Five Years’ Experience with Gene Panel Sequencing in Hereditary Hemolytic Anemia Screened by Routine Peripheral Blood Smear Examination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and DNA Extraction
2.2. Panel Design and Library Preparation
2.3. Gene Panel Sequencing and Bioinformatics Analysis
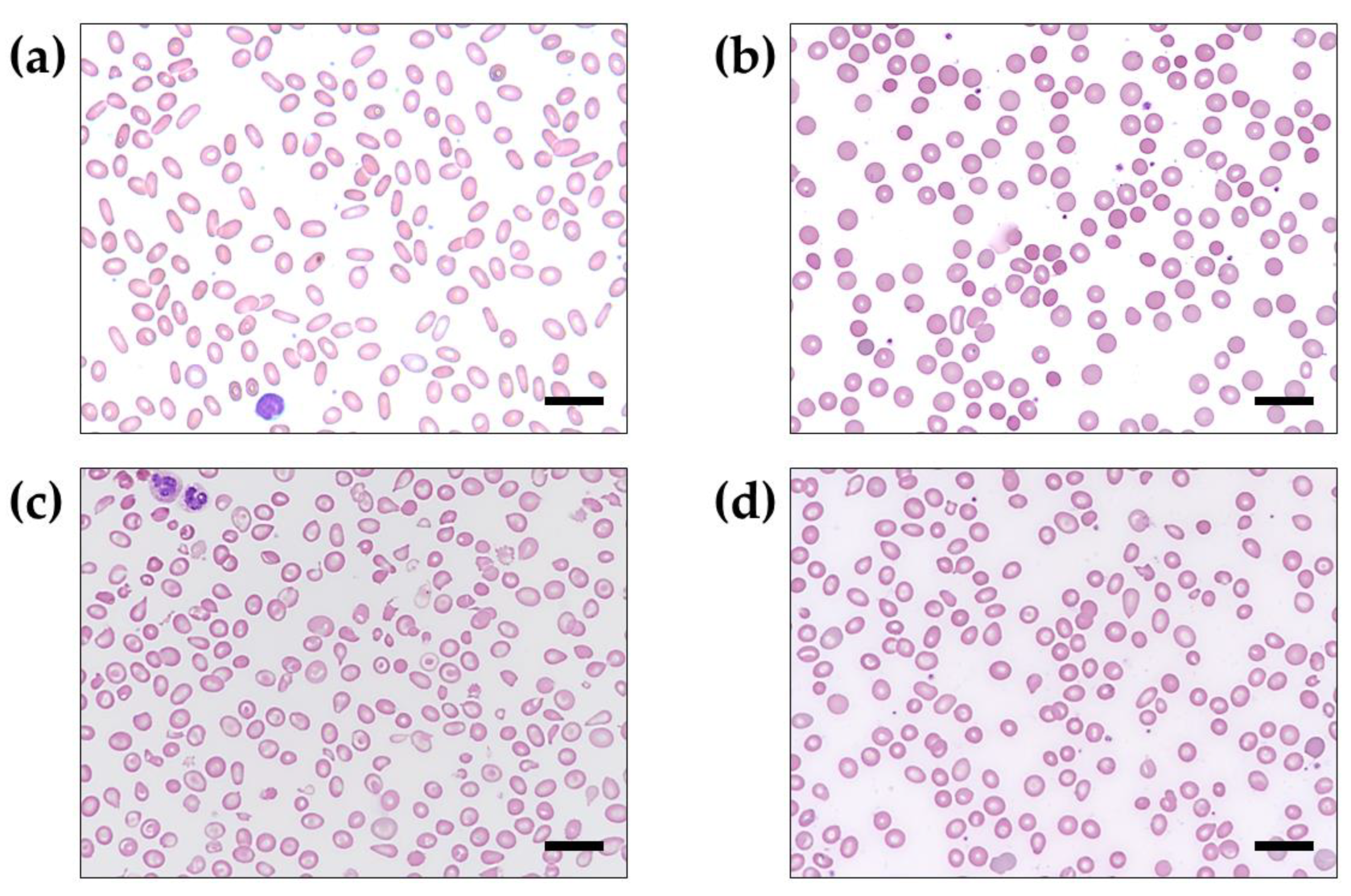
3. Results
3.1. Sequencing Quality Metrics and Overall Custom Designed Performance
3.2. Molecular Diagnosis of the Suspected Hereditary Hemolytic Anemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Park, J.; Kim, M. Diagnostic approaches for inherited hemolytic anemia in the genetic era. Blood Res. 2017, 52, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, R.F.; Glader, B. Red Blood Cell Enzyme Disorders. Pediatr. Clin. N. Am. 2018, 65, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Sabath, D.E. Molecular Diagnosis of Thalassemias and Hemoglobinopathies: An ACLPS Critical Review. Am. J. Clin. Pathol. 2017, 148, 6–15. [Google Scholar] [CrossRef]
- Shim, Y.J.; Jung, H.L.; Shin, H.Y.; Kang, H.J.; Choi, J.Y.; Hah, J.O.; Lee, J.M.; Lim, Y.T.; Yang, E.J.; Baek, H.J.; et al. Epidemiological Study of Hereditary Hemolytic Anemia in the Korean Pediatric Population during 1997-2016: A Nationwide Retrospective Cohort Study. J. Korean Med. Sci. 2020, 35, e279. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, Q.; Kim, J.A.; Im, K.O.; Park, S.N.; Park, Y.; Shin, H.Y.; Kang, H.J.; Kook, H.; Kim, S.Y.; et al. Molecular diagnosis of hereditary spherocytosis by multi-gene target sequencing in Korea: Matching with osmotic fragility test and presence of spherocyte. Orphanet. J. Rare Dis. 2019, 14, 114. [Google Scholar] [CrossRef] [Green Version]
- Fermo, E.; Vercellati, C.; Bianchi, P. Screening tools for hereditary hemolytic anemia: New concepts and strategies. Expert Rev. Hematol. 2021, 14, 281–292. [Google Scholar] [CrossRef]
- Russo, R.; Andolfo, I.; Manna, F.; Gambale, A.; Marra, R.; Rosato, B.E.; Caforio, P.; Pinto, V.; Pignataro, P.; Radhakrishnan, K.; et al. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am. J. Hematol. 2018, 93, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Fermo, E.; Vercellati, C.; Marcello, A.P.; Keskin, E.Y.; Perrotta, S.; Zaninoni, A.; Brancaleoni, V.; Zanella, A.; Giannotta, J.A.; Barcellini, W.; et al. Targeted Next Generation Sequencing and Diagnosis of Congenital Hemolytic Anemias: A Three Years Experience Monocentric Study. Front. Physiol. 2021, 12, 684569. [Google Scholar] [CrossRef]
- Li, X.; Buckton, A.J.; Wilkinson, S.L.; John, S.; Walsh, R.; Novotny, T.; Valaskova, I.; Gupta, M.; Game, L.; Barton, P.J.; et al. Towards clinical molecular diagnosis of inherited cardiac conditions: A comparison of bench-top genome DNA sequencers. PLoS ONE 2013, 8, e67744. [Google Scholar] [CrossRef] [Green Version]
- Vives-Corrons, J.L.; Krishnevskaya, E.; Rodriguez, I.H.; Ancochea, A. Characterization of hereditary red blood cell membranopathies using combined targeted next-generation sequencing and osmotic gradient ektacytometry. Int. J. Hematol. 2021, 113, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Hwang, K.A.; Paik, K.; Park, J. A novel EPB41 p.Trp704* mutation in a Korean patient with hereditary elliptocytosis: A case report. Hematology 2020, 25, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, W.; Kim, M.; Kim, Y.; Park, S.Y.; Park, J.; Yang, Y.J. Targeted next-generation sequencing identifies a novel nonsense mutation in SPTB for hereditary spherocytosis: A case report of a Korean family. Medicine 2018, 97, e9677. [Google Scholar] [CrossRef]
- Bain, B.J. Diagnosis from the blood smear. N. Engl. J. Med. 2005, 353, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Constantino, B.T. Reporting and grading of abnormal red blood cell morphology. Int. J. Lab. Hematol. 2015, 37, 1–7. [Google Scholar] [CrossRef]
- Katz, B.Z.; Feldman, M.D.; Tessema, M.; Benisty, D.; Toles, G.S.; Andre, A.; Shtreker, B.; Paz, F.M.; Edwards, J.; Jengehino, D.; et al. Evaluation of Scopio Labs X100 Full Field PBS: The first high-resolution full field viewing of peripheral blood specimens combined with artificial intelligence-based morphological analysis. Int. J. Lab. Hematol. 2021, 43, 1408–1416. [Google Scholar] [CrossRef]
- Jamwal, M.; Aggarwal, A.; Palodhi, A.; Sharma, P.; Bansal, D.; Trehan, A.; Malhotra, P.; Maitra, A.; Das, R. Next-Generation Sequencing-Based Diagnosis of Unexplained Inherited Hemolytic Anemias Reveals Wide Genetic and Phenotypic Heterogeneity. J. Mol. Diagn. 2020, 22, 579–590. [Google Scholar] [CrossRef]
- Andolfo, I.; Russo, R.; Gambale, A.; Iolascon, A. New insights on hereditary erythrocyte membrane defects. Haematologica 2016, 101, 1284–1294. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.M.; Nussenzveig, R.H.; Reading, N.S.; Patel, J.L.; Sangle, N.; Salama, M.E.; Prchal, J.T.; Perkins, S.L.; Yaish, H.M.; Christensen, R.D. Clinical utility of next-generation sequencing in the diagnosis of hereditary haemolytic anaemias. Br. J. Haematol. 2016, 174, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Roy, N.B.; Wilson, E.A.; Henderson, S.; Wray, K.; Babbs, C.; Okoli, S.; Atoyebi, W.; Mixon, A.; Cahill, M.R.; Carey, P.; et al. A novel 33-Gene targeted resequencing panel provides accurate, clinical-grade diagnosis and improves patient management for rare inherited anaemias. Br. J. Haematol. 2016, 175, 318–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koralkova, P.; van Solinge, W.W.; van Wijk, R. Rare hereditary red blood cell enzymopathies associated with hemolytic anemia—Pathophysiology, clinical aspects, and laboratory diagnosis. Int. J. Lab. Hematol. 2014, 36, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, R.; Nakajima, H.; Noguchi, T.; Imamura, K.; Hamaguchi, T.; Tomita, K.; Yamada, K.; Kuwajima, M.; Kono, N.; Tanaka, T.; et al. Compound heterozygous mutations affecting both hepatic and erythrocyte isozymes of pyruvate kinase. Biochem. Biophys. Res. Commun. 1995, 208, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Gelbart, T. Estimating the prevalence of pyruvate kinase deficiency from the gene frequency in the general white population. Blood 2000, 95, 3585–3588. [Google Scholar] [CrossRef]
- Vives Corrons, J.L.; Krishnevskaya, E.; Montllor, L.; Leguizamon, V.; Garcia Bernal, M. Concomitant Hereditary Spherocytosis and Pyruvate Kinase Deficiency in a Spanish Family with Chronic Hemolytic Anemia: Contribution of Laser Ektacytometry to Clinical Diagnosis. Cells 2022, 11, 1133. [Google Scholar] [CrossRef]
- van Zwieten, R.; van Oirschot, B.A.; Veldthuis, M.; Dobbe, J.G.; Streekstra, G.J.; van Solinge, W.W.; Schutgens, R.E.; van Wijk, R. Partial pyruvate kinase deficiency aggravates the phenotypic expression of band 3 deficiency in a family with hereditary spherocytosis. Am. J. Hematol. 2015, 90, E35–E39. [Google Scholar] [CrossRef]
- Andres, O.; Loewecke, F.; Morbach, H.; Kraus, S.; Einsele, H.; Eber, S.; Speer, C.P. Hereditary spherocytosis is associated with decreased pyruvate kinase activity due to impaired structural integrity of the red blood cell membrane. Br. J. Haematol. 2019, 187, 386–395. [Google Scholar] [CrossRef]
- Zarza, R.; Moscardó, M.; Alvarez, R.; García, J.; Morey, M.; Pujades, A.; Vives-Corrons, J.L. Co-existence of hereditary spherocytosis and a new red cell pyruvate kinase variant: PK mallorca. Haematologica 2000, 85, 227–232. [Google Scholar]
- Hong, C.R.; Kang, H.J.; Lee, J.W.; Kim, H.; Kim, N.H.; Park, K.D.; Park, J.D.; Seong, M.W.; Park, S.S.; Shin, H.Y.; et al. Clinical characteristics of pediatric thalassemia in Korea: A single institute experience. J. Korean Med. Sci. 2013, 28, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Park, E.S.; Jung, H.L.; Kim, H.J.; Park, S.S.; Bae, S.H.; Shin, H.Y.; Song, S.H.; Koh, K.N.; Lyu, C.J.; Lim, Y.T.; et al. Hereditary hemolytic anemia in Korea from 2007 to 2011: A study by the Korean Hereditary Hemolytic Anemia Working Party of the Korean Society of Hematology. Blood Res. 2013, 48, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Shin, K.H.; Kim, H.H.; Yang, E.J.; Park, K.H.; Kim, M.J.; Kwon, J.R.; Choi, Y.S.; Kim, J.N.; Shin, M.G.; et al. Increased Prevalence of Thalassemia in Young People in Korea: Impact of Increasing Immigration. Ann. Lab. Med. 2019, 39, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Location | Disease Phenotypes | # OMIM | Inheritance |
|---|---|---|---|---|
| RBC membranopathy (N = 10) | ||||
| EPB41 | 1p35.3 | Elliptocytosis-1 | # 611804 | AD, AR |
| SPTA1 | 1q23.1 | Elliptocytosis-2 Pyropoikilocytosis Spherocytosis-3 | # 130600 # 266140 # 270970 | AD AR AR |
| SPTB | 14q23.3 | Elliptocytosis-3 Spherocytosis-2 | # 617948 # 616649 | AD |
| ANK1 | 8p11.21 | Spherocytosis-1 | # 182900 | AD, AR |
| SLC4A1 | 17q21.31 | Spherocytosis-4 | # 612653 | AD |
| EPB42 | 15q15.2 | Spherocytosis-5 | # 612690 | AD |
| PIEZO1 | 16q24.3 | Dehydrated hereditary stomatocytosis | # 194380 | AD |
| KCNN4 | 19q13.31 | Dehydrated hereditary stomatocytosis-2 | # 616689 | AD |
| RHAG | 6p12.3 | Overhydrated hereditary stomatocytosis | # 185000 | AD |
| SEC23B | 20p11.23 | Dyserythropoietic anemia, congenital, 2 | # 224100 | AR |
| RBC enzymopathy (N = 17) | ||||
| ABCB7 | Xq13.3 | Anemia, sideroblastic, with ataxia | # 301310 | XLR |
| AK1 | 9q34.11 | Hemolytic anemia due to adenylate kinase deficiency | # 612631 | AR |
| ALAS2 | Xp11.21 | Anemia, sideroblastic, 1 Protoporphyria, erythropoietic, X-linked | # 300751 # 300752 | XLR XL |
| ALDOA | 16p11.2 | Glycogen storage disease XII | # 611881 | AR |
| CYB5R3 | 22q13.2 | Methemoglobinemia | # 250800 | AR |
| G6PD | Xq28 | Hemolytic anemia, G6PD deficient | # 300908 | XLD |
| GCLC | 6p12.1 | Hemolytic anemia due to gamma-glutamylcysteine synthetase deficiency | # 230450 | AR |
| GPI | 19q13.11 | Hemolytic anemia, nonspherocytic, due to glucose phosphate isomerase deficiency | # 613470 | AR |
| GSS | 20q11.22 | Hemolytic anemia due to glutathione synthetase deficiency | # 231900 | AR |
| HK1 | 10q22.1 | Hemolytic anemia due to hexokinase deficiency | # 235700 | AR |
| NT5C3A | 7p14.3 | Anemia, hemolytic, due to UMPH1 deficiency | # 266120 | AR |
| PFKM | 12q13.11 | Glycogen storage disease VII | # 232800 | AR |
| PGK1 | Xq21.1 | Phosphoglycerate kinase 1 deficiency | # 300653 | XLR |
| PKLR | 1q22 | Pyruvate kinase deficiency | # 266200 | AR |
| SLC25A38 | 3p22.1 | Anemia, sideroblastic, 2, pyridoxine-refractory | # 205950 | AR |
| TPI1 | 12p13.31 | Hemolytic anemia due to triosephosphate isomerase deficiency | # 615512 | AR |
| UGT1A1 | 2q37.1 | Crigler–Najjar syndrome, type I Crigler–Najjar syndrome, type II Hyperbilirubinemia, familial transient neonatal | # 218800 # 606785 # 237900 | AR AR AR |
| Hemoglobinopathy (N = 6) | ||||
| HBA1 | 16p13.3 | Thalassemia, alpha | # 604131 | AR |
| HBA2 | 16p13.3 | Thalassemia, alpha | # 604131 | AR |
| HBB | 11p15.4 | Thalassemia, beta Delta-beta thalassemia Sickle cell anemia | # 613985 # 141749 # 603903 | AD, AR AD AR |
| HBD | 11p15.4 | Thalassemia, delta | na | AR |
| HBG1 | 11p15.4 | Fetal hemoglobin quantitative trait locus 1 | # 141749 | AD |
| HBG2 | 11p15.4 | Fetal hemoglobin quantitative trait locus 1 | # 141749 | AD |
| Case ID | S/A | Dx | Gene | Transcript | Base Change | AA Change | Variant Type | Zygosity | Effect | gnomAD |
|---|---|---|---|---|---|---|---|---|---|---|
| D099 | F/89 | HE | EPB41 | NM_001166005.2 | c.2112G > A | p.Trp704Ter | Nonsense | Het | P | nf |
| D050 | M/89 | HE | SPTA1 | NM_003126.4 | c.452G > A | p.Gly151Asp | Missense | Het | VUS | 0.000158 |
| D131 | M/62 | HS | ANK1 | NM_001142446.2 | c.2642_2645dup | p.Leu884GlyfsTer27 | Frameshift | Het | P | nf |
| D112 * | F/70 | HS | SPTB | NM_001024858.3 | c.1956G > A | p.Trp652Ter | Nonsense | Het | P | nf |
| D114 * | M/48 | HS | SPTB | NM_001024858.3 | c.1956G > A | p.Trp652Ter | Nonsense | Het | P | nf |
| D034 | F/89 | HS | PKLR | NM_000298.6 | c.1468C > T | p.Arg490Trp | Missense | Het | P | 0.0000637 |
| D136 | M/27 | BTM | HBB | NM_000518.5 | c.79G > A | p.Glu27Lys | Missense | Het | P | 0.0000637 |
| HBB | NM_000518.5 | c.92 + 1G > T | Splicing error | Het | P | nf | ||||
| D129 | M/76 | BTm | HBB | NM_000518.5 | c.315 + 1G > A | Splicing error | Het | P | 0.0000319 | |
| D009 | F/81 | BTm | HBB | NM_000518.5 | c.315 + 1G > A | Splicing error | Het | P | 0.0000319 | |
| D888 | F/41 | BTm | HBB | NM_000518.5 | c.52A > T | p.Lys18Ter | Nonsense | Het | P | 0.0000637 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Kim, T.Y.; Han, J.Y.; Park, J. Five Years’ Experience with Gene Panel Sequencing in Hereditary Hemolytic Anemia Screened by Routine Peripheral Blood Smear Examination. Diagnostics 2023, 13, 770. https://doi.org/10.3390/diagnostics13040770
Kim N, Kim TY, Han JY, Park J. Five Years’ Experience with Gene Panel Sequencing in Hereditary Hemolytic Anemia Screened by Routine Peripheral Blood Smear Examination. Diagnostics. 2023; 13(4):770. https://doi.org/10.3390/diagnostics13040770
Chicago/Turabian StyleKim, Namsu, Tae Yun Kim, Ji Yoon Han, and Joonhong Park. 2023. "Five Years’ Experience with Gene Panel Sequencing in Hereditary Hemolytic Anemia Screened by Routine Peripheral Blood Smear Examination" Diagnostics 13, no. 4: 770. https://doi.org/10.3390/diagnostics13040770





