An Intra-Individual Comparison of Low-keV Photon-Counting CT versus Energy-Integrating-Detector CT Angiography of the Aorta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Compliance
2.2. Study Protocol and Patient Population
2.3. CTA Protocols
2.4. Image Reconstruction
2.5. Contrast Agent Protocol
2.6. Quantitative Image Comparisons
2.7. Qualitative Image Comparisons
2.8. Statistical Analysis
3. Results
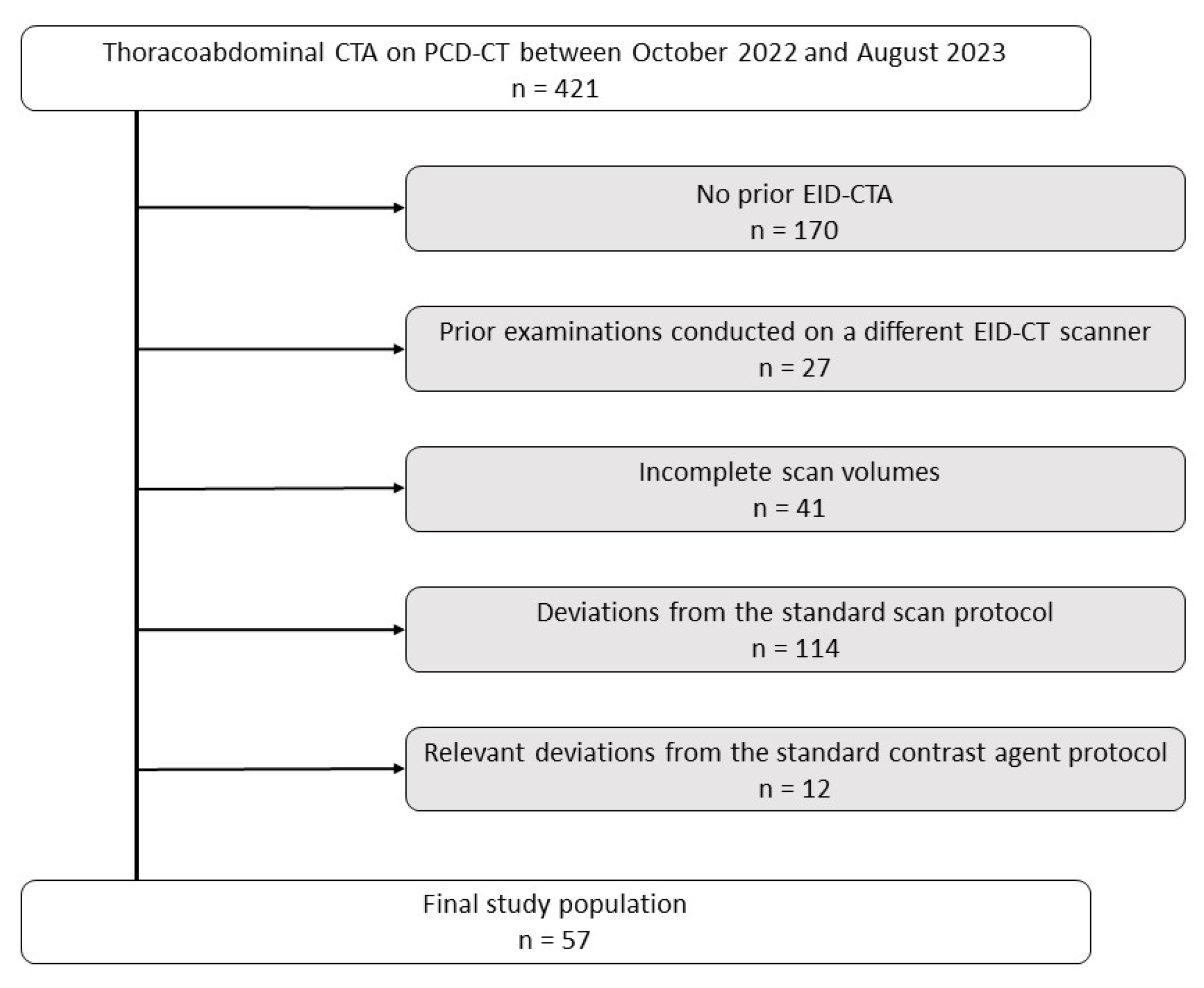
3.1. Patient Characteristics and Radiation Dose
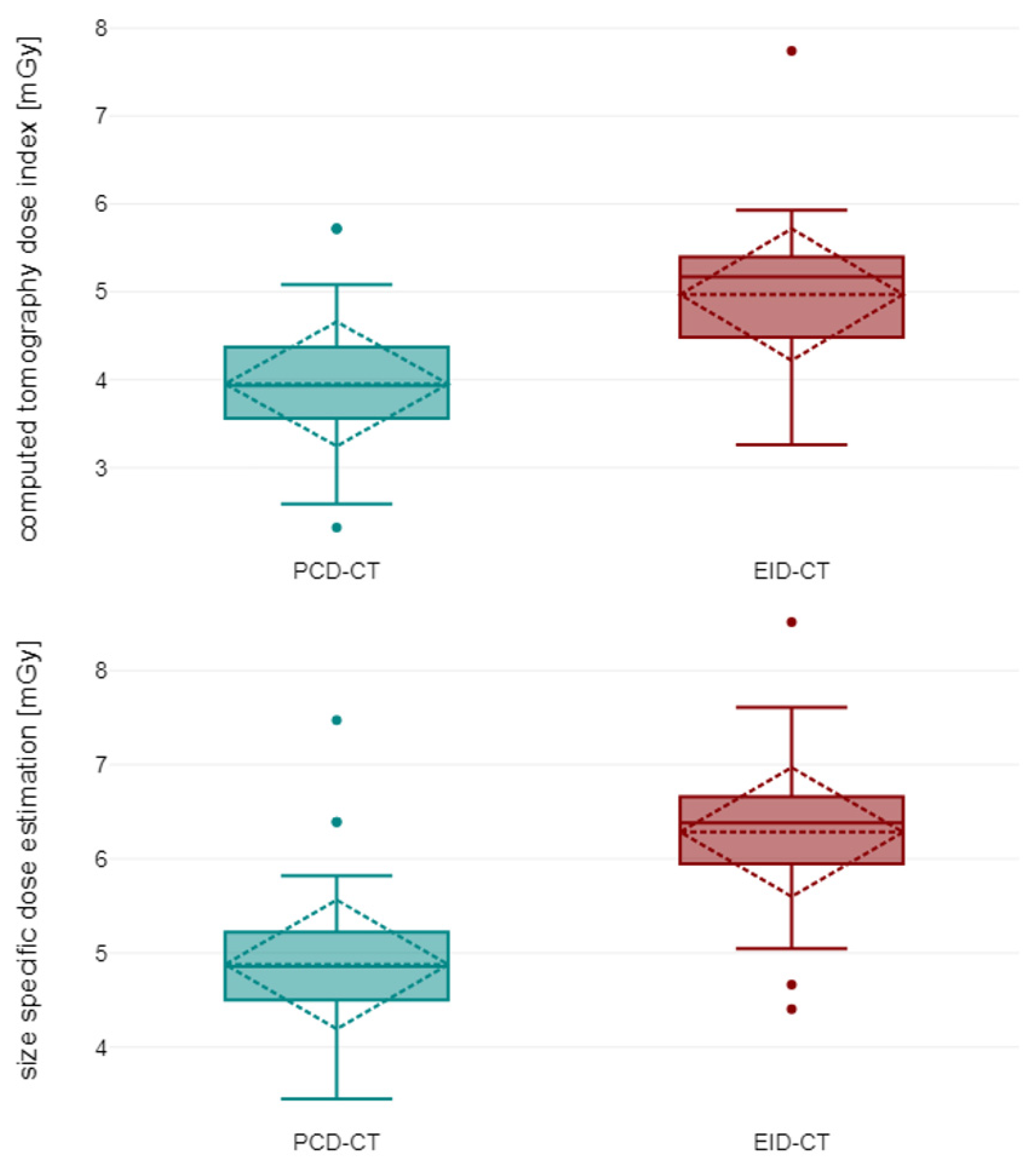
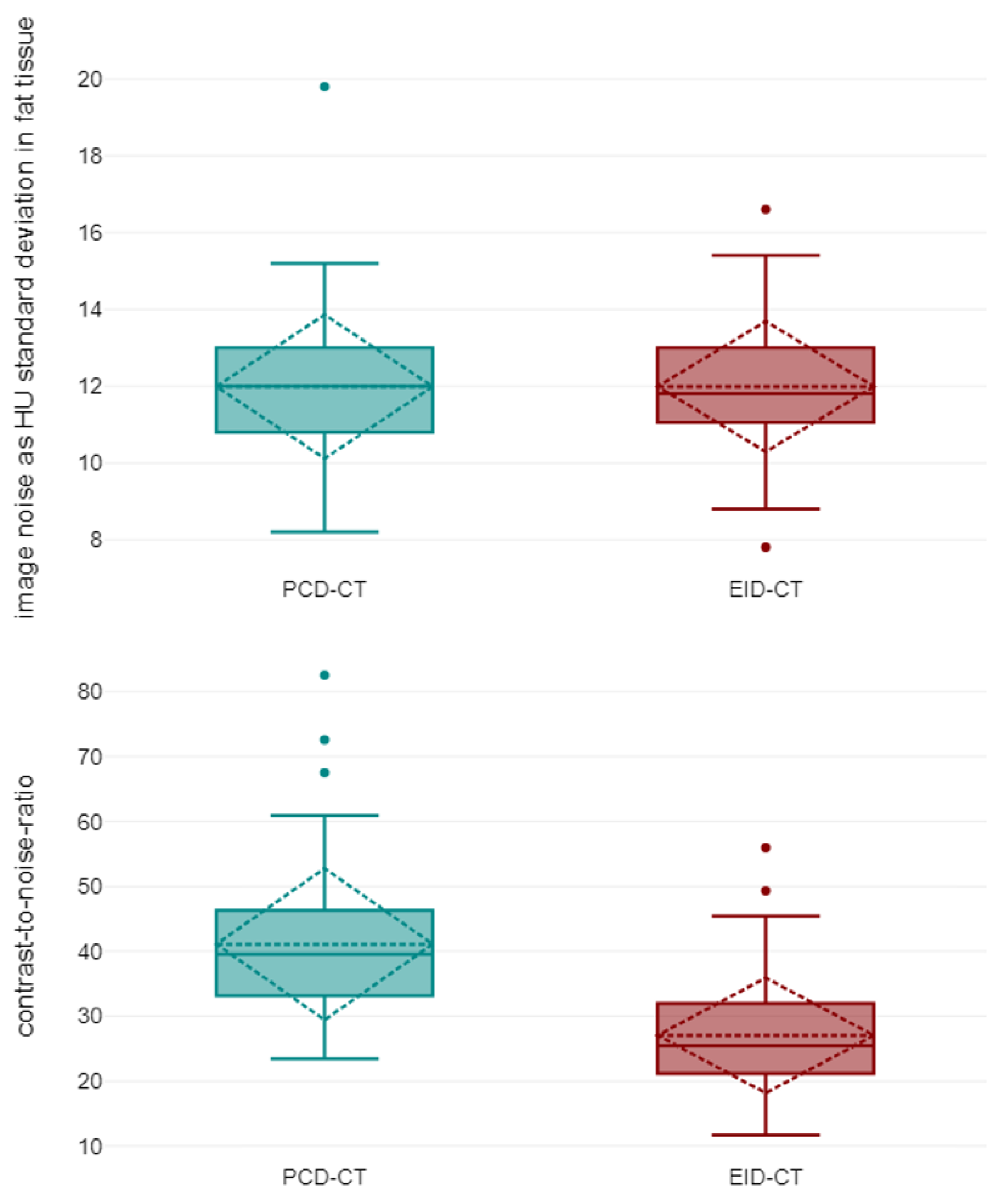
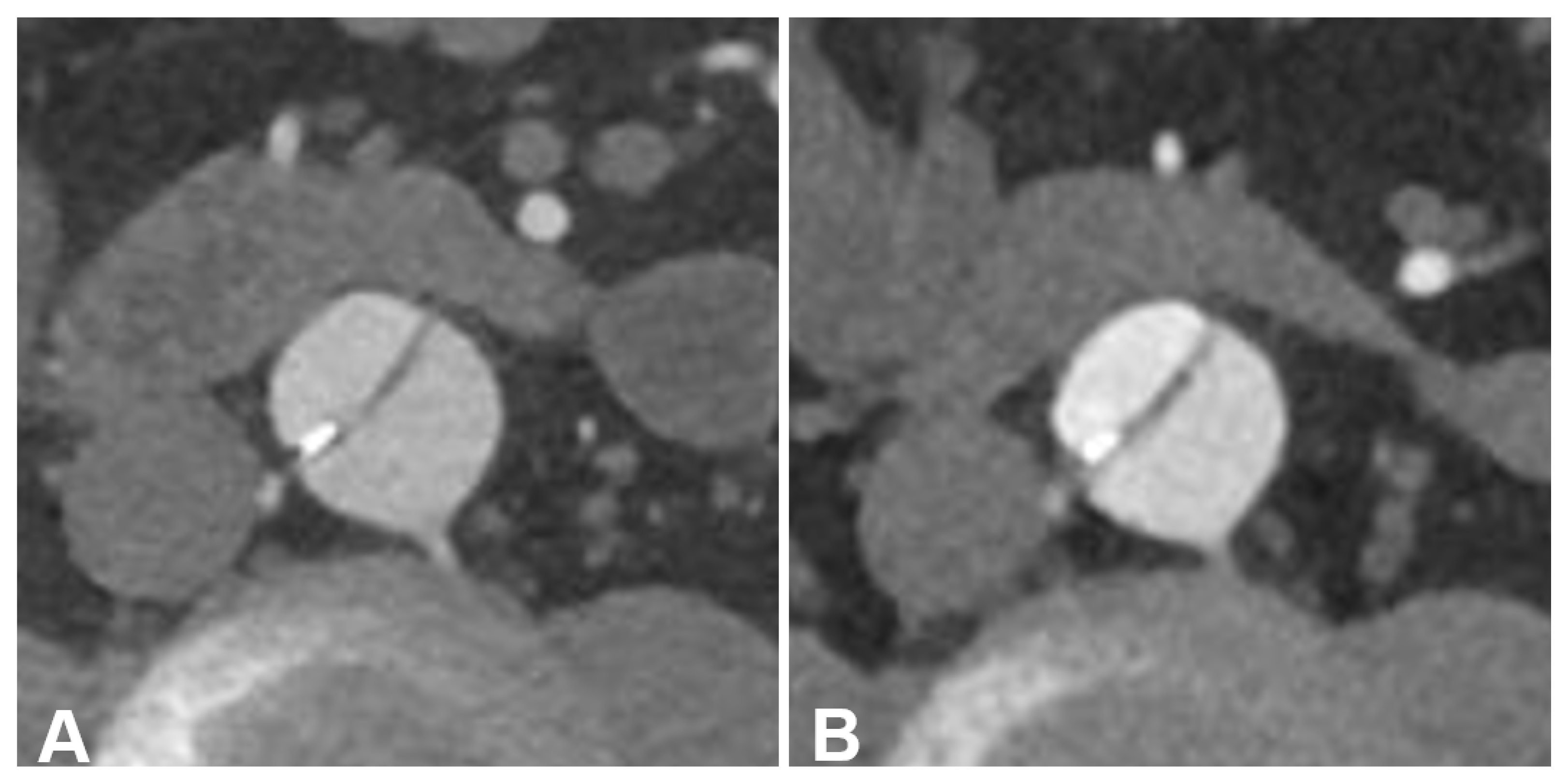
3.2. Objective Image Quality
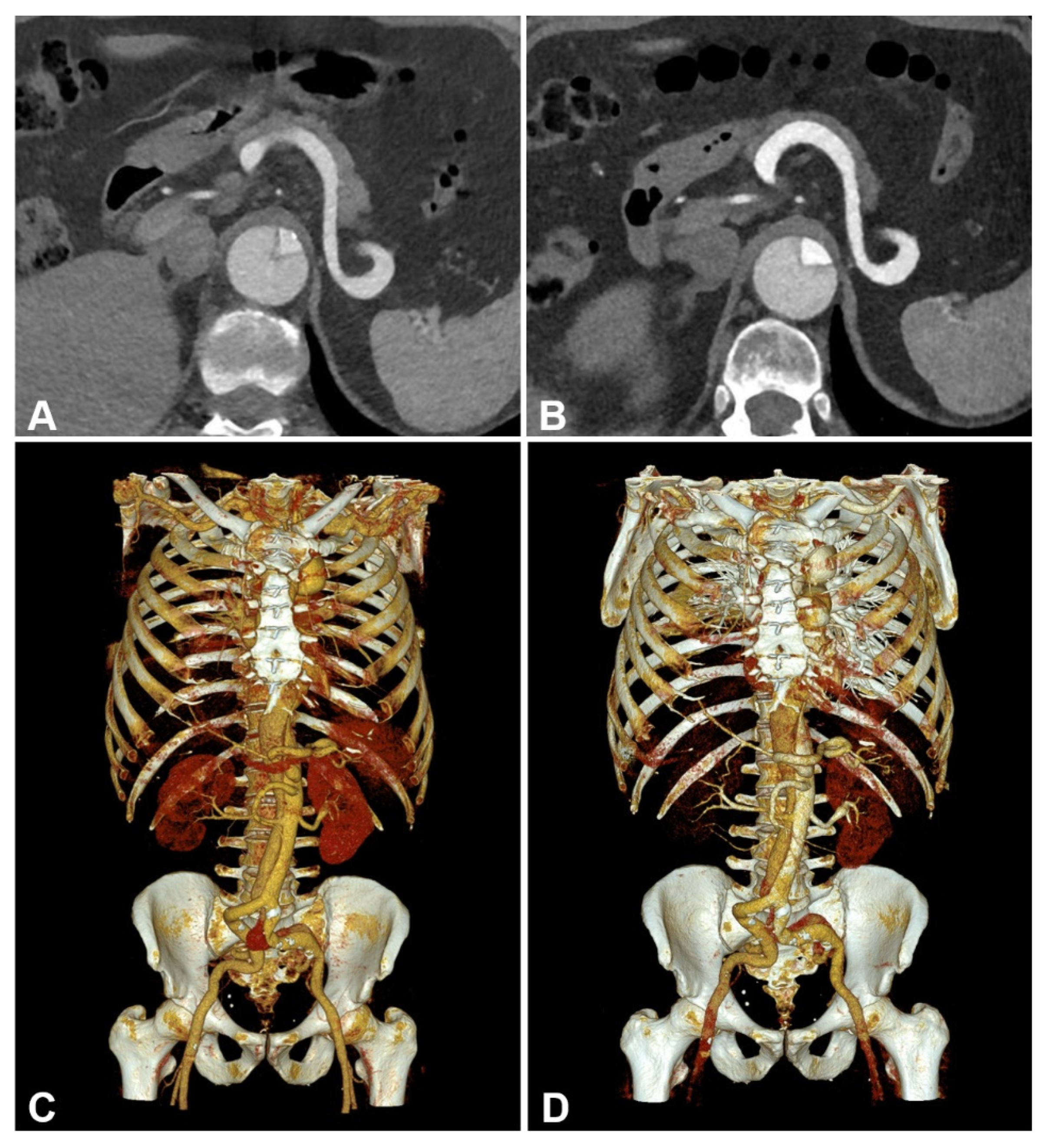
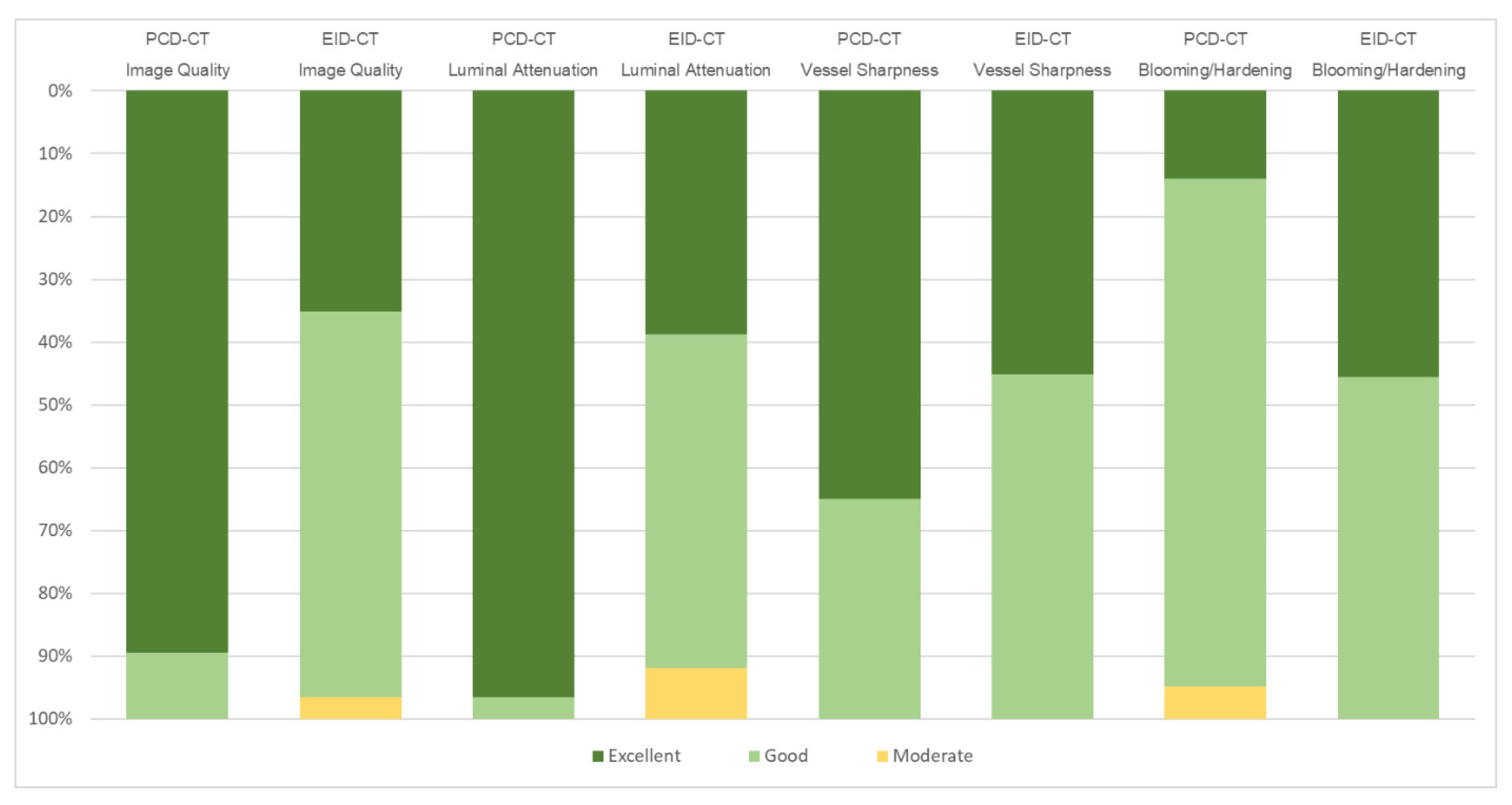
3.3. Subjective Image Quality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Sampson, U.K.; Norman, P.E.; Fowkes, F.G.; Aboyans, V.; Yanna, S.; Harrell, F.E., Jr.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M.; et al. Global and regional burden of aortic dissection and aneurysms: Mortality trends in 21 world regions, 1990 to 2010. Glob. Heart 2014, 9, 171–180.e110. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Freyhardt, P.; Solowjowa, N.; Böning, G.; Kahn, J.; Aufmesser, B.; Haage, P.; Streitparth, F. CT-angiography of the aorta in patients with Marfan disease—High-pitch MDCT at different levels of tube voltage combined with Sinogram Affirmed Iterative Reconstruction. Clin. Imaging 2018, 51, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Johnson, P.T.; Dietz, H.C.; Brooke, B.S.; Arnaoutakis, G.J.; Black, J.H., 3rd; Fishman, E.K. Vascular complications of Ehlers-Danlos syndrome: CT findings. AJR Am. J. Roentgenol. 2012, 198, 482–487. [Google Scholar] [CrossRef]
- Winklehner, A.; Goetti, R.; Baumueller, S.; Karlo, C.; Schmidt, B.; Raupach, R.; Flohr, T.; Frauenfelder, T.; Alkadhi, H. Automated attenuation-based tube potential selection for thoracoabdominal computed tomography angiography: Improved dose effectiveness. Investig. Radiol. 2011, 46, 767–773. [Google Scholar] [CrossRef]
- Johansen, C.B.; Martinsen, A.C.T.; Enden, T.R.; Svanteson, M. The potential of iodinated contrast reduction in dual-energy CT thoracic angiography; an evaluation of image quality. Radiography 2022, 28, 2–7. [Google Scholar] [CrossRef]
- Dubourg, B.; Caudron, J.; Lestrat, J.P.; Bubenheim, M.; Lefebvre, V.; Godin, M.; Tron, C.; Eltchaninoff, H.; Bauer, F.; Dacher, J.N. Single-source dual-energy CT angiography with reduced iodine load in patients referred for aortoiliofemoral evaluation before transcatheter aortic valve implantation: Impact on image quality and radiation dose. Eur. Radiol. 2014, 24, 2659–2668. [Google Scholar] [CrossRef]
- Bos, D.; König, B.; Blex, S.; Zensen, S.; Opitz, M.; Maier, S.; Forsting, M.; Zylka, W.; Kühl, H.; Wetter, A.; et al. Experimental examination of radiation doses of dual- and single-energy computed tomography in chest and upper abdomen in a phantom study. Radiat. Prot. Dosim. 2021, 193, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Solomon, J.; Marin, D.; Nelson, R.C.; Samei, E. A Third-Generation Adaptive Statistical Iterative Reconstruction Technique: Phantom Study of Image Noise, Spatial Resolution, Lesion Detectability, and Dose Reduction Potential. Am. J. Roentgenol. 2018, 210, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Geng, D.; Zhang, J. Noise-optimized virtual monoenergetic imaging technology of the third-generation dual-source computed tomography and its clinical applications. Quant. Imaging Med. Surg. 2021, 11, 4627–4643. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Investig. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hagen, F.; Hofmann, J.; Wrazidlo, R.; Gutjahr, R.; Schmidt, B.; Faby, S.; Nikolaou, K.; Horger, M. Image quality and dose exposure of contrast-enhanced abdominal CT on a 1st generation clinical dual-source photon-counting detector CT in obese patients vs. a 2nd generation dual-source dual energy integrating detector CT. Eur. J. Radiol. 2022, 151, 110325. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ishii, K.; Hosokawa, C.; Hyodo, T.; Kashiwagi, N.; Matsuki, M.; Ashikaga, R.; Murakami, T. Clinical Application of 3D Arterial Spin-Labeled Brain Perfusion Imaging for Alzheimer Disease: Comparison with Brain Perfusion SPECT. Am. J. Neuroradiol. 2014, 35, 906–911. [Google Scholar] [CrossRef]
- Higashigaito, K.; Mergen, V.; Eberhard, M.; Jungblut, L.; Hebeisen, M.; Rätzer, S.; Zanini, B.; Kobe, A.; Martini, K.; Euler, A.; et al. CT Angiography of the Aorta Using Photon-counting Detector CT with Reduced Contrast Media Volume. Radiol. Cardiothorac. Imaging 2023, 5, e220140. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Higashigaito, K.; Mergen, V.; Sartoretti, T.; Zanini, B.; Schmidt, B.; Flohr, T.G.; Ulzheimer, S.; Eberhard, M.; Alkadhi, H. High-Pitch Photon-Counting Detector Computed Tomography Angiography of the Aorta: Intraindividual Comparison to Energy-Integrating Detector Computed Tomography at Equal Radiation Dose. Investig. Radiol. 2022, 57, 115–121. [Google Scholar] [CrossRef]
- Dunning, C.A.S.; Rajendran, K.; Inoue, A.; Rajiah, P.; Weber, N.; Fletcher, J.G.; McCollough, C.H.; Leng, S. Optimal Virtual Monoenergetic Photon Energy (keV) for Photon-Counting-Detector Computed Tomography Angiography. J. Comput. Assist. Tomogr 2023, 47, 569–575. [Google Scholar] [CrossRef]
- Dillinger, D.; Overhoff, D.; Booz, C.; Kaatsch, H.L.; Piechotka, J.; Hagen, A.; Froelich, M.F.; Vogl, T.J.; Waldeck, S. Impact of CT Photon-Counting Virtual Monoenergetic Imaging on Visualization of Abdominal Arterial Vessels. Diagnostics 2023, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Beeres, M.; Wichmann, J.L.; Frellesen, C.; Bucher, A.M.; Albrecht, M.; Scholtz, J.E.; Nour-Eldin, N.E.; Gruber-Rouh, T.; Lee, C.; Vogl, T.J.; et al. ECG-gated Versus Non-ECG-gated High-pitch Dual-source CT for Whole Body CT Angiography (CTA). Acad. Radiol. 2016, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Talei Franzesi, C.R.; Ippolito, D.; Riva, L.; Fior, D.; Cangiotti, C.; Sironi, S. Diagnostic value of iterative reconstruction algorithm in low kV CT angiography (CTA) with low contrast medium volume for transcatheter aortic valve implantation (TAVI) planning: Image quality and radiation dose exposure. Br. J. Radiol. 2018, 91, 20170802. [Google Scholar] [CrossRef] [PubMed]
- Gruschwitz, P.; Hartung, V.; Kleefeldt, F.; Ergün, S.; Huflage, H.; Peter, D.; Hendel, R.; Patzer, T.S.; Pannenbecker, P.; Kuhl, P.J.; et al. Photon-Counting Versus Energy-Integrating Detector CT Angiography of the Lower Extremity in a Human Cadaveric Model with Continuous Extracorporeal Perfusion. Investig. Radiol. 2023, 58, 740–745. [Google Scholar] [CrossRef]
- Mileto, A.; Ramirez-Giraldo, J.C.; Nelson, R.C.; Hurwitz, L.M.; Choudhury, K.R.; Seaman, D.M.; Marin, D. High-Pitch Dual-Source MDCT for Imaging of the Thoracoabdominal Aorta: Relationships Among Radiation Dose, Noise, Pitch, and Body Size in a Phantom Experiment and Clinical Study. AJR Am. J. Roentgenol. 2015, 205, 834–839. [Google Scholar] [CrossRef]
| Parameters/Settings | EID-CT | PCD-CT |
|---|---|---|
| Collimation | 192 mm × 0.6 mm * | 144 mm × 0.4 mm |
| Tube voltage | 90/100 kVp (ATVS) | 120 kVp |
| Rotation time | 0.25 s | 0.25 s |
| Pitch | 3.2 | 3.2 |
| Reconstruction kernel | Bv36 | Bv36 |
| Slice thickness/increment | 3.0 mm | 3.0 mm |
| Iterative reconstruction level | ADMIRE, level 3 | QIR, level 3 |
| Characteristics | EID-CT | PCD-CT | p-Value |
|---|---|---|---|
| Age (years) | 62.84 ± 10.13 | 64.19 ± 10.31 | <0.001 |
| Weight (kg) | 78.00 (67.00–89.00) | 75.00 (65.50–84.50) | 0.285 |
| Height (m) | 1.78 (1.68–1.88) | 1.76 (1.62–1.90) | 0.657 |
| BMI (kg/m2) | 24.92 ± 2.36 | 24.68 ± 2.20 | 0.187 |
| CTDIVol (mGy) | 4.97 ± 0.57 | 3.95 ± 0.54 | <0.001 |
| SSDE (mGy) | 6.28 ± 0.50 | 4.88 ± 0.48 | <0.001 |
| Image Quality Parameter | EID-CT | PCD-CT | p-Value |
|---|---|---|---|
| Vessel attenuation avg. (HU) | 368.80 ± 62.63 | 528.53 ± 77.28 | <0.001 |
| Attenuation muscle avg. (HU) | 53.80 ± 5.02 | 47.57 ± 5.80 | <0.001 |
| SD attenuation fat avg. (HU) | 11.99 ± 1.32 | 11.99 ± 1.40 | 0.943 |
| Contrast-to-noise-ratio | 27.05 ± 6.73 | 41.11 ± 8.68 | <0.001 |
| Dose-corrected contrast-to-noise-ratio | 12.33 ± 3.44 | 21.25 ± 5.54 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennes, J.-L.; Huflage, H.; Grunz, J.-P.; Hartung, V.; Augustin, A.M.; Patzer, T.S.; Pannenbecker, P.; Petritsch, B.; Bley, T.A.; Gruschwitz, P. An Intra-Individual Comparison of Low-keV Photon-Counting CT versus Energy-Integrating-Detector CT Angiography of the Aorta. Diagnostics 2023, 13, 3645. https://doi.org/10.3390/diagnostics13243645
Hennes J-L, Huflage H, Grunz J-P, Hartung V, Augustin AM, Patzer TS, Pannenbecker P, Petritsch B, Bley TA, Gruschwitz P. An Intra-Individual Comparison of Low-keV Photon-Counting CT versus Energy-Integrating-Detector CT Angiography of the Aorta. Diagnostics. 2023; 13(24):3645. https://doi.org/10.3390/diagnostics13243645
Chicago/Turabian StyleHennes, Jan-Lucca, Henner Huflage, Jan-Peter Grunz, Viktor Hartung, Anne Marie Augustin, Theresa Sophie Patzer, Pauline Pannenbecker, Bernhard Petritsch, Thorsten Alexander Bley, and Philipp Gruschwitz. 2023. "An Intra-Individual Comparison of Low-keV Photon-Counting CT versus Energy-Integrating-Detector CT Angiography of the Aorta" Diagnostics 13, no. 24: 3645. https://doi.org/10.3390/diagnostics13243645





