A Validated Composite Score Demonstrates Potential Superiority to MELD-Based Systems in Predicting Short-Term Survival in Patients with Liver Cirrhosis and Spontaneous Bacterial Peritonitis—A Preliminary Study
Abstract
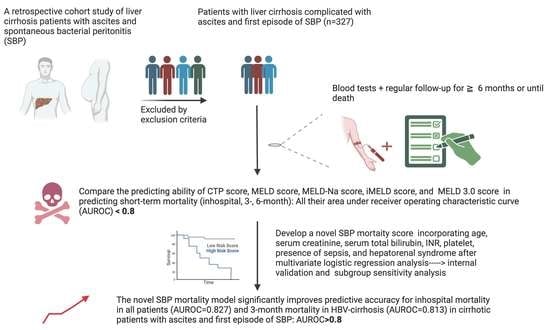
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Diagnosis, Definition, and Management of Liver Cirrhosis with Ascites and Spontaneous Bacterial Peritonitis
2.3. Primary Outcomes and Scheduled Follow-Up Periods
2.4. Calculation of the Child–Turcotte–Pugh, MELD, MELD-Na, iMELD, and MELD 3.0 Scores
2.5. Statistical Analysis
2.6. Construction of a Novel Prognostic Model
2.7. Model Validation and Sensitivity Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Comparison of Score Differences among CTP and Four MELD-Based Models between In-Hospital, 3-Month, and 6-Month Mortality and Non-Mortality Groups
3.3. Comparison of the Predicting Performance of the Original CTP, MELD, MELD-Na, iMELD, MELD 3.0, and the New SBP Scores for In-Hospital, 3-Month, and 6-Month Mortality Prediction Using AUROC and DeLong Test
3.4. Development of the New SBP Score Model
3.5. Comparative Analysis of the Predictive Performance between the New SBP Score and the Original CTP, MELD, MELD-Na, iMELD, and MELD 3.0 Scores for In-Hospital, 3-Month, and 6-Month Mortality Prediction Utilizing AUROC and DeLong Test
3.6. Model Validation
3.7. Comparative Subgroup Sensitivity Analysis of the Predictive Performance between the New SBP Score and the Original CTP, MELD, MELD-Na, iMELD, and MELD 3.0 Scores for 3-Month Mortality Prediction in Patients with HBV-Related Cirrhosis and SBP
3.8. The Cut-Off Values for the New SBP Score Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| AUROC | area under the receiver operating characteristic curve |
| GI | gastrointestinal |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HRS | hepatorenal syndrome |
| LC | liver cirrhosis |
| PLT | platelet |
| PMN | polymorphonuclear neutrophils |
| PPV | positive predictive value |
| PT-INR | Prothrombin time–International Normalized Ratio |
| SBP | spontaneous bacterial peritonitis |
| WBC | white blood cell |
References
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.; Navasa, M.; Gomez, J.; Colmenero, J.; Vila, J.; Arroyo, V.; Rodes, J. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002, 35, 140–148. [Google Scholar] [CrossRef]
- Gines, P.; Cardenas, A.; Arroyo, V.; Rodes, J. Management of cirrhosis and ascites. N. Engl. J. Med. 2004, 350, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Lee, C.; Chang, C. Spontaneous Bacterial Peritonitis in Decompensated Liver Cirrhosis—A Literature Review. Livers 2022, 2, 214–232. [Google Scholar] [CrossRef]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Gines, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Tsai, C.C.; Hsieh, Y.H.; Tsai, C.C. The long-term mortality of spontaneous bacterial peritonitis in cirrhotic patients: A 3-year nationwide cohort study. Turk. J. Gastroenterol. 2015, 26, 159–162. [Google Scholar] [CrossRef]
- Andreu, M.; Sola, R.; Sitges-Serra, A.; Alia, C.; Gallen, M.; Vila, M.C.; Coll, S.; Oliver, M.I. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology 1993, 104, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tseng, H.J.; Amodio, P.; Chen, Y.L.; Wang, S.F.; Chang, S.H.; Hsieh, S.Y.; Lin, C.Y. Hepatic Encephalopathy and Spontaneous Bacterial Peritonitis Improve Cirrhosis Outcome Prediction: A Modified Seven-Stage Model as a Clinical Alternative to MELD. J. Pers. Med. 2020, 10, 186. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Asrani, S.K.; Kim, W.R. Model for end-stage liver disease: End of the first decade. Clin. Liver Dis. 2011, 15, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Biggins, S.W.; Kim, W.R.; Terrault, N.A.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef]
- Luca, A.; Angermayr, B.; Bertolini, G.; Koenig, F.; Vizzini, G.; Ploner, M.; Peck-Radosavljevic, M.; Gridelli, B.; Bosch, J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transplant. 2007, 13, 1174–1180. [Google Scholar] [CrossRef]
- Huo, T.I.; Wang, Y.W.; Yang, Y.Y.; Lin, H.C.; Lee, P.C.; Hou, M.C.; Lee, F.Y.; Lee, S.D. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int. 2007, 27, 498–506. [Google Scholar]
- Leise, M.D.; Kim, W.R.; Kremers, W.K.; Larson, J.J.; Benson, J.T.; Therneau, T.M. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology 2011, 140, 1952–1960. [Google Scholar]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.E4. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.I.; Lin, H.C.; Huo, S.C.; Lee, P.C.; Wu, J.C.; Lee, F.Y.; Hou, M.C.; Lee, S.D. Comparison of four model for end-stage liver disease-based prognostic systems for cirrhosis. Liver Transplant. 2008, 14, 837–844. [Google Scholar] [CrossRef]
- Koo, J.K.; Kim, J.H.; Choi, Y.J.; Lee, C.I.; Yang, J.H.; Yoon, H.Y.; Choi, H.J.; Ko, S.Y.; Choe, W.H.; Kwon, S.Y.; et al. Predictive value of Refit Model for End-Stage Liver Disease, Refit Model for End-Stage Liver Disease-Na, and pre-existing scoring system for 3-month mortality in Korean patients with cirrhosis. J. Gastroenterol. Hepatol. 2013, 28, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.I.; Lin, H.C.; Wu, J.C.; Hou, M.C.; Lee, F.Y.; Lee, P.C.; Chang, F.Y.; Lee, S.D. Limitation of the model for end-stage liver disease for outcome prediction in patients with cirrhosis-related complications. Clin. Transplant. 2006, 20, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Chawla, Y.K.; Kashinath, R.C.; Duseja, A.; Dhiman, R.K. Predicting Mortality Across a Broad Spectrum of Liver Disease—An Assessment of Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and Creatinine-Modified CTP Scores. J. Clin. Exp. Hepatol. 2011, 1, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Huang, C.H.; Hsieh, S.Y. More immunosuppressive, more immunotherapy responsive? A double-edged sword of HBV-induced immune response in HCC. Hepatology, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Chen, P.C.; Chen, B.H.; Huang, C.H.; Jeng, W.J.; Hsieh, Y.C.; Teng, W.; Chen, Y.C.; Ho, Y.P.; Sheen, I.S.; Lin, C.Y. Integrated model for end-stage liver disease maybe superior to some other model for end-stage liver disease-based systems in addition to Child-Turcotte-Pugh and albumin-bilirubin scores in patients with hepatitis B virus-related liver cirrhosis and spontaneous bacterial peritonitis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, S.F.; Lee, C.H.; Wu, Y.M.; Chang, C.; Chen, B.H.; Huang, Y.T.; Ho, Y.P. Bacteremia (Sepsis), Hepatorenal Syndrome, and Serum Creatinine Levels Rather than Types or Microbial Patterns Predicted the Short-Term Survival of Cirrhotic Patients Complicated with Spontaneous Bacterial Peritonitis. Diagnostics 2023, 13, 94. [Google Scholar] [CrossRef]
- Lin, D.Y.; Sheen, I.S.; Chiu, C.T.; Lin, S.M.; Kuo, Y.C.; Liaw, Y.F. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: A longitudinal study. J. Clin. Ultrasound 1993, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA 2023, 329, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Runyon, B.A.; Montano, A.A.; Akriviadis, E.A.; Antillon, M.R.; Irving, M.A.; McHutchison, J.G. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann. Intern. Med. 1992, 117, 215–220. [Google Scholar] [CrossRef]
- Runyon, B.A. Management of adult patients with ascites caused by cirrhosis. Hepatology 1998, 27, 264–272. [Google Scholar] [CrossRef]
- Rimola, A.; García-Tsao, G.; Navasa, M.; Piddock, L.J.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. J. Hepatol. 2000, 32, 142–153. [Google Scholar]
- Lee, Y.Y.; Tee, H.P.; Mahadeva, S. Role of prophylactic antibiotics in cirrhotic patients with variceal bleeding. World J. Gastroenterol. 2014, 20, 1790–1796. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Angeli, P.; Gines, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.F.; Leung, N.; Kao, J.H.; Piratvisuth, T.; Gane, E.; Han, K.H.; Guan, R.; Lau, G.K.; Locarnini, S. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatol. Int. 2008, 2, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.F.; Kao, J.H.; Piratvisuth, T.; Chan, H.L.; Chien, R.N.; Liu, C.J.; Gane, E.; Locarnini, S.; Lim, S.G.; Han, K.H.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol. Int. 2012, 6, 531–561. [Google Scholar] [CrossRef] [Green Version]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef]
- Pugh, R.; Murray-Lyon, I.; Dawson, J.; Pietroni, M.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar]
- Maldonado, G.; Greenland, S. Simulation study of confounder-selection strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmast, N.; Ogola, G.O.; Kouznetsova, M.; Leise, M.D.; Bahirwani, R.; Maiwall, R.; Tapper, E.; Trotter, J.; Bajaj, J.S.; Thacker, L.R.; et al. Model for End-Stage Liver Disease-Lactate and Prediction of Inpatient Mortality in Patients With Chronic Liver Disease. Hepatology 2020, 72, 1747–1757. [Google Scholar] [CrossRef]
- Lata, J.; Stiburek, O.; Kopacova, M. Spontaneous bacterial peritonitis: A severe complication of liver cirrhosis. World J. Gastroenterol. 2009, 15, 5505–5510. [Google Scholar] [CrossRef]
- Aithal, G.P.; Palaniyappan, N.; China, L.; Harmala, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Morss, S.; Thompson, R. Spontaneous bacterial peritonitis—In-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am. J. Gastroenterol. 2001, 96, 1232–1236. [Google Scholar] [CrossRef]
- Huang, C.H.; Lin, C.Y.; Sheen, I.S.; Chen, W.T.; Lin, T.N.; Ho, Y.P.; Chiu, C.T. Recurrence of spontaneous bacterial peritonitis in cirrhotic patients non-prophylactically treated with norfloxacin: Serum albumin as an easy but reliable predictive factor. Liver Int. 2011, 31, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001, 120, 726–748. [Google Scholar] [CrossRef]
- Kaplan, D.E.; Dai, F.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Knott, A.; Pedrosa, M.; Pocha, C.; Mehta, R.; et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin. Gastroenterol. Hepatol. 2015, 13, 2333–2341.E6. [Google Scholar] [CrossRef] [Green Version]
- Oellerich, M.; Burdelski, M.; Lautz, H.U.; Rodeck, B.; Duewel, J.; Schulz, M.; Schmidt, F.W.; Brodehl, J.; Pichlmayr, R. Assessment of pretransplant prognosis in patients with cirrhosis. Transplantation 1991, 51, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; McKinley, C.; Showalter, R.; Wilner, K.; Marsano, L.; Vivian, B.; Everson, G.T. Quantitative liver function tests define the functional severity of liver disease in early-stage cirrhosis. Liver Transplant. Surg. 1997, 3, 166–173. [Google Scholar] [CrossRef]
- Testa, R.; Valente, U.; Risso, D.; Caglieris, S.; Giannini, E.; Fasoli, A.; Botta, F.; Dardano, G.; Lantieri, P.B.; Celle, G. Can the MEGX test and serum bile acids improve the prognostic ability of Child-Pugh’s score in liver cirrhosis? Eur. J. Gastroenterol. Hepatol. 1999, 11, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, K.M.; Brown, R.S., Jr.; Delmonico, F.L.; Freeman, R.B.; McDiarmid, S.V.; Merion, R.M.; Millis, J.M.; Roberts, J.P.; Shaked, A.; Wiesner, R.H.; et al. Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era. Liver Transplant. 2004, 10 (Suppl. S2), A6–A22. [Google Scholar] [CrossRef]
- Said, A.; Williams, J.; Holden, J.; Remington, P.; Gangnon, R.; Musat, A.; Lucey, M.R. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J. Hepatol. 2004, 40, 897–903. [Google Scholar] [CrossRef]
- Chalasani, N.; Kahi, C.; Francois, F.; Pinto, A.; Marathe, A.; Bini, E.J.; Pandya, P.; Sitaraman, S.; Shen, J. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology 2002, 35, 1282–1284. [Google Scholar] [CrossRef]
- Biggins, S.W.; Rodriguez, H.J.; Bacchetti, P.; Bass, N.M.; Roberts, J.P.; Terrault, N.A. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005, 41, 32–39. [Google Scholar] [CrossRef]
- Ruf, A.E.; Kremers, W.K.; Chavez, L.L.; Descalzi, V.I.; Podesta, L.G.; Villamil, F.G. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transplant. 2005, 11, 336–343. [Google Scholar] [CrossRef]
- Yang, H.I.; Lu, S.N.; Liaw, Y.F.; You, S.L.; Sun, C.A.; Wang, L.Y.; Hsiao, C.K.; Chen, P.J.; Chen, D.S.; Chen, C.J.; et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 2002, 347, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, A.J.; Zhang, K.Y.; Ebel, N.; Mannalithara, A.; Kim, W.R. MELD 3.0 for adolescent liver transplant candidates. Hepatology 2023, 78, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Sort, P.; Navasa, M.; Arroyo, V.; Aldeguer, X.; Planas, R.; Ruiz-del-Arbol, L.; Castells, L.; Vargas, V.; Soriano, G.; Guevara, M.; et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 1999, 341, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Baseline Parameters | Values |
|---|---|
| Clinical Parameters | |
| Age, mean ± SD | 57.1 ± 13.6 |
| Male No. (%) | 236 (72%) |
| Etiology No. (%) | |
| Alcohol | 90 (27.5%) |
| HBV | 148 (45.3%) |
| HCV | 78 (23.9%) |
| Others | 11 (3.4%) |
| Blood culture positive | 42(12.8%) |
| Hepatorenal syndrome | 31(9.5%) |
| Hepatic encephalopathy | |
| Grade 0 | 243(74.3%) |
| Grade 1 | 29(8.9%) |
| Grade 2 | 32(9.8%) |
| Grade 3 | 15(4.6%) |
| Grade 4 | 8(2.4%) |
| Ascites | |
| Mild | 43(13.2%) |
| Moderate | 76(23.2%) |
| Severe | 208(63.6%) |
| Laboratory Parameters Median (IQR) | |
| Creatinine (mg/dL) | 1.2 (0.86–2.41) |
| Bilirubin Total (mg/dL) | 4.1 (1.9–9.8) |
| Sodium (mEq/L) | 135 (131–139) |
| Albumin (g/dL) | 2.4 (2.2–2.8) |
| INR | 1.64 (1.39–2.20) |
| WBC (103/μL) | 7.9 (5.20–12.80) |
| Hemoglobin (g/dL) | 9.6 (8.4–11.0) |
| PLT (103/μL) | 74.0 (48.0–122.0) |
| Ascites PMN (cells/mm3) | 1393.5 (344.3–4203.0) |
| CTP score | 10.1 ± 1.9 |
| MELD score | 22.7 ± 9.3 |
| MELD-Na score | 25.1 ± 8.5 |
| iMELD score | 45.6 ± 10.4 |
| MELD 3.0 score | 25.4 ± 8.5 |
| Mortality | Non-Mortality | p | |
|---|---|---|---|
| In-hospital | n = 129 (39.4%) | n = 198 (60.6%) | |
| CTP score | 10.95 ± 1.785 | 9.58 ± 1.839 | <0.001 |
| MELD score | 28.3977 ± 10.28562 | 19.0414 ± 6.37866 | <0.001 |
| MELD-Na score | 30.3214 ± 8.59616 | 21.7216 ± 6.57153 | <0.001 |
| iMELD score | 51.8581 ± 10.17405 | 41.4468 ± 8.26332 | <0.001 |
| MELD 3.0 score | 30.6357 ± 8.50546 | 21.9747 ± 6.52013 | <0.001 |
| 3-Month | n = 168 (51.4%) | n = 159 (48.6%) | |
| CTP score | 10.64 ± 1.865 | 9.57 ± 1.861 | <0.001 |
| MELD score | 26.5179 ± 10.19027 | 18.7327 ± 6.21459 | <0.001 |
| MELD-Na score | 28.7469 ± 8.74900 | 21.2759 ± 6.36454 | <0.001 |
| iMELD score | 50.0182 ± 10.50086 | 40.8371 ± 790264 | <0.001 |
| MELD 3.0 score | 29.0714 ± 8.70169 | 21.5031 ± 6.24892 | <0.001 |
| 6-Month | n = 199 (60.9%) | n = 128 (39.1%) | |
| CTP score | 10.50 ± 1.864 | 9.52 ± 1.903 | <0.001 |
| MELD score | 25.5815 ± 9.90270 | 18.3030 ± 6.18074 | <0.001 |
| MELD-Na score | 27.8953 ± 8.7364 | 20.7905 ± 6.43326 | <0.001 |
| iMELD score | 49.1069 ± 10.34567 | 40.0304 ± 7.71442 | <0.001 |
| MELD 3.0 score | 28.1457 ± 8.52293 | 21.1094 ± 6.43224 | <0.001 |
| Predicting In-Hospital Mortality | ||||||
|---|---|---|---|---|---|---|
| CTP Score | MELD Score | MELD-Na Score | iMELD Score | MELD 3.0 Score | New SBP Score | |
| AUROC | 0.701 | 0.775 | 0.783 | 0.780 | 0.786 | 0.827 |
| (95% CI) | 0.64–0.76 | 0.72–0.83 | 0.73–0.83 | 0.73–0.83 | 0.73–0.84 | 0.78–0.87 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CTP score | ||||||
| MELD score | 0.0013 | |||||
| MELD-Na score | 0.0003 | 0.4608 | ||||
| iMELD score | 0.0041 | 0.7843 | 0.8778 | |||
| MELD 3.0 score | 0.0001 | 0.2789 | 0.5814 | 0.7370 | ||
| New SBP score | <0.0001 | 0.0196 | 0.0471 | 0.0240 | 0.0651 | |
| Predicting 3-Month Mortality | ||||||
|---|---|---|---|---|---|---|
| CTP Score | MELD Score | MELD-Na Score | iMELD Score | MELD 3.0 Score | New SBP Score | |
| AUROC | 0.656 | 0.737 | 0.755 | 0.753 | 0.760 | 0.789 |
| (95% CI) | 0.60–0.71 | 0.68–0.79 | 0.70–0.81 | 0.70–0.81 | 0.71–0.81 | 0.74–0.84 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CTP score | ||||||
| MELD score | 0.0007 | |||||
| MELD-Na score | <0.0001 | 0.0799 | ||||
| iMELD score | 0.0007 | 0.4011 | 0.8833 | |||
| MELD 3.0 score | <0.0001 | 0.0209 | 0.4002 | 0.6702 | ||
| New SBP score | <0.0001 | 0.0266 | 0.1499 | 0.1074 | 0.2160 | |
| Predicting 6-Month Mortality | ||||||
|---|---|---|---|---|---|---|
| CTP Score | MELD Score | MELD-Na Score | iMELD Score | MELD 3.0 Score | New SBP Score | |
| AUROC | 0.640 | 0.728 | 0.745 | 0.752 | 0.742 | 0.762 |
| (95% CI) | 0.58–0.70 | 0.67–0.78 | 0.69–0.80 | 0.70–0.80 | 0.69–0.80 | 0.71–0.81 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CTP score | ||||||
| MELD score | 0.0007 | |||||
| MELD-Na score | <0.0001 | 0.0623 | ||||
| iMELD score | 0.0001 | 0.1345 | 0.5149 | |||
| MELD 3.0 score | <0.0001 | 0.0772 | 0.8966 | 0.4950 | ||
| New SBP score | 0.0008 | 0.1115 | 0.4330 | 0.6602 | 0.4094 | |
| Predicting 3-Month Mortality in HBV Subgroup | ||||||
|---|---|---|---|---|---|---|
| CTP Score | MELD Score | MELD-Na Score | iMELD Score | MELD 3.0 Score | New SBP Score | |
| AUC | 0.700 | 0.755 | 0.760 | 0.736 | 0.765 | 0.813 |
| (95% CI) | 0.62–0.78 | 0.68–0.83 | 0.68–0.84 | 0.66–0.82 | 069–0.84 | 0.73–0.89 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CTP score | ||||||
| MELD score | 0.1442 | |||||
| MELD-Na score | 0.1014 | 0.6895 | ||||
| iMELD score | 0.4245 | 0.5233 | 0.2928 | |||
| MELD 3.0 score | 0.0711 | 0.4827 | 0.5569 | 0.2254 | ||
| New SBP score | 0.0291 | 0.0476 | 0.0886 | 0.0446 | 0.2035 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Chen, W.-T.; Wu, T.-H.; Liu, Y.; Liu, L.-T.; Teng, W.; Hsieh, Y.-C.; Wu, Y.-M.; Huang, C.-H.; Hsu, C.-W.; et al. A Validated Composite Score Demonstrates Potential Superiority to MELD-Based Systems in Predicting Short-Term Survival in Patients with Liver Cirrhosis and Spontaneous Bacterial Peritonitis—A Preliminary Study. Diagnostics 2023, 13, 2578. https://doi.org/10.3390/diagnostics13152578
Lin Y-T, Chen W-T, Wu T-H, Liu Y, Liu L-T, Teng W, Hsieh Y-C, Wu Y-M, Huang C-H, Hsu C-W, et al. A Validated Composite Score Demonstrates Potential Superiority to MELD-Based Systems in Predicting Short-Term Survival in Patients with Liver Cirrhosis and Spontaneous Bacterial Peritonitis—A Preliminary Study. Diagnostics. 2023; 13(15):2578. https://doi.org/10.3390/diagnostics13152578
Chicago/Turabian StyleLin, Yan-Ting, Wei-Ting Chen, Tsung-Han Wu, Yu Liu, Li-Tong Liu, Wei Teng, Yi-Chung Hsieh, Yen-Mu Wu, Chien-Hao Huang, Chao-Wei Hsu, and et al. 2023. "A Validated Composite Score Demonstrates Potential Superiority to MELD-Based Systems in Predicting Short-Term Survival in Patients with Liver Cirrhosis and Spontaneous Bacterial Peritonitis—A Preliminary Study" Diagnostics 13, no. 15: 2578. https://doi.org/10.3390/diagnostics13152578