Detecting Multiple Myeloma Infiltration of the Bone Marrow on CT Scans in Patients with Osteopenia: Feasibility of Radiomics Analysis
Abstract
:1. Introduction
2. Materials and Methods
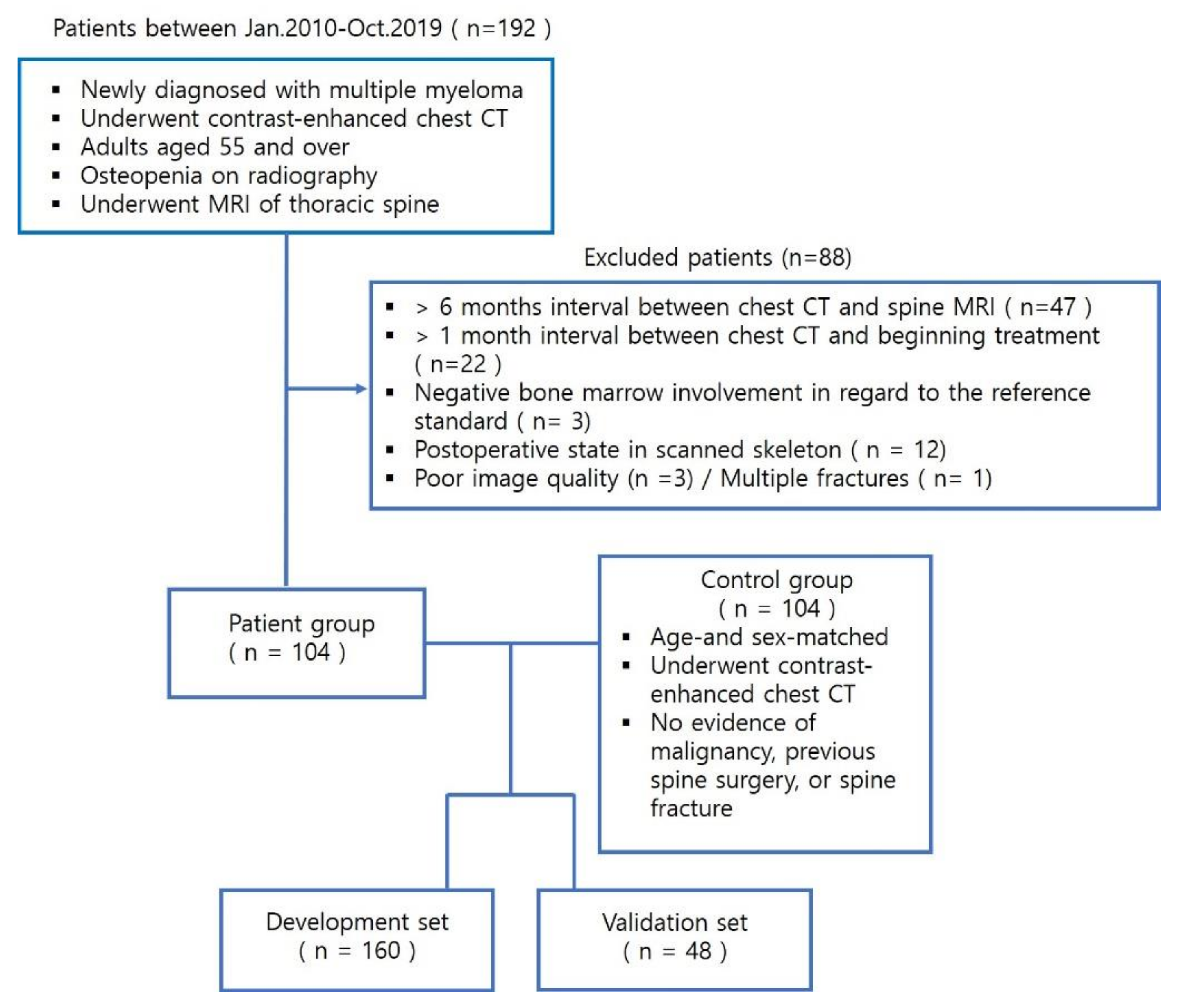
2.1. Patient Population
2.2. CT Data Acquisition
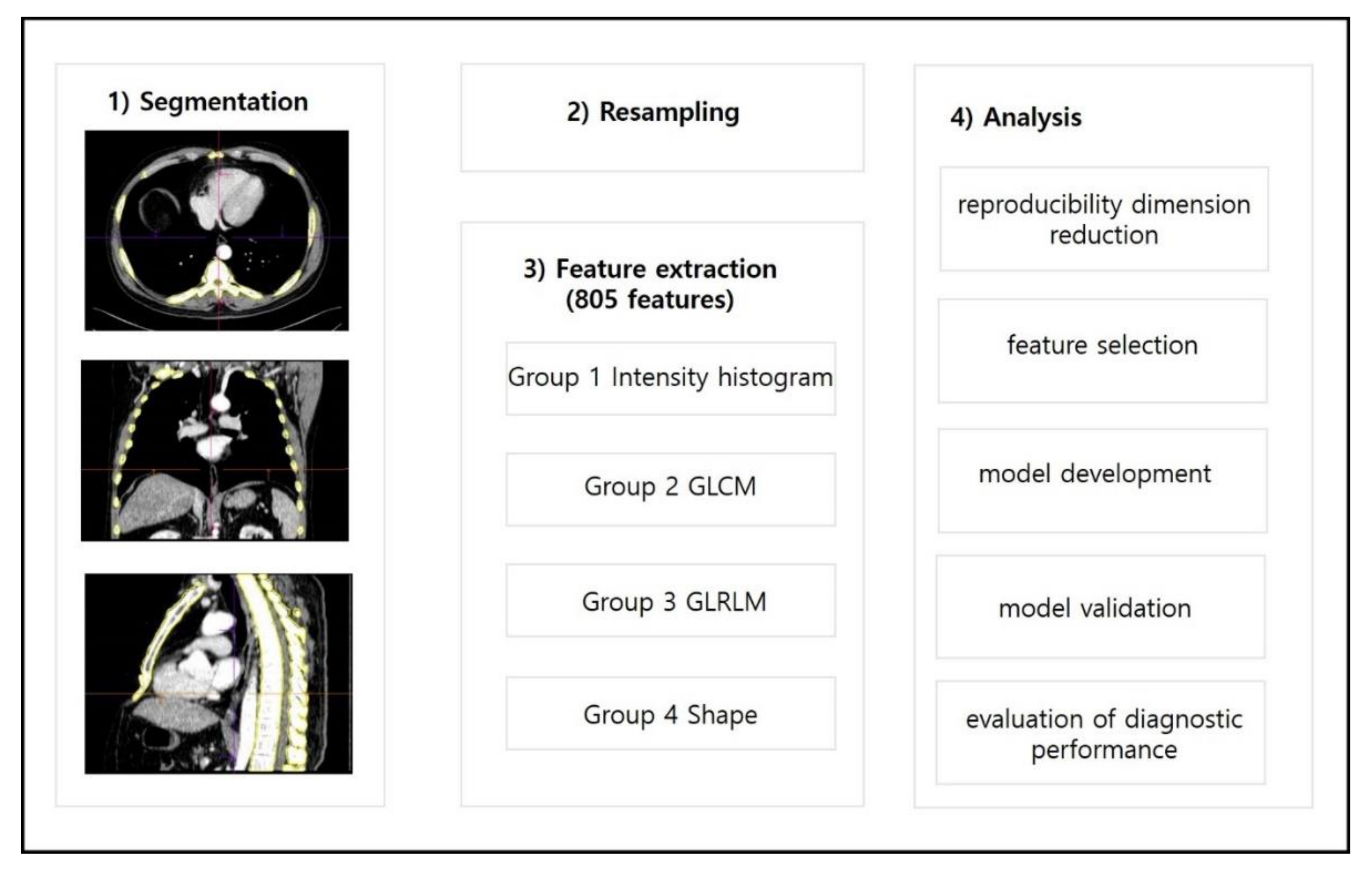
2.3. Lesion Segmentation and Radiomics Feature Extraction
2.4. Dimensionality Reduction and Radiomics Feature Selection
2.5. Radiomics Model Development
2.6. Radiomics Model Validation
2.7. Evaluation of the Usefulness of Adding a Radiomics Model to Conventional Reading
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Radiomics Feature Selection and Model Development
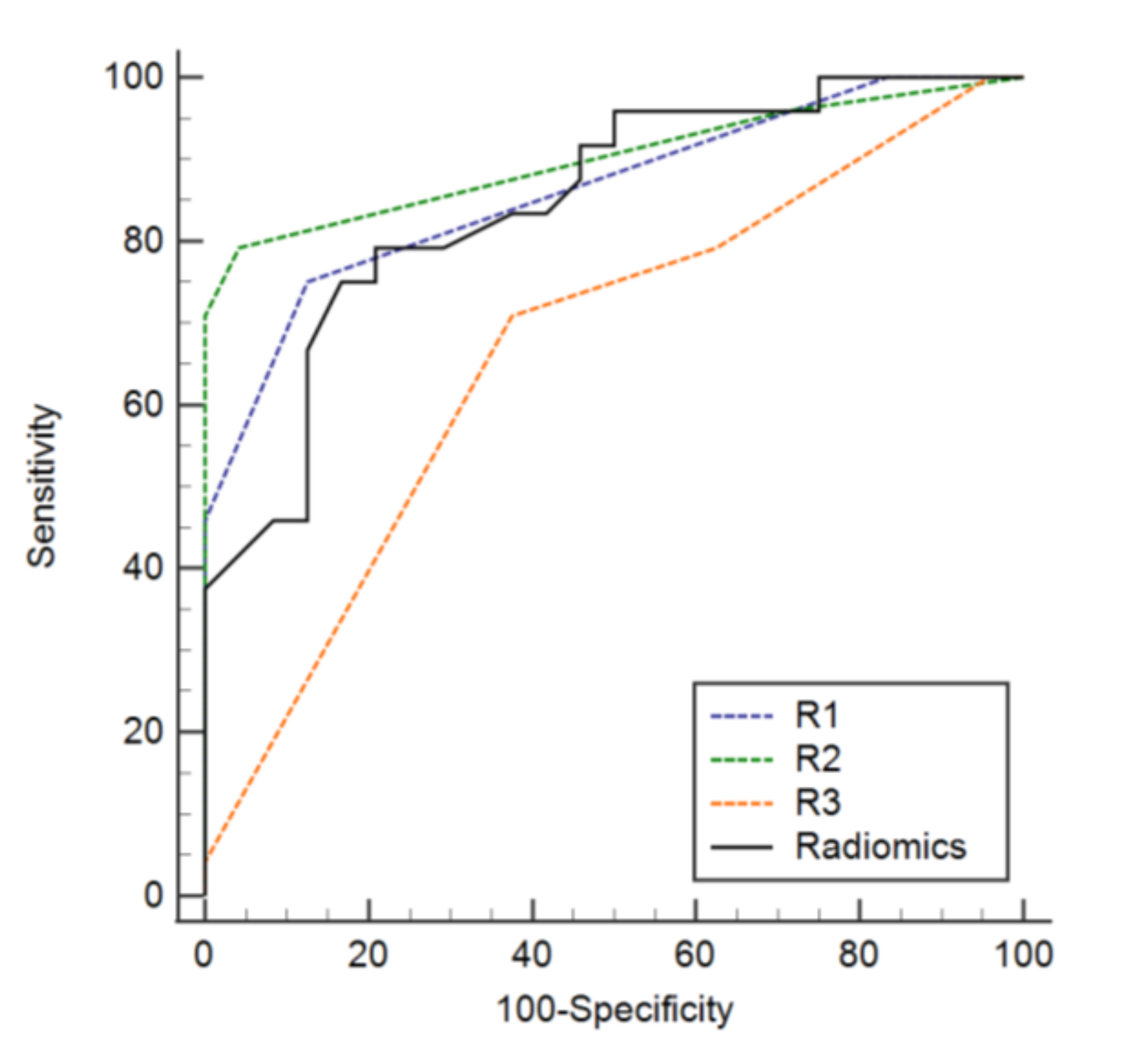
3.3. Diagnostic Performance of the Radiomics Model
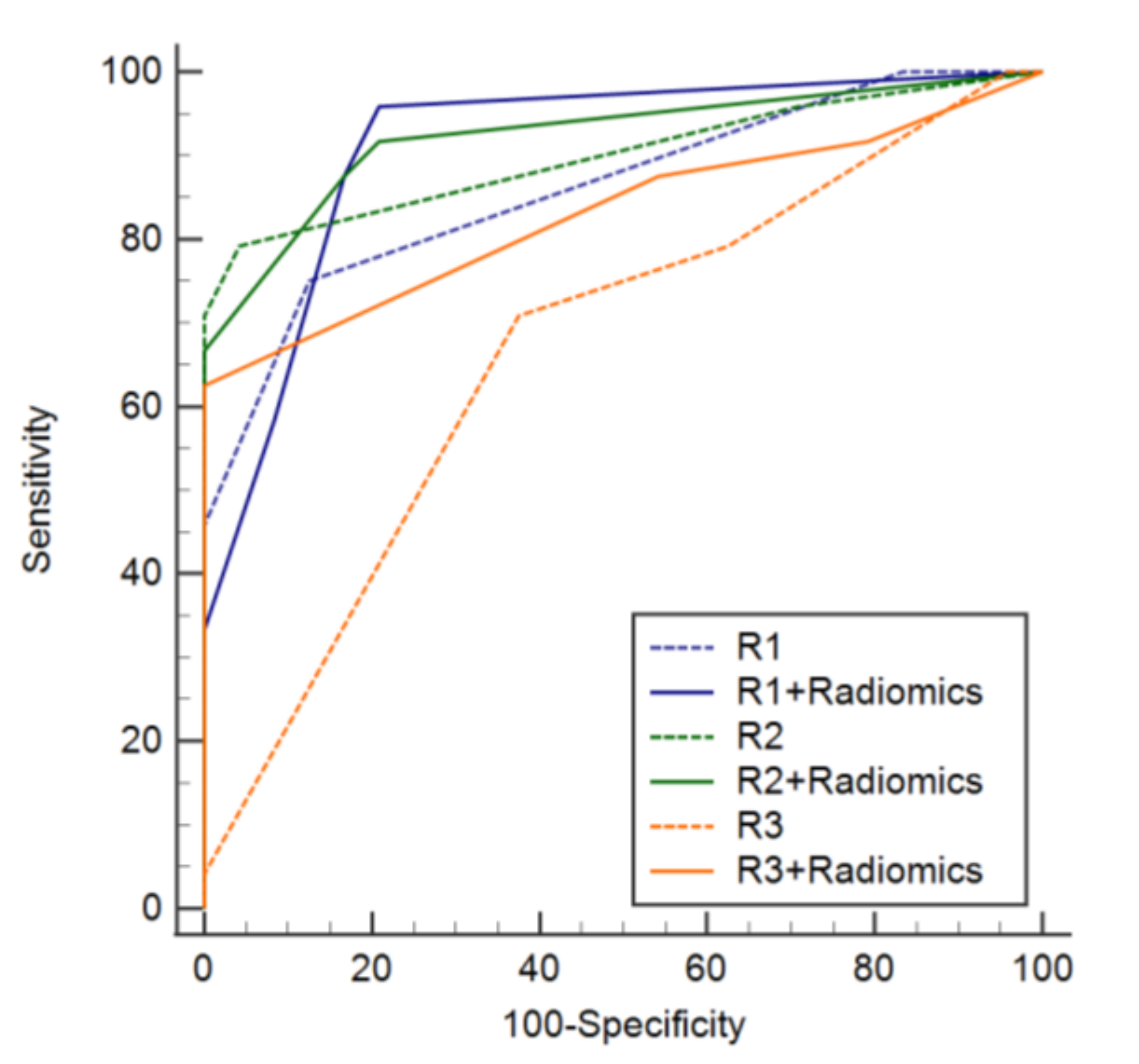
3.4. Added Value of a Radiomics Model to Conventional Readings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Multiple myeloma. Blood 2008, 111, 2962–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Ormond Filho, A.G.; Carneiro, B.C.; Pastore, D.; Silva, I.P.; Yamashita, S.R.; Consolo, F.D.; Hungria, V.T.M.; Sandes, A.F.; Rizzatti, E.G.; Nico, M.A.C. Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. Radiographics 2019, 39, 1077–1097. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Silbermann, R.; Roodman, G.D. Myeloma bone disease: Pathophysiology and management. J. Bone Oncol. 2013, 2, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.V.; Lonial, S.; Joao, C.; Anderson, K.C.; Garcia-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Hillengass, J.; Moulopoulos, L.A.; Delorme, S.; Koutoulidis, V.; Mosebach, J.; Hielscher, T.; Drake, M.; Rajkumar, S.V.; Oestergaard, B.; Abildgaard, N.; et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: A study of the International Myeloma Working Group. Blood Cancer J. 2017, 7, e599. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.B.; Murray, F.; Kilk, K.; Hillengass, J.; Delorme, S.; Heiss, C.; Neben, K.; Goldschmidt, H.; Kauczor, H.U.; Weber, M.A. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur. J. Radiol. 2014, 83, 1222–1230. [Google Scholar] [CrossRef]
- Kropil, P.; Fenk, R.; Fritz, L.B.; Blondin, D.; Kobbe, G.; Modder, U.; Cohnen, M. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur. Radiol. 2008, 18, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hinge, M.; Andersen, K.T.; Lund, T.; Jorgensen, H.B.; Holdgaard, P.C.; Ormstrup, T.E.; Ostergaard, L.L.; Plesner, T. Baseline bone involvement in multiple myeloma—A prospective comparison of conventional X-ray, low-dose computed tomography, and 18flourodeoxyglucose positron emission tomography in previously untreated patients. Haematologica 2016, 101, e415–e418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.; Yoon, C.S.; Ryu, Y.H.; Yun, M.J.; Suh, J.S. Efficacy of multidetector row computed tomography of the spine in patients with multiple myeloma: Comparison with magnetic resonance imaging and fluorodeoxyglucose-positron emission tomography. J. Comput. Assist. Tomogr. 2007, 31, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Weng, A.M.; Heidemeier, A.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Multiple Myeloma and Dual-Energy CT: Diagnostic Accuracy of Virtual Noncalcium Technique for Detection of Bone Marrow Infiltration of the Spine and Pelvis. Radiology 2018, 286, 205–213. [Google Scholar] [CrossRef]
- Horger, M.; Pereira, P.; Claussen, C.D.; Kanz, L.; Vonthein, R.; Denecke, B.; Driessen, C. Hyperattenuating bone marrow abnormalities in myeloma patients using whole-body non-enhanced low-dose MDCT: Correlation with haematological parameters. Br. J. Radiol. 2008, 81, 386–396. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reason. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [Green Version]
- Muehlematter, U.J.; Mannil, M.; Becker, A.S.; Vokinger, K.N.; Finkenstaedt, T.; Osterhoff, G.; Fischer, M.A.; Guggenberger, R. Vertebral body insufficiency fractures: Detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur. Radiol. 2019, 29, 2207–2217. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Cea, M.; Rossi, F.; Valdora, F.; Bignotti, B.; Succio, G.; Gualco, S.; Conte, A.; Dominietto, A. Differentiating diffuse from focal pattern on Computed Tomography in multiple myeloma: Added value of a Radiomics approach. Eur. J. Radiol. 2019, 121, 108739. [Google Scholar] [CrossRef]
- Kakigi, T.K.H.; Qureshi, M.; Chapman, M.; Li, B.; Sakai, O. Bone Texture Anaylsis of lumbar spine on CT to detect early finding of multiple myeloma: Comparison of Control subjects with normal bone density, patients with osteoporosis, and patients with multiple myeloma. In Proceedings of the American Society of Neuroradiology (ASNR) Annual Meeting, Washington, DC, USA, 23–26 May 2016. [Google Scholar]
- Ligero, M.; Jordi-Ollero, O.; Bernatowicz, K.; Garcia-Ruiz, A.; Delgado-Munoz, E.; Leiva, D.; Mast, R.; Suarez, C.; Sala-Llonch, R.; Calvo, N.; et al. Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Eur. Radiol. 2021, 31, 1460–1470. [Google Scholar] [CrossRef]
- Park, J.E.; Park, S.Y.; Kim, H.J.; Kim, H.S. Reproducibility and Generalizability in Radiomics Modeling: Possible Strategies in Radiologic and Statistical Perspectives. Korean J. Radiol. 2019, 20, 1124–1137. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Tha, K.K.; Terasaka, S.; Yamaguchi, S.; Wang, J.; Kudo, K.; Xing, L.; Shirato, H.; Li, R. Prognostic Imaging Biomarkers in Glioblastoma: Development and Independent Validation on the Basis of Multiregion and Quantitative Analysis of MR Images. Radiology 2016, 278, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahnken, A.H.; Wildberger, J.E.; Gehbauer, G.; Schmitz-Rode, T.; Blaum, M.; Fabry, U.; Gunther, R.W. Multidetector CT of the spine in multiple myeloma: Comparison with MR imaging and radiography. AJR Am. J. Roentgenol. 2002, 178, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Lutje, S.; de Rooy, J.W.; Croockewit, S.; Koedam, E.; Oyen, W.J.; Raymakers, R.A. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann. Hematol. 2009, 88, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Healy, C.F.; Murray, J.G.; Eustace, S.J.; Madewell, J.; O’Gorman, P.J.; O’Sullivan, P. Multiple myeloma: A review of imaging features and radiological techniques. Bone Marrow Res. 2011, 2011, 583439. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, D.; Giandola, T.; Maino, C.; Gandola, D.; Ragusi, M.; Bonaffini, P.A.; Sironi, S. Whole Body Low Dose Computed Tomography (WBLDCT) Can Be Comparable to Whole-Body Magnetic Resonance Imaging (WBMRI) in the Assessment of Multiple Myeloma. Diagnostics 2021, 11, 857. [Google Scholar] [CrossRef]
- Homann, G.; Weisel, K.; Mustafa, D.F.; Ditt, H.; Nikolaou, K.; Horger, M. Improvement of diagnostic confidence for detection of multiple myeloma involvement of the ribs by a new CT software generating rib unfolded images: Comparison with 5- and 1-mm axial images. Skelet. Radiol. 2015, 44, 971–979. [Google Scholar] [CrossRef]
- Messina, C.; Usuelli, F.G.; Maccario, C.; Di Silvestri, C.A.; Gitto, S.; Cortese, M.C.; Albano, D.; Sconfienza, L.M. Precision of Bone Mineral Density Measurements Around Total Ankle Replacement Using Dual Energy X-ray Absorptiometry. J. Clin. Densitom. 2020, 23, 656–663. [Google Scholar] [CrossRef]
- Mariette, X.; Bergot, C.; Ravaud, P.; Roux, C.; Laval-Jeantet, M.; Brouet, J.C.; Fermand, J.P. Evolution of bone densitometry in patients with myeloma treated with conventional or intensive therapy. Cancer 1995, 76, 1559–1563. [Google Scholar] [CrossRef]
- Mackin, D.; Ger, R.; Dodge, C.; Fave, X.; Chi, P.C.; Zhang, L.; Yang, J.; Bache, S.; Dodge, C.; Jones, A.K.; et al. Effect of tube current on computed tomography radiomic features. Sci. Rep. 2018, 8, 2354. [Google Scholar] [CrossRef]
- Foy, J.J.; Al-Hallaq, H.A.; Grekoski, V.; Tran, T.; Guruvadoo, K.; Armato Iii, S.G.; Sensakovic, W.F. Harmonization of radiomic feature variability resulting from differences in CT image acquisition and reconstruction: Assessment in a cadaveric liver. Phys. Med. Biol. 2020, 65, 205008. [Google Scholar] [CrossRef] [PubMed]

| Radiomics Features | Importance |
|---|---|
| wavelet_HLL_glcm_Imc2 | 6.284976 |
| wavelet_LLL_glcm_Imc2 | 3.911729 |
| wavelet_HHH_glszm_SmallArea Emphasis | 3.730171 |
| wavelet_LLL_gldm_Dependence Entropy | 3.620143 |
| wavelet_LHL_glcm_Imc1 | 3.452086 |
| wavelet_HLH_glcm_Correlation | 2.614805 |
| wavelet_HHL_glcm_Idmn | 2.315703 |
| wavelet_LHH_glszm_SmallAreaLowGrayLevelEmphasis | 1.965419 |
| wavelet_HLH_glcm_MCC | 1.776747 |
| wavelet_LHH_glrlm_LongRunLowGrayLevelEmphasis | 1.77367 |
| Sensitivity | Specificity | Accuracy | AUC | |
|---|---|---|---|---|
| Development set | 0.76 (0.65–0.85) | 0.78 (0.67–0.86) | 0.77 (0.70–0.83) | 0.858 (0.801–0.916) |
| Validation set | 0.75 (0.53–0.90) | 0.83 (0.63–0.95) | 0.79 (0.65–0.90) | 0.846 (0.737–0.955) |
| Diagnostic Performance | Sensitivity | Specificity | Accuracy | AUC |
|---|---|---|---|---|
| Radiomics model (A) | 75% (18/24) | 83% (20/24) | 79% (38/48) | 0.846 (0.737–0.955) |
| Readers(B) | ||||
| R1 | 75% (18/24) | 88% (21/24) | 81% (39/48) | 0.862 (0.770–0.954) |
| R2 | 79% (19/24) | 96% (23/24) | 88% (42/48) | 0.900 (0.811–0.989) |
| R3 | 79% (19/24) | 38% (9/24) | 58% (28/48) | 0.668 (0.526–0.810) |
| Comparison of A and B | ||||
| R1 | 1.000 | 1.000 | 1.000 | 0.821 |
| R2 | 1.000 | 0.375 | 0.424 | 0.451 |
| R3 | 1.000 | 0.019 * | 0.076 | 0.056 |
| Diagnostic Performance | Sensitivity | Specificity | Accuracy | AUC |
|---|---|---|---|---|
| Readers R1 | 88% (21/24) | 83% (20/24) | 85% (41/48) | 0.912 (0.832–0.993) |
| R2 | 88% (21/24) | 83% (20/24) | 85% (41/48) | 0.924 (0.851–0.998) |
| R3 | 88% (21/24) | 46% (11/24) | 67% (32/48) | 0.83 (0.712–0.947) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Lee, S.-Y.; Lee, J.; Pak, J.; Lee, K.; Lee, S.-E.; Jung, J.-Y. Detecting Multiple Myeloma Infiltration of the Bone Marrow on CT Scans in Patients with Osteopenia: Feasibility of Radiomics Analysis. Diagnostics 2022, 12, 923. https://doi.org/10.3390/diagnostics12040923
Park H, Lee S-Y, Lee J, Pak J, Lee K, Lee S-E, Jung J-Y. Detecting Multiple Myeloma Infiltration of the Bone Marrow on CT Scans in Patients with Osteopenia: Feasibility of Radiomics Analysis. Diagnostics. 2022; 12(4):923. https://doi.org/10.3390/diagnostics12040923
Chicago/Turabian StylePark, Hyerim, So-Yeon Lee, Jooyeon Lee, Juyoung Pak, Koeun Lee, Seung-Eun Lee, and Joon-Yong Jung. 2022. "Detecting Multiple Myeloma Infiltration of the Bone Marrow on CT Scans in Patients with Osteopenia: Feasibility of Radiomics Analysis" Diagnostics 12, no. 4: 923. https://doi.org/10.3390/diagnostics12040923






