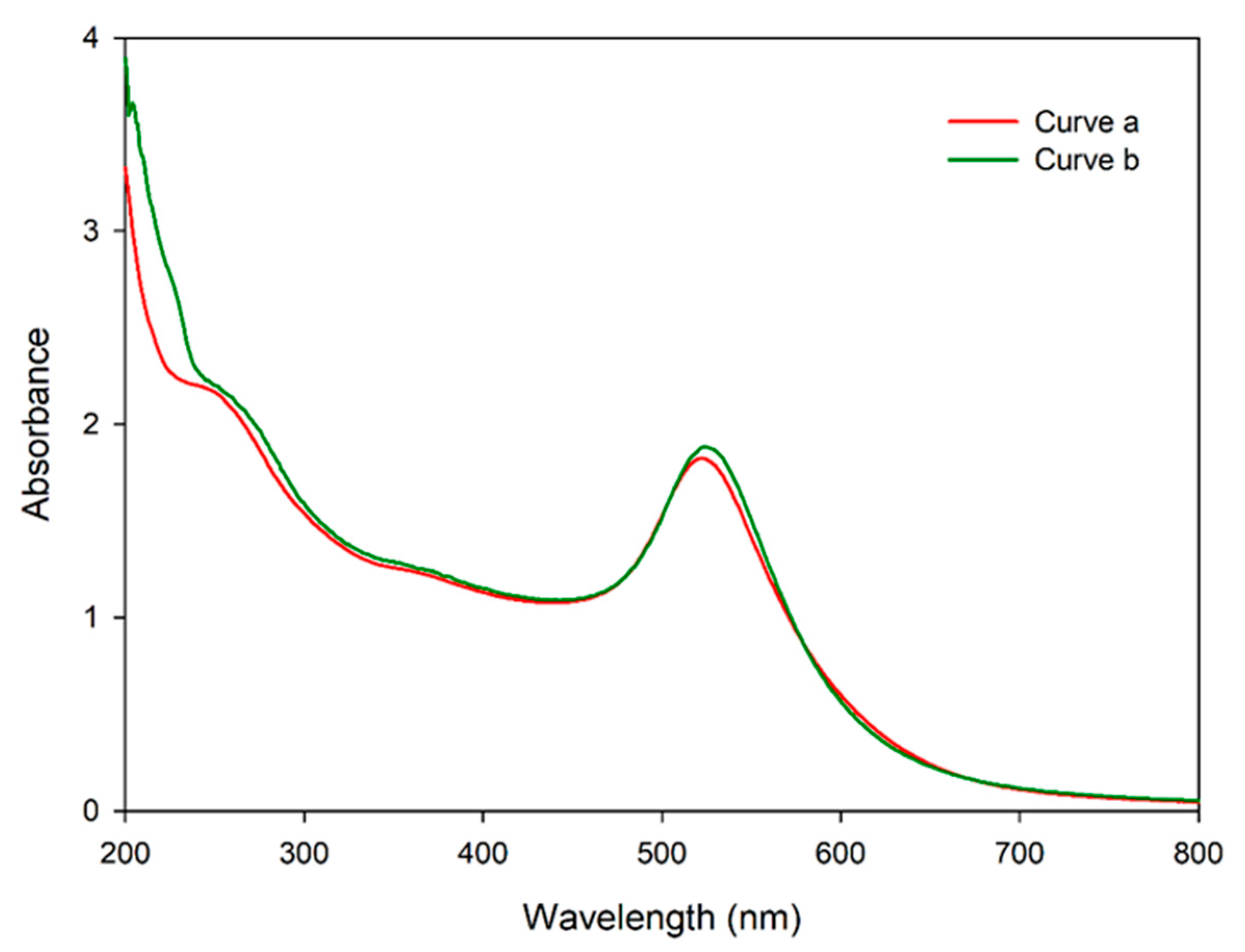
3.4. Validation of mSA-CGC/Biotinylated-IFN-γ Complex
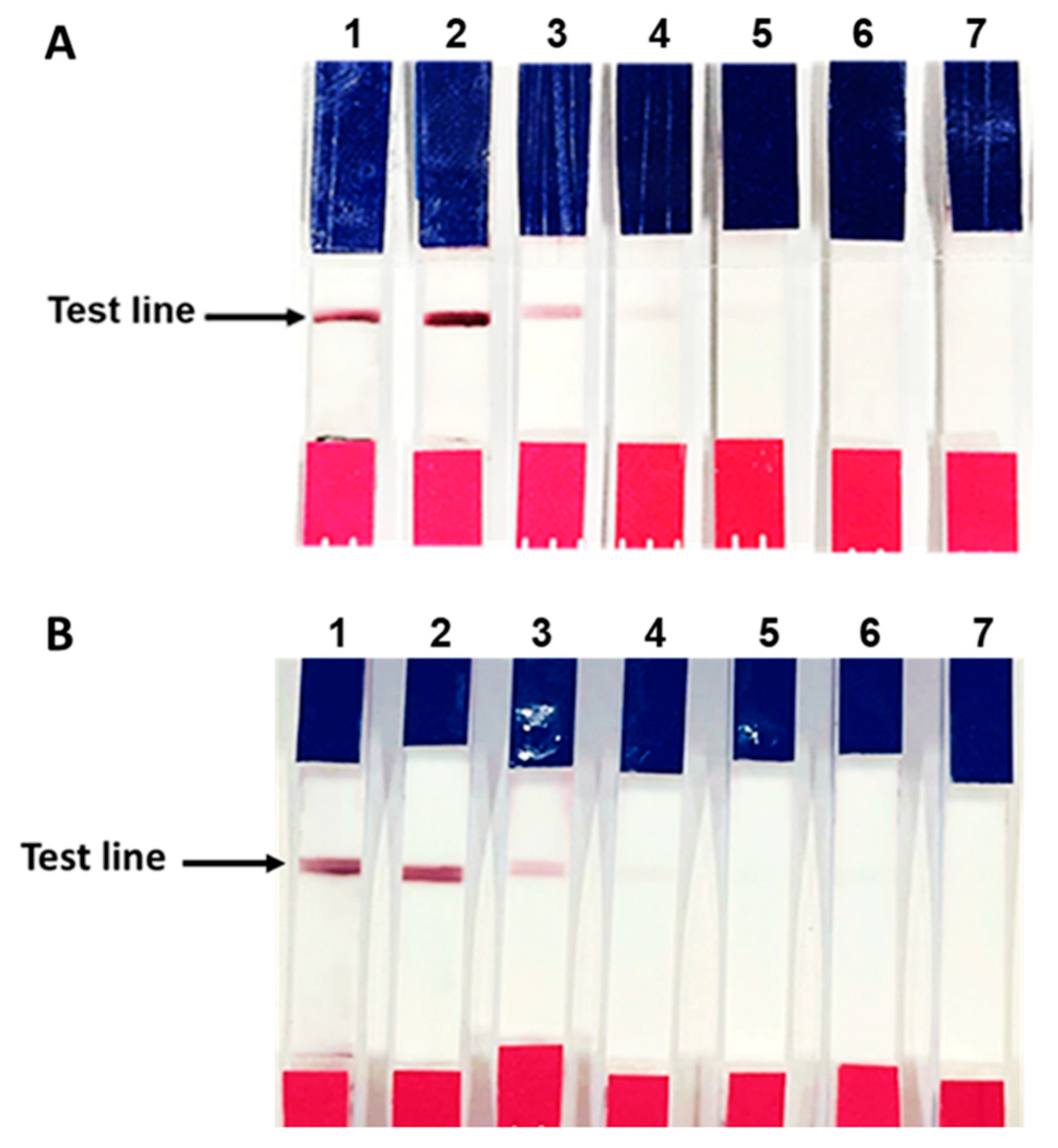
3.5. Verification of the Fabricated IC Strip Platform
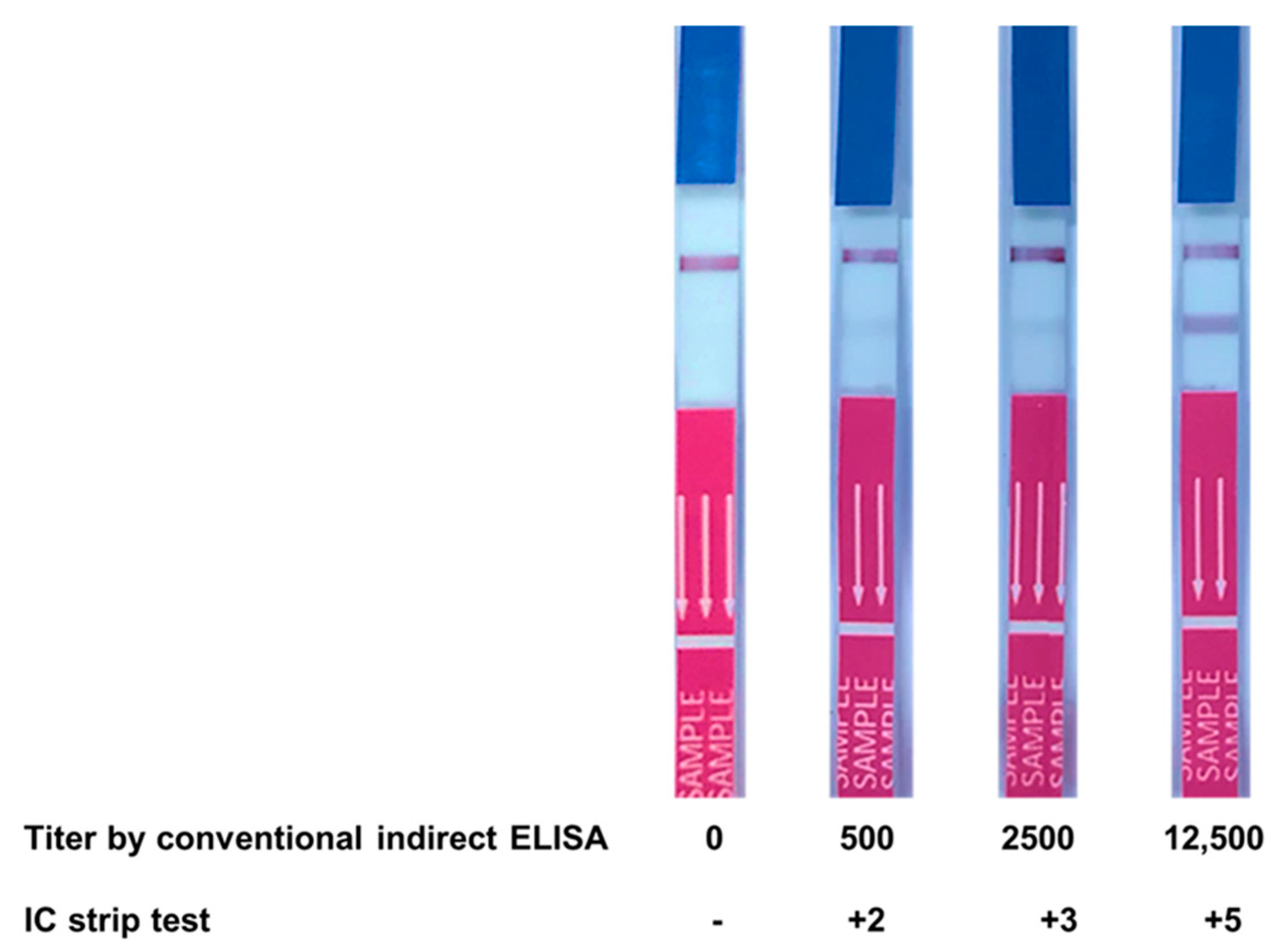
3.6. Evaluation of Clinical Samples Using IC Strip Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Billiau, A.; Matthys, P. Interferon-gamma: A historical perspective. Cytokine Growth Factor Rev. 2009, 20, 97–113. [Google Scholar] [CrossRef]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef] [Green Version]
- Camargo, J.F.; Lobo, S.A.; Hsu, A.P.; Zerbe, C.S.; Wormser, G.P.; Holland, S.M. MonoMAC syndrome in a patient with a GATA2 mutation: Case report and review of the literature. Clin. Infect. Dis. 2013, 57, 697–699. [Google Scholar] [CrossRef] [Green Version]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Wongkulab, P.; Wipasa, J.; Chaiwarith, R.; Supparatpinyo, K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS ONE 2013, 8, e76371. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.L.; Rosen, L.B.; Sereti, I.; Barber, D.L.; Chen, R.Y.; Hsu, D.C.; Qasba, S.S.; Zerbe, C.S.; Holland, S.M.; Browne, S.K. Severe Paradoxical Reaction During Treatment of Disseminated Tuberculosis in a Patient With Neutralizing Anti-IFNgamma Autoantibodies. Clin. Infect. Dis. 2016, 62, 770–773. [Google Scholar] [CrossRef] [Green Version]
- Wipasa, J.; Wongkulab, P.; Chawansuntati, K.; Chaiwarit, R.; Supparatpinyo, K. Cellular immune responses in HIV-negative immunodeficiency with anti-interferon-gamma antibodies and opportunistic intracellular microorganisms. PLoS ONE 2014, 9, e110276. [Google Scholar] [CrossRef]
- Chruewkamlow, N.; Mahasongkram, K.; Pata, S.; Chaiwarith, R.; Salee, P.; Supparatpinyo, K.; Kasinrerk, W. Immune Alterations in Patients with Anti-Interferon-gamma Autoantibodies. PLoS ONE 2016, 11, e0145983. [Google Scholar] [CrossRef]
- Wongtrakul, J.; Thongtan, T.; Roytrakul, S.; Praparattanapan, J.; Wipasa, J.; Kumrapich, B.; Supparatpinyo, K. Identification of novel biomarkers for adult-onset-immunodeficiency (AOID) syndrome using serum proteomics. Asian Pac. J. Trop. Med. 2017, 10, 445–452. [Google Scholar] [CrossRef]
- Browne, S.K.; Zaman, R.; Sampaio, E.P.; Jutivorakool, K.; Rosen, L.B.; Ding, L.; Pancholi, M.J.; Yang, L.M.; Priel, D.L.; Uzel, G.; et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood 2012, 119, 3933–3939. [Google Scholar] [CrossRef]
- Doffinger, R.; Helbert, M.R.; Barcenas-Morales, G.; Yang, K.; Dupuis, S.; Ceron-Gutierrez, L.; Espitia-Pinzon, C.; Barnes, N.; Bothamley, G.; Casanova, J.L.; et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 2004, 38, e10–e14. [Google Scholar] [CrossRef]
- Koya, T.; Tsubata, C.; Kagamu, H.; Koyama, K.; Hayashi, M.; Kuwabara, K.; Itoh, T.; Tanabe, Y.; Takada, T.; Gejyo, F. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J. Infect. Chemother. 2009, 15, 118–122. [Google Scholar] [CrossRef]
- Holland, S.M.; Eisenstein, E.M.; Kuhns, D.B.; Turner, M.L.; Fleisher, T.A.; Strober, W.; Gallin, J.I. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N. Engl. J. Med. 1994, 330, 1348–1355. [Google Scholar] [CrossRef]
- Patel, S.Y.; Ding, L.; Brown, M.R.; Lantz, L.; Gay, T.; Cohen, S.; Martyak, L.A.; Kubak, B.; Holland, S.M. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 2005, 175, 4769–4776. [Google Scholar] [CrossRef] [Green Version]
- Aoki, A.; Sakagami, T.; Yoshizawa, K.; Shima, K.; Toyama, M.; Tanabe, Y.; Moro, H.; Aoki, N.; Watanabe, S.; Koya, T.; et al. Clinical Significance of Interferon-gamma Neutralizing Autoantibodies Against Disseminated Nontuberculous Mycobacterial Disease. Clin. Infect. Dis. 2018, 66, 1239–1245. [Google Scholar] [CrossRef]
- Wu, U.I.; Chuang, Y.C.; Sheng, W.H.; Sun, H.Y.; Jhong, Y.T.; Wang, J.Y.; Chang, S.C.; Wang, J.T.; Chen, Y.C. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-gamma autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin. Microbiol. Infect. 2018, 24, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Rattanathammethee, K.; Chawansuntati, K.; Chaiwarith, R.; Praparattanapan, J.; Supparatpinyo, K.; Wipasa, J. Dot enzyme-linked immunosorbent assay strip as a screening tool for detection of autoantibody to interferon gamma in sera of suspected cases of adult-onset immunodeficiency. J. Clin. Lab. Anal. 2018, 32, e22460. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.-I.; Lee, A.S.-Y.; Lee, S.-S.; Chung, M.-H.; Liu, M.-W.; Lee, C.-K. Enhanced Signal and Quantitative Detection of Anti-Interferon-Gamma Antibody by Using a Nanometer Biolinker. PLoS ONE 2016, 11, e0160031. [Google Scholar] [CrossRef] [PubMed]
- Mainet-González, D.; Palenzuela-Gardon, D.O.; Dayz-Argudín, T. Development of a immunochromatographic test with avidin-biotin for the detection of antibodies against antigen e of hepatitis B in human plasma. Biotecnol. Apl. 2007, 24, 265–275. [Google Scholar]
- Samokhvalov, A.V.; Razo, S.C.; Safenkova, I.V.; Slutskaya, E.S.; Pridvorova, S.M.; Zherdev, A.V.; Dzantiev, B.B. Synthesis of the Most Effective Streptavidin Conjugates with Small Gold Nanoparticles for Indirect Labeling in Lateral Flow Assay. Int. J. Appl. Eng. Res. 2017, 12, 13847–13860. [Google Scholar]
- Demonte, D.; Dundas, C.M.; Park, S. Expression and purification of soluble monomeric streptavidin in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 6285–6295. [Google Scholar] [CrossRef]
- Kroetsch, A.; Chin, B.; Nguyen, V.; Gao, J.; Park, S. Functional expression of monomeric streptavidin and fusion proteins in Escherichia coli: Applications in flow cytometry and ELISA. Appl. Microbiol. Biotechnol. 2018, 102, 10079–10089. [Google Scholar] [CrossRef] [PubMed]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis–A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Sánchez, C.; McNeil, C.J.; Rawson, K.; Nilsson, O. Disposable noncompetitive immunosensor for free and total prostate-specific antigen based on capacitance measurement. Anal. Chem. 2004, 76, 5649–5656. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zeng, G.-M.; Huang, D.-L.; Feng, C.-L.; Hu, S.; Su, F.-F.; Zhao, M.-H.; Huang, C.; Wei, Z. Detection based on immunogold labeling technique and its expected application in composting. Chin. J. Anal. Chem. 2010, 38, 909–914. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcenas-Morales, G.; Cortes-Acevedo, P.; Doffinger, R. Anticytokine autoantibodies leading to infection: Early recognition, diagnosis and treatment options. Curr. Opin. Infect. Dis. 2019, 32, 330. [Google Scholar] [CrossRef]
- Madariaga, L.; Amurrio, C.; Martin, G.; Garcia-Cebrian, F.; Bicandi, J.; Lardelli, P.; Suarez, M.D.; Cisterna, R. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 62–68. [Google Scholar]
- Merck. Human Autoimmune Autoantibody Magnetic Bead Panel. Protocol. 2019. Available online: https://www.merckmillipore.com/INTL/en/product/MILLIPLEX-MAP-Human-Cytokine-Autoantibody-Panel,MM_NF-HCYTAAB-17K (accessed on 17 January 2021).
- Thobhani, S.; Attree, S.; Boyd, R.; Kumarswami, N.; Noble, J.; Szymanski, M.; Porter, R.A. Bioconjugation and characterisation of gold colloid-labelled proteins. J. Immunol. Methods 2010, 356, 60–69. [Google Scholar] [CrossRef]
- Fam, C.M.; Eisenberg, S.P.; Carlson, S.J.; Chlipala, E.A.; Cox, G.N.; Rosendahl, M.S. PEGylation improves the pharmacokinetic properties and ability of interferon gamma to inhibit growth of a human tumor xenograft in athymic mice. J. Interferon Cytokine Res. 2014, 34, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yi, C.; Lv, S.; Sheng, Y.; Wen, W.; Zhang, X.; Wang, S. Development of a lateral flow strip biosensor based on copper oxide nanoparticles for rapid and sensitive detection of HPV16 DNA. Sens. Actuators B Chem. 2019, 285, 326–332. [Google Scholar] [CrossRef]
- Muangsuwan, W.; Promptmas, C.; Jeamsaksiri, W.; Bunjongpru, W.; Srisuwan, A.; Hruanun, C.; Poyai, A.; Wongchitrat, P.; Yasawong, M. Development of an immunoFET biosensor for the detection of biotinylated PCR product. Heliyon 2016, 2, e00188. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, F.; Peng, F.; Xu, H.; Luo, W.; Xu, H.; Xiong, Y. High-yield production of Streptavidin with native C-terminal in Escherichia coli. Afr. J. Biotechnol. 2012, 11, 8250–8258. [Google Scholar]
- Jouybari, R.M.; Sadeghi, A.; Khansarinejad, B.; Abbasian, S.S.; Abtahi, H. Production of recombinant streptavidin and optimization of refolding conditions for recovery of biological activity. Rep. Biochem. Mol. Biol. 2018, 6, 178. [Google Scholar]
- Wang, S.; Zhang, Y.; Gao, D.; Zi, J.; Wang, W.; Zhang, N.; Wan, Y.; Wang, L. Refolding with Simultaneous Purification of Recombinant Core Streptavidin Using Single-step High-performance Hydrophobic Interaction Chromatography. Biotechnol. Bioprocess Eng. 2019, 24, 658–665. [Google Scholar] [CrossRef]
- O’Farrell, B. Lateral flow immunoassay systems: Evolution from the current state of the art to the next generation of highly sensitive, quantitative rapid assays. In The Immunoassay Handbook, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 89. [Google Scholar]
- Serebrennikova, K.; Samsonova, J.; Osipov, A. Enhancement of the sensitivity of a lateral flow immunoassay by using the biotin–streptavidin system. Mosc. Univ. Chem. Bull. 2018, 73, 131–134. [Google Scholar] [CrossRef]
- Zherdev, A.V.; Dzantiev, B.B. Ways to reach lower detection limits of lateral flow immunoassays. In Rapid Test—Advances in Design, Format and Diagnostic Applications; Anfossi, L., Ed.; IntechOpen: London, UK, 2018; pp. 9–43. [Google Scholar]
- Abbexa. Human Anti-Interferon Gamma Antibody (Anti-IFNg) ELISA Kit. Instructions for Use. Available online: https://www.abbexa.com/human-anti-interferon-gamma-antibody-anti-ifng-elisa-kit (accessed on 18 February 2021).
- Yoshizawa, K.; Aoki, A.; Shima, K.; Tanabe, Y.; Koya, T.; Hasegawa, T.; Kikuchi, T.; Sakagami, T. Serum Anti-interferon-gamma Autoantibody Titer as a Potential Biomarker of Disseminated Non-tuberculous Mycobacterial Infection. J. Clin. Immunol. 2020, 40, 399–405. [Google Scholar] [CrossRef]
- Chetchotisakd, P.; Anunnatsiri, S.; Nanagara, R.; Nithichanon, A.; Lertmemongkolchai, G. Intravenous Cyclophosphamide Therapy for Anti-IFN-Gamma Autoantibody-Associated Mycobacterium abscessus Infection. J. Immunol. Res. 2018, 2018, 6473629. [Google Scholar] [CrossRef] [Green Version]
- Pithukpakorn, M.; Roothumnong, E.; Angkasekwinai, N.; Suktitipat, B.; Assawamakin, A.; Luangwedchakarn, V.; Umrod, P.; Thongnoppakhun, W.; Foongladda, S.; Suputtamongkol, Y. HLA-DRB1 and HLA-DQB1 Are Associated with Adult-Onset Immunodeficiency with Acquired Anti-Interferon-Gamma Autoantibodies. PLoS ONE 2015, 10, e0128481. [Google Scholar] [CrossRef]
- Lin, C.H.; Chi, C.Y.; Shih, H.P.; Ding, J.Y.; Lo, C.C.; Wang, S.Y.; Kuo, C.Y.; Yeh, C.F.; Tu, K.H.; Liu, S.H.; et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat. Med. 2016, 22, 994–1001. [Google Scholar] [CrossRef]

| IC-Strip | ELISA | Total | |
|---|---|---|---|
| Titer ≥ 2500 | Titer < 2500 | ||
| Positive | 21 | 4 | 25 |
| Negative | 4 | 37 | 41 |
| Total | 25 | 41 | 66 |
| IC-Strip | ELISA | Total | |
|---|---|---|---|
| Titer ≥ 500 | Titer < 500 | ||
| Positive | 25 | 0 | 25 |
| Negative | 9 | 32 | 41 |
| Total | 34 | 32 | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongkum, W.; Yasamut, U.; Chupradit, K.; Sakkhachornphop, S.; Wipasa, J.; Sornsuwan, K.; Juntit, O.-a.; Pornprasit, R.; Thongkamwitoon, W.; Chaichanan, J.; et al. Latticed Gold Nanoparticle Conjugation via Monomeric Streptavidin in Lateral Flow Assay for Detection of Autoantibody to Interferon-Gamma. Diagnostics 2021, 11, 987. https://doi.org/10.3390/diagnostics11060987
Thongkum W, Yasamut U, Chupradit K, Sakkhachornphop S, Wipasa J, Sornsuwan K, Juntit O-a, Pornprasit R, Thongkamwitoon W, Chaichanan J, et al. Latticed Gold Nanoparticle Conjugation via Monomeric Streptavidin in Lateral Flow Assay for Detection of Autoantibody to Interferon-Gamma. Diagnostics. 2021; 11(6):987. https://doi.org/10.3390/diagnostics11060987
Chicago/Turabian StyleThongkum, Weeraya, Umpa Yasamut, Koollawat Chupradit, Supachai Sakkhachornphop, Jiraprapa Wipasa, Kanokporn Sornsuwan, On-anong Juntit, Rawiwan Pornprasit, Wanwisa Thongkamwitoon, Jirapan Chaichanan, and et al. 2021. "Latticed Gold Nanoparticle Conjugation via Monomeric Streptavidin in Lateral Flow Assay for Detection of Autoantibody to Interferon-Gamma" Diagnostics 11, no. 6: 987. https://doi.org/10.3390/diagnostics11060987