Diagnosis of Indigenous Non-Malarial Vector-Borne Infections from Malaria Negative Samples from Community and Rural Hospital Surveillance in Dhalai District, Tripura, North-East India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.2.1. Records of Fever and Malaria Cases
2.2.2. Exclusion Criteria
2.2.3. Village and PHC Collections
2.2.4. Medical History Records
2.3. Sample Processing
2.4. Demographic Census
2.5. Meteorological Data Collection
2.6. Preparation of Ecological Maps
2.7. Data Analysis
3. Results
3.1. Fever and Malaria Infections
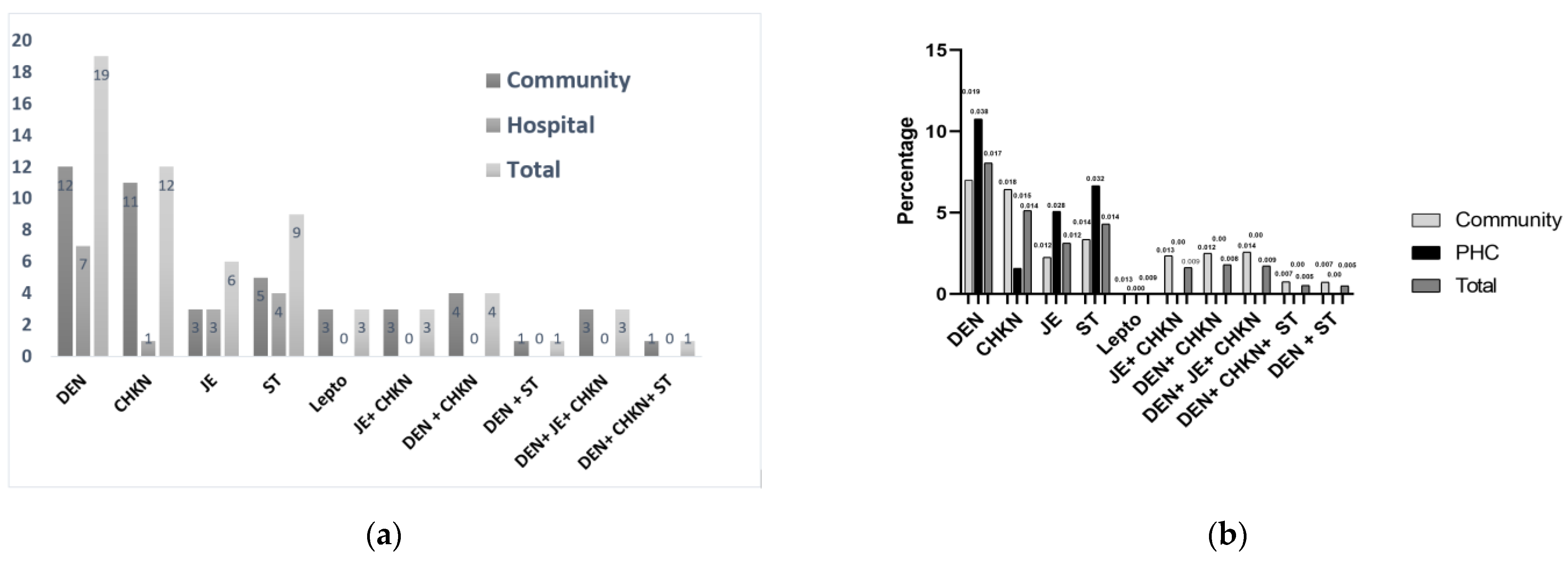
3.2. Vector-Borne Non-Malarial Fevers
3.3. Percentage Prevalence of the Symptoms for Each Disease as Reported by the Patients Clustering Analysis between the Symptoms
3.4. Diseases Tested Outside Criteria
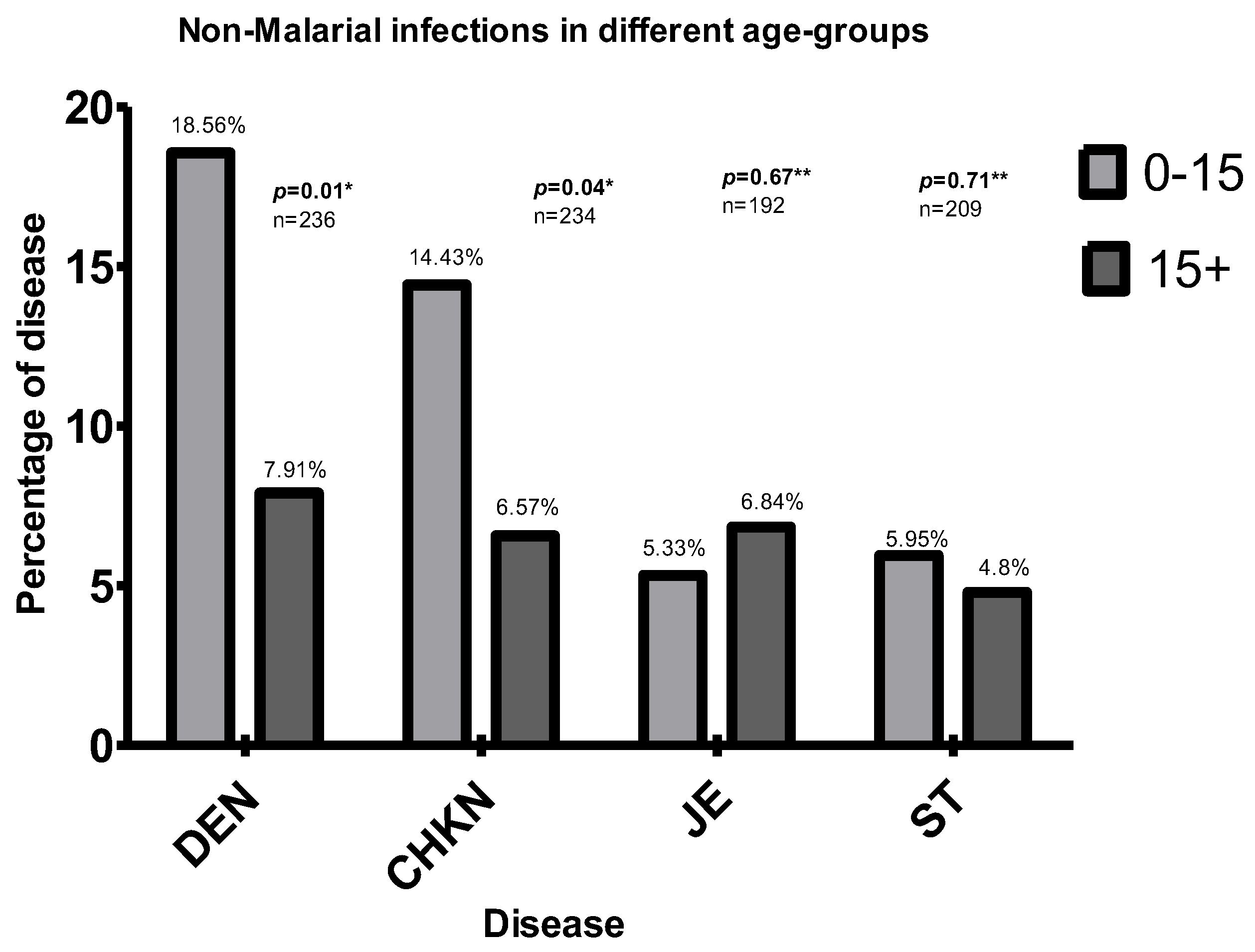
3.5. Association with Age
3.6. Demographic Pattern and Vector Control Practices of All Tested and Positive Cases from the Villages
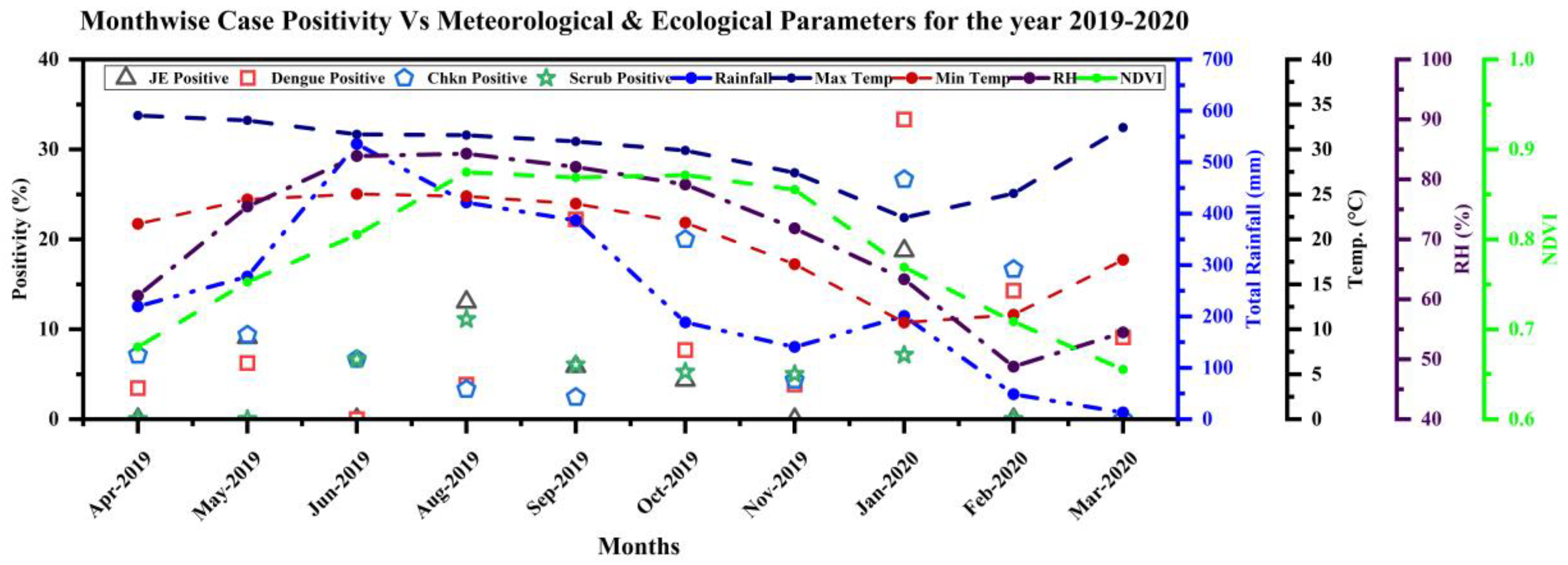
3.7. Ecological and Meteorological Correlates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, T.J.; Dandona, L.; Sharma, V.P.; Kakkar, M. Continuing challenge of infectious diseases in India. Lancet 2011, 377, 252–269. [Google Scholar] [CrossRef]
- Shelke, Y.P.; Deotale, V.S.; Maraskolhe, D.L. Spectrum of Infections in Acute Febrile Illness in Central India. Ind. J. Med. Microbiol. 2017, 35, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.P.; Bhowmik, I.P.; Sarma, D.K.; Sharma, C.K.; Medhi, G.K.; Mohapatra, P.K.; Mahanta, J.; Bhattacharyya, D.R. Role of Anopheles baimaii: Potential vector of epidemic outbreak in Tripura, North-East India. J. Glob. Health Rep. 2019, 3, e2019036. [Google Scholar] [CrossRef]
- Joshi, R.; Colford, J.M.; Kalantri, S.; Reingold, A.L. Nonmalarial acute undifferentiated fever in a rural hospital in central India: Diagnostic uncertainty and overtreatment with antimalarial agents. Am. J. Trop. Med. Hyg. 2008, 78, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Kabir, M.M.; Hossain, M.S.; Naher, S.; Ferdous, N.E.N.; Khan, W.A.; Mondal, D.; Karim, J.; Shamsuzzaman, A.K.M.; Ahmed, B.-N.; et al. Reduction in malaria prevalence and increase in malaria awareness in endemic districts of Bangladesh. Malar. J. 2016, 15, 1603. [Google Scholar] [CrossRef] [Green Version]
- Naing, C.; Kassim, A.I.B.M. Scaling-up attention to non-malaria acute undifferentiated fever. Trans. R Soc. Trop. Med. Hyg. 2012, 106, 331–332. [Google Scholar] [CrossRef]
- D’Acremont, V.; Lengeler, C.; Genton, B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: A systematic review. Malar. J. 2010, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Murhekar, M.V.; Mittal, M.; Prakash, J.A.J.; Pillai, V.M.; Kumar, C.G.; Shinde, S.; Ranjan, P.; Oak, C.; Gupta, N.; Mehendale, S.; et al. Acute encephalitis syndrome in Gorakhpur, Uttar Pradesh, India—Role of scrub typhus. J. Infect. 2016, 73, 623–626. [Google Scholar] [CrossRef]
- Murhekar, M.; Joshua, V.; Kanagasabai, K.; Shete, V.; Ravi, M.; Ramachandran, R.; Sabarinathan, R.; Kirubakaran, B.; Gupta, N.; Mehendale, S. Epidemiology of dengue fever in India, based on laboratory surveillance data, 2014–2017. Int. J. Infect. Dis. 2019, 84, S10–S14. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, P.; Dahal, P.; Ogbonnaa-Njoku, C.; Das, D.; Stepniewska, K.; Thomas, N.V.; Hopkins, H.; Crump, J.A.; Bell, D.; Newton, P.N.; et al. Non-malarial febrile illness: A systematic review of published aetiological studies and case reports from Southern Asia and South-eastern Asia, 1980–2015. BMC Med. 2020, 18, 1745. [Google Scholar] [CrossRef]
- Maude, R.; Ghose, A.; Samad, R.; De Jong, H.K.; Fukushima, M.; Wijedoru, L.; Hassan, M.U.; Hossain, A.; Karim, R.; Abu Sayeed, A.; et al. A prospective study of the importance of enteric fever as a cause of non-malarial febrile illness in patients admitted to Chittagong Medical College Hospital, Bangladesh. BMC Infect. Dis. 2016, 16, 1886. [Google Scholar] [CrossRef] [Green Version]
- Maude, R.; Maude, R.; Ghose, A.; Amin, M.R.; Islam, M.B.; Ali, M.; Bari, S.; Majumder, M.I.; Tanganuchitcharnchai, A.; Dondorp, A.M.; et al. Serosurveillance of Orientia tsutsugamushi and Rickettsia typhi in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 91, 580–583. [Google Scholar] [CrossRef]
- Dutta, P.; Khan, S.A.; Chetry, S.; Abdul, M. Incrimination of Aedes aegypti for dengue virus serotype-1 in Assam, Northeast India. J. Vector Borne Dis. 2018, 55, 330–333. [Google Scholar] [CrossRef]
- Jain, P.; Prakash, S.; Tripathi, P.K.; Chauhan, A.; Gupta, S.; Sharma, U.; Jaiswal, A.K.; Sharma, D.; Jain, A. Emergence of Orientia tsutsugamushi as an important cause of Acute Encephalitis Syndrome in India. PLoS Neglec. Trop. Dis. 2018, 12, e0006346. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kamaraj, P.; Khan, S.A.; Allam, R.R.; Barde, P.V.; Dwibedi, B.; Kanungo, S.; Mohan, U.; Mohanty, S.S.; Roy, S.; et al. Seroprevalence of chikungunya virus infection in India, 2017: A cross-sectional population-based serosurvey. Lancet Microbe 2021, 2, e41–e47. [Google Scholar] [CrossRef]
- Murhekar, M.; Kanagasabai, K.; Shete, V.; Joshua, V.; Ravi, M.; Kirubakaran, B.K.; Ramachandran, R.; Sabarinathan, R.; Gupta, N. Epidemiology of chikungunya based on laboratory surveillance data—India, 2016–2018. Trans. R Soc. Trop. Med. Hyg. 2019, 113, 259–262. [Google Scholar] [CrossRef]
- Narain, J.P.; Dhariwal, A.C.; MacIntyre, C.R. Acute encephalitis in India: An unfolding tragedy. Ind. J. Med. Res. 2017, 145, 584–587. [Google Scholar]
- Rao, P.N.; Van Eijk, A.M.; Choubey, S.; Ali, S.Z.; Dash, A.; Barla, P.; Oraon, R.R.; Patel, G.; Nandini, P.; Acharya, S.; et al. Dengue, chikungunya, and scrub typhus are important etiologies of non-malarial febrile illness in Rourkela, Odisha, India. BMC Infect. Dis. 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Tripathy, S.K.; Dwibedi, B.; Priyadarshini, L.; Das, R. Clinico-epidemiological study of viral acute encephalitis syndrome cases and comparison to nonviral cases in children from Eastern India. J. Glob. Infect. Dis. 2019, 11, 7–12. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Kamaraj, P.; Kumar, M.S.; Khan, S.A.; Allam, R.R.; Barde, P.; Dwibedi, B.; Kanungo, S.; Mohan, U.; Mohanty, S.S.; et al. Burden of dengue infection in India, 2017: A cross-sectional population based serosurvey. Lancet Glob. Health 2019, 7, e1065–e1073. [Google Scholar] [CrossRef] [Green Version]
- Borkakoty, B.; Jakharia, A.; Biswas, D.; Mahanta, J. Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Ind. J. Med. Microbiol. 2016, 34, 88–91. [Google Scholar] [CrossRef]
- Borkakoty, B.; Das, M.; Sarma, K.; Jakharia, A.; Das, P.K.; Bhattacharya, C.; Apum, B.; Biswas, D. Molecular Characterisation and Phylogenetic Analysis of Dengue Outbreak in Pasighat, Arunachal Pradesh, Northeast India. Ind. J. Med. Microbiol. 2018, 36, 37–42. [Google Scholar] [CrossRef]
- Khan, S.A.; Bora, T.; Laskar, B.; Khan, A.M.; Dutta, P. Scrub Typhus Leading to Acute Encephalitis Syndrome, Assam, India. Emerg. Infect. Dis. 2017, 23, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Mørch, K.; Manoharan, A.; Chandy, S.; Chacko, N.; Alvarez-Uria, G.; Patil, S.; Henry, A.; Nesaraj, J.; Kuriakose, C.; Singh, A.; et al. Acute undifferentiated fever in India: A multicentre study of aetiology and diagnostic accuracy. BMC Infect. Dis. 2017, 17, 665. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, I.P.; Nirmolia, T.; Pandey, A.; Subbarao, S.K.; Nath, A.; Senapati, S.; Tripathy, N.; Pebam, R.; Nag, S.; Roy, R.; et al. Dry Post Wintertime Mass Surveillance Unearths a Huge Burden of P. vivax, and Mixed Infection with P. vivax P. falciparum, a Threat to Malaria Elimination, in Dhalai, Tripura, India. Pathogens 2021, 10, 1259. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef]
- Reiczigel, J.; Földi, J.; Ózsvári, L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol. Infect. 2010, 138, 1674–1678. [Google Scholar] [CrossRef]
- Huang, J. A Fast Clustering Algorithm to Cluster Very Large Categorical Data Sets in Data Mining. In KDD: Techniques and Applications; Lu, H., Motoda, H., Luu, E.H., Eds.; World Scientific: Singapore, 1997; pp. 21–34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: https://www.R-project.org/ (accessed on 12 March 2020).
- Tantawichien, T. Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr. Int. Child Health 2012, 32, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Thangaraj, J.W.V.; Zaman, K.; Shete, V.; Pandey, A.K.; Velusamy, S.; Deoshatwar, A.; Mittal, M.; Gupta, N.; Murhekar, M. Effectiveness of Presumptive Treatment of Acute Febrile Illness with Doxycycline or Azithromycin in Preventing Acute Encephalitis Syndrome in Gorakhpur, India: A Cohort Study; Springer: Berlin, Germany, 2020. [Google Scholar]
- Diallo, M.; Ba, Y.; Sall, A.A.; Diop, O.M.; Ndione, J.A.; Mondo, M.; Girault, L.; Mathiot, C. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999–2000: Entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 2003, 9, 362–367. [Google Scholar] [CrossRef]
- Young, K.I.; Mundis, S.; Widen, S.G.; Wood, T.G.; Tesh, R.B.; Cardosa, J.; Vasilakis, N.; Perera, D.; Hanley, K.A. Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasites Vectors 2017, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Hanley, K.A.; Monath, T.P.; Weaver, S.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic cycles of arboviruses in non-human primates. Parasites Vectors 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Siwal, N.; Singh, U.; Dash, M.; Kar, S.; Rani, S.; Rawal, C.; Singh, R.; Anvikar, A.R.; Pande, V.; Das, A. Malaria diagnosis by PCR revealed differential distribution of mono and mixed species infections by Plasmodium falciparum and P. vivax in India. PLoS ONE 2018, 13, e0193046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.K.; Kong, T.F.; Ng, C.S.; Chen, L.; Huang, Y.; Bhagat, A.A.; Nguyen, N.-T.; Preiser, P.R.; Han, J. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat. Med. 2014, 20, 1069–1073. [Google Scholar] [CrossRef]
- Saito, T.; Kikuchi, A.; Kaneko, A.; Isozumi, R.; Teramoto, I.; Kimura, M.; Hirasawa, N.; Hiratsuka, M. Rapid and sensitive multiplex single-tube nested PCR for the identification of five human Plasmodium species. Parasitol. Int. 2018, 67, 277–283. [Google Scholar] [CrossRef]
- Chen, K.; Yuen, C.; Aniweh, Y.; Preiser, P.; Liu, Q. Towards ultrasensitive malaria diagnosis using surface enhanced Raman spectroscopy. Sci. Rep. 2016, 6, 20177. [Google Scholar] [CrossRef]
- Arndt, L.; Koleala, T.; Ibam, C.; Lufele, E.; Timinao, L.; Lorry, L.; Kaman, P.; Molnár, A.P. Magneto-optical diagnosis of symptomatic malaria in Papua New Guinea. Nat. Commun. 2021, 12, 110. [Google Scholar] [CrossRef]
- Aggarwal, S.; Peng, W.K.; Srivastava, S. Multi-Omics Advancements towards Plasmodium vivax Malaria Diagnosis. Diagnostics 2021, 11, 2222. [Google Scholar] [CrossRef]
- Bhatt, M.; Soneja, M.; Gupta, N. Approach to acute febrile illness during the COVID-19 pandemic. Drug Discov. Ther. 2020, 14, 282–286. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Tanganuchitcharnchai, A.; Nawtaisong, P.; Kantipong, P.; Laongnualpanich, A.; Day, N.P.J.; Paris, D.H. Diagnostic Accuracy of the InBios Scrub Typhus Detect Enzyme-Linked Immunoassay for the Detection of IgM Antibodies in Northern Thailand. Clin. Vaccine Immunol. 2016, 23, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, P.L.S.; Chinnachamy, R.; Rajamani, N. Diagnostic dilemmas in the detection of dengue specific immunoglobulin M by different kits. J. Sci. Soc. 2015, 42, 126. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Kingston, H.W.F.; Tanganuchitcharnchai, A.; Phanichkrivalkosil, M.; Hossain, M.; Hossain, M.; Ghose, A.; Leopold, S.J.; Dondrop, A.M. Diagnostic Accuracy of the InBios Scrub Typhus DetectTM ELISA for the Detection of IgM Antibodies in Chittagong, Bangladesh. Trop. Med. Infect. Dis. 2018, 3, 95. [Google Scholar] [CrossRef] [Green Version]




| Ambassa PHC (Population ~61,000) | IPD * | OPD * | Total IPD + OPD | All SC * Cases | Total Ambassa PHC (IPD + OPD + SCs) |
|---|---|---|---|---|---|
| Fever cases | 422 | 3100 | 3500 | 14,537 | 18,037 |
| Malaria cases | 155 | 3 | 158 | 431 | 589 |
| Gurudhanpara SC (Population ~1900; Villages: Dhansinghpara, Bidyapara, Sambhuram, Sudhiram, Old Sudhiram, Khagendra, Tilakkumar) | ||||||
| MPW * | ASHA * | State and Project Village Health Volunteers and Project Staff | Health Camp | PHC IPD | Total | |
| Fever | 489 | 564 | 525 | 65 | 33 ** | 1676 |
| Malaria positive (by RDT *) | 52 | 36 | 115 | 4 | 26** | 233 (API ~122) |
| Shikaribari SC (Population ~1800; Villages: Dankarai, Birendra, Lakhindra, Annaram, Satiram, Forest Village, Mahedra Debbarma, Rangachara, Sriratan, Mainayaram-Ananta 1, Mainayaram-Ananta 2) | ||||||
| Fever | 479 | 115 | 279 | 7 | 28 * | 908 |
| Malaria Positive (by RDT) | 34 | 6 | 51 | 0 | 11 ** | 102 (API ~57) |
| Total Gurudhanpara + Shikaribari SC Fever Surveillance | ||||||
| Fever | 968 | 679 | 804 | 72 | 61 | 2584 |
| Malaria Positive (by RDT) | 86 | 42 | 166 | 4 | 37 | 335 |
| Mosquito Net Type | JE * N, (%) | DEN * N, (%) | CHKN * N, (%) | ST * N, (%) | JE * + CHKN * N, (%) | DEN * + CHKN * N, (%) | DEN *+ ST * N, (%) | DEN * + JE * + CHKN * N, (%) | DEN * + CHKN * + ST * N, (%) |
|---|---|---|---|---|---|---|---|---|---|
| New LLIN N, (%) | 3 (100) | 12 (92) | 6 (60) | 5 (100) | 3 (100) | 3 (75) | 1 (100) | 3 (100) | 1 (100) |
| Old LLIN N, (%) | 0 | 1 (7.7) | 3 (30) | 0 | 0 | 1 (25) | 0 | 0 | 0 |
| No LLIN | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 |
| Period of sleep under a mosquito net | |||||||||
| Always | 3 (100) | 12 (92.3) | 7 (70) | 5 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) |
| Sometimes | 0 | 1 (7.7) | 2 (20) | 0 | 0 | 0 | 0 | 0 | 0 |
| Never | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmick, I.P.; Pandey, A.; Subbarao, S.K.; Pebam, R.; Majumder, T.; Nath, A.; Nandi, D.; Basu, A.; Sarkar, A.; Majumder, S.; et al. Diagnosis of Indigenous Non-Malarial Vector-Borne Infections from Malaria Negative Samples from Community and Rural Hospital Surveillance in Dhalai District, Tripura, North-East India. Diagnostics 2022, 12, 362. https://doi.org/10.3390/diagnostics12020362
Bhowmick IP, Pandey A, Subbarao SK, Pebam R, Majumder T, Nath A, Nandi D, Basu A, Sarkar A, Majumder S, et al. Diagnosis of Indigenous Non-Malarial Vector-Borne Infections from Malaria Negative Samples from Community and Rural Hospital Surveillance in Dhalai District, Tripura, North-East India. Diagnostics. 2022; 12(2):362. https://doi.org/10.3390/diagnostics12020362
Chicago/Turabian StyleBhowmick, Ipsita Pal, Apoorva Pandey, Sarala K. Subbarao, Rocky Pebam, Tapan Majumder, Aatreyee Nath, Diptarup Nandi, Analabha Basu, Apurba Sarkar, Saikat Majumder, and et al. 2022. "Diagnosis of Indigenous Non-Malarial Vector-Borne Infections from Malaria Negative Samples from Community and Rural Hospital Surveillance in Dhalai District, Tripura, North-East India" Diagnostics 12, no. 2: 362. https://doi.org/10.3390/diagnostics12020362









