Application of Speckle Tracking Echocardiography for Evaluating Ventricular Function after Transcatheter Pulmonary Valve Replacement
Abstract
:1. Introduction
2. The Influence of Severe PR on Ventricular Structure and Function
3. Two-Dimensional Echocardiography and Tissue Doppler Imaging
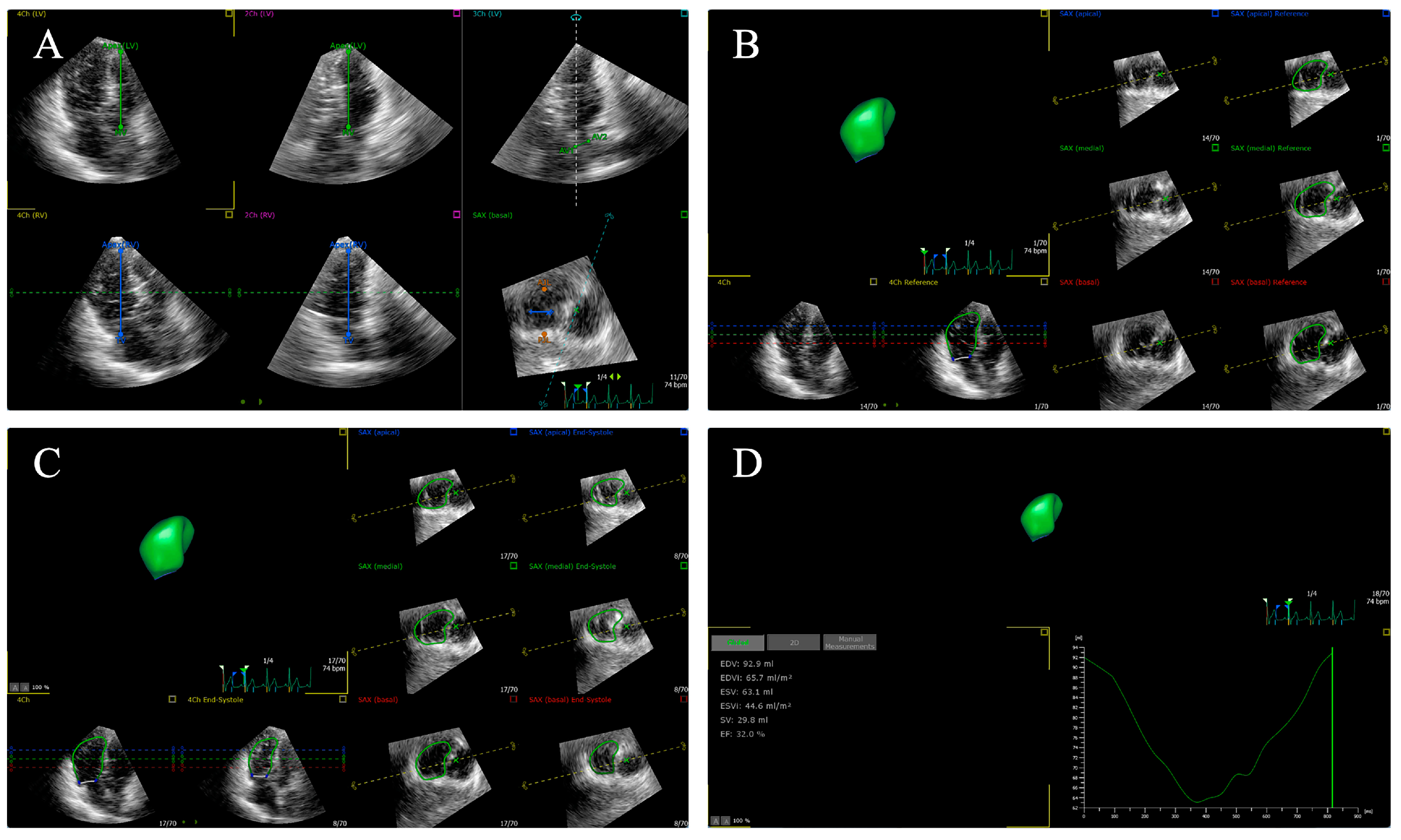
4. Speckle Tracking Echocardiography
4.1. General
4.2. Strain Parameters
4.3. Two-Dimensional Speckle Tracking Echocardiography
4.4. Three-Dimensional Speckle Tracking Echocardiography
5. The Application of Speckle Tracking Echocardiography in Patients after TPVR
5.1. The Global Longitudinal Strain and Strain Rate of the Right Ventricle
| Study | N | Age (Y) | Diagnosis | TPVR Indication | Follow Up | RVLS (%) | RVLSR (s−1) | LVLS (%) | LVLSR (s−1) | IVSLS (%) | IVSLSR (s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moiduddin et al. [52] | 10 | 15.56 ± 2.22 | TOF: 7 Other: 3 | PR: 4 PR+PS: 6 | pre-discharge | RVFWLS: −23.4 ± 6.2 | −2.1 ± 0.7 | −20.0 ± 11.2 | −1.5 ± 0.5 | −15.6 ± 6.7 | −1.13 ± 0.5 |
| Chowdhury et al. [94] | 24 | 32.3 ± 17.0 | TOF: 12 Other: 12 | PR: 7 PR+PS: 17 | 1 month | RVGLS: −17.8 ± 0.6 RVFWLS: −19.1 ± 4.8 | RVGLSR: −1.03 ± 0.05 RVGLSRe: 1.12± 0.09 RVFWLSR: −1.11 ± 0.30 RVFWLSR: 1.27 ± 0.61 | LVGLS: −18.0 ± 1.1 | LVGLSR: −1.11 ± 0.08 LVGLSRe: 1.30 ± 1.10 | −15.9 ± 2.9 | −0.93 ± 0.27 |
| 6-month | RVGLS: −19.6 ± 0.9 RVFWLS: −21.9 ± 6.2 | RVGLSR: −1.16 ± 0.08 RVGLSRe: 1.31± 0.10 RVFWLSR: −1.31 ± 0.68 RVFWLSRe: 1.43 ± 0.64 | LVGLS: −18.2 ± 0.9 | LVGLSR: −1.06 ± 0.15 LVGLSRe: 1.32 ± 0.09 | −17.8 ± 5.4 | −1.05 ± 0.42 | |||||
| Chowdhury et al. [96] | 24 | 32.3 ± 17.0 | TOF: 12 Other: 12 | PR: 7 PR+PS: 17 | 6-month | −19.6 | −1.16 | −18.2 | - | - | - |
| Moiduddin et al. [40] | 10 | 24.4 ± 7.6 | TOF: 9 Other: 1 | PR: 6 PS: 2 PR+PS: 2 | pre-discharge | RVGLS: −17.13 ± 2.71 basal: −19.71 ± 5.18 mid: −15.96 ± 5.45 apical: −16.96 ± 6.65 | RVGLSRs: −0.97 ± 0.22 RVGLSRe: 1.05 ± 0.32 RVGLSRa: 0.61 ± 0.14 | LVGLS: −19.23 ± 1.49 basal: −22.87 ± 5.09 mid: −18.64 ± 3.68 apical: −19.64 ± 4.69 | LVGLSRs: −1.06 ± 0.10 LVGLSRe: 1.23 ± 0.32 LVGLSRa: 0.63 ± 0.21 | basal: −17.83 ± 3.73 mid: −20.55 ± 2.19 apical: −20.27 ± 4.27 | - |
| 3-month | RVGLS: −16.96 ± 5.17 basal: −20.27 ± 5.60 mid: −15.55 ± 7.57 apical: −16.22 ± 9.21 | RVGLSRs: −0.87 ± 0.28 RVGLSRe: 1.03 ± 0.36 RVGLSRa: 0.49 ± 0.29 | LVGLS: −14.72 ± 3.62 basal: −19.67 ± 10.46 mid: −12.29 ± 5.64 apical: −15.20 ± 6.89 | LVGLSRs: −0.84 ± 0.16 LVGLSRe: 1.11 ± 0.44 LVGLSRa: 0.29 ± 0.28 | basal: −15.41 ± 3.16 mid: −17.77 ± 3.47 apical: −15.71 ± 3.99 | - | |||||
| 6-month | RVGLS: −16.95 ± 4.20 basal: −20.97 ± 7.68 mid: −15.04 ± 7.02 apical: −16.49 ± 5.88 | RVGLSRs: −0.83 ± 0.22 RVGLSRe: 1.08 ± 0.28 RVGLSRa: 0.52 ± 0.21 | LVGLS: −17.18 ± 3.08 basal: −24.72 ± 9.31 mid: −14.00 ± 6.60 apical: −17.10 ± 3.92 | LVGLSRs: −0.94 ± 0.23 LVGLSRe: 1.23 ± 0.28 LVGLSRa: 0.50 ± 0.15 | basal: −16.09 ± 5.47 mid: −18.66 ± 3.33 apical: −18.23 ± 6.20 | - | |||||
| Hasan et al. [41] | 20 | 18 | TOF:13 Other: 7 | PR+ obstructed RVOT conduit: 9 obstructed RVOT conduit: 11 | 6-month | RVGLS: −17.0 (−12, −22) a; −18.8 (−13, −24) b RV lateral wall strain: −18.3 (−6.8, −28.3) a; −18.6 (−11, −32) b | - | LVGLS: −18.6 (−14.6, −22.0) a; −20.8 (−15.4, −23.0) b LV lateral wall strain: −20.3 (−16.7, −25) a; −22.3 (−18.5,1 −27) b | - | −16.6 (−11, −21) a; −17.2 (−12.3, −23.0) b | - |
5.2. The Global Longitudinal Strain and Strain Rate of the Left Ventricle
5.3. The Regional Longitudinal Strain Values of the Left and Right Ventricles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuypers, J.A.; Menting, M.E.; Konings, E.E.; Opić, P.; Utens, E.M.; Helbing, W.A.; Witsenburg, M.; van den Bosch, A.E.; Ouhlous, M.; van Domburg, R.T.; et al. Unnatural history of tetralogy of Fallot: Prospective follow-up of 40 years after surgical correction. Circulation 2014, 130, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.J.; Veldtman, G.; Bradley, T.J.; Gengsakul, A.; Manlhiot, C.; Williams, W.G.; Webb, G.D.; McCrindle, B.W. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur. J. Cardiothorac. Surg. 2009, 35, 156–164, discussion 164. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, M.S.; Hurwitz, R.A. Course of right and left ventricular function in patients with pulmonary insufficiency after repair of Tetralogy of Fallot. Pediatr. Cardiol. 2000, 21, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gatzoulis, M.A.; Balaji, S.; Webber, S.A.; Siu, S.C.; Hokanson, J.S.; Poile, C.; Rosenthal, M.; Nakazawa, M.; Moller, J.H.; Gillette, P.C.; et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet Br. Ed. 2000, 356, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.; Labbio, G.D.; Darwish, A.; Kadem, L. How pulmonary valve regurgitation after tetralogy of fallot repair changes the flow dynamics in the right ventricle: An in vitro study. Med. Eng. Phys. 2020, 83, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mauger, C.A.; Govil, S.; Chabiniok, R.; Gilbert, K.; Hegde, S.; Hussain, T.; McCulloch, A.D.; Occleshaw, C.J.; Omens, J.; Perry, J.C.; et al. Right-left ventricular shape variations in tetralogy of Fallot: Associations with pulmonary regurgitation. J. Cardiovasc. Magn. Reson. 2021, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Bonhoeffer, P.; De Groot, N.M.; de Haan, F.; Deanfield, J.E.; Galie, N.; Gatzoulis, M.A.; Gohlke-Baerwolf, C.; Kaemmerer, H.; Kilner, P.; et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur. Heart J. 2010, 31, 2915–2957. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e81–e192. [Google Scholar] [CrossRef]
- Suradi, H.S.; Hijazi, Z.M. Percutaneous pulmonary valve implantation. Glob. Cardiol. Sci. Pract. 2015, 2015, 23. [Google Scholar] [CrossRef]
- Alkashkari, W.; Albugami, S.; Abbadi, M.; Niyazi, A.; Alsubei, A.; Hijazi, Z.M. Transcatheter pulmonary valve replacement in pediatric patients. Expert Rev. Med. Devices 2020, 17, 541–554. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Jalal, Z.; Valdeolmillos, E.; Malekzadeh-Milani, S.; Eicken, A.; Georgiev, S.; Hofbeck, M.; Sieverding, L.; Gewillig, M.; Ovaert, C.; Bouvaist, H.; et al. Mid-Term Outcomes Following Percutaneous Pulmonary Valve Implantation Using the “Folded Melody Valve” Technique. Circ. Cardiovasc. Interv. 2021, 14, e009707. [Google Scholar] [CrossRef] [PubMed]
- Hribernik, I.; Thomson, J.; Ho, A.; English, K.; Van Doorn, C.; Jaber, O.; Bentham, J. Comparative analysis of surgical and percutaneous pulmonary valve implants over a 20-year period. Eur. J. Cardiothorac. Surg. 2022, 61, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, S.; Ewert, P.; Eicken, A.; Hager, A.; Hörer, J.; Cleuziou, J.; Meierhofer, C.; Tanase, D. Munich Comparative Study: Prospective Long-Term Outcome of the Transcatheter Melody Valve Versus Surgical Pulmonary Bioprosthesis With Up to 12 Years of Follow-Up. Circ. Cardiovasc. Interv. 2020, 13, e008963. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Asian expert consensus statement on transcatheter pulmonary valve replacement. Chin. J. Interv. Cardiol. 2023, 31, 404–412. [CrossRef]
- Luis, S.A.; Chan, J.; Pellikka, P.A. Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography. Mayo Clin. Proc. 2019, 94, 125–138. [Google Scholar] [CrossRef]
- Badano, L.P.; Muraru, D.; Ciambellotti, F.; Caravita, S.; Guida, V.; Tomaselli, M.; Parati, G. Assessment of left ventricular diastolic function by three-dimensional transthoracic echocardiography. Echocardiography 2020, 37, 1951–1956. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Li, J.; Li, G.; Kong, F.; Mu, L.; Jia, D.; Li, Y.; Yang, J.; Ma, C. Incremental Value of Three-dimensional Speckle-tracking Echocardiography for Evaluating Left Ventricular Systolic Function in Patients with Coronary Slow Flow. Curr. Probl. Cardiol. 2022, 47, 100928. [Google Scholar] [CrossRef]
- Kopetz, V.; Kennedy, J.; Heresztyn, T.; Stafford, I.; Willoughby, S.R.; Beltrame, J.F. Endothelial function, oxidative stress and inflammatory studies in chronic coronary slow flow phenomenon patients. Cardiology 2012, 121, 197–203. [Google Scholar] [CrossRef]
- Houard, L.; Benaets, M.B.; de Meester de Ravenstein, C.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A.; et al. Additional Prognostic Value of 2D Right Ventricular Speckle-Tracking Strain for Prediction of Survival in Heart Failure and Reduced Ejection Fraction: A Comparative Study With Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Citarelli, G.; Antoncecchi, V.; Romito, R.; Monitillo, F.; Leone, M.; Puzzovivo, A.; Lattarulo, M.S.; Rizzo, C.; Caldarola, P.; et al. Right Ventricular Longitudinal Strain Measures Independently Predict Chronic Heart Failure Mortality. Echocardiogr.-J. Cardiovasc. Ultrasound Allied Tech. 2016, 33, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Dufendach, K.A.; Zhu, T.; Castrillon, C.D.; Hong, Y.; Countouris, M.E.; Hickey, G.; Keebler, M.; Thoma, F.W.; Kilic, A. Pre-implant right ventricular free wall strain predicts post-LVAD right heart failure. J. Card. Surg. 2021, 36, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, D.; Gruchała, M.; Sobiczewski, W. Echocardiographic evaluation of right ventricular systolic function: The traditional and innovative approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C. Right Ventricular Function: Imaging Techniques. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Potus, F.; Martin, A.Y.; Snetsinger, B.; Archer, S.L. Biventricular Assessment of Cardiac Function and Pressure-Volume Loops by Closed-Chest Catheterization in Mice. J. Vis. Exp. 2020, 160, e61088. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, L.; Xie, Y.; Zhang, Y.; Zhu, S.; Wu, C.; Sun, W.; Li, M.; Gao, Y.; Wang, B.; et al. 3-Dimensional Versus 2-Dimensional Speckle-Tracking Echocardiography for Right Ventricular Myocardial Fibrosis in Patients With End-Stage Heart Failure. JACC Cardiovasc. Imaging 2021, 14, 1309–1320. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Carigi, S.; De Gennaro, L.; Gentile, P.; De Maria, R.; Di Giannuario, G.; Khoury, G.; Polizzi, V.; Gori, M.; Orso, F.; Tinti, M.D.; et al. Ten questions on cardiac magnetic resonance in patients with heart failure: From etiological diagnosis to prognostic stratification. G. Ital. Cardiol. 2022, 23, 912–923. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Daraban, A.M.; Mada, R.O.; Duchenne, J.; Mirea, O.; Cools, B.; Heying, R.; Boshoff, D.; Bogaert, J.; Budts, W.; et al. Right ventricular remodelling after transcatheter pulmonary valve implantation. Catheter. Cardiovasc. Interv. 2017, 90, 407–417. [Google Scholar] [CrossRef]
- Zhang, X.L.; Peng, Y. Echocardiographic Assessment Progress of Pulmonary Regurgitation after Surgical Repair of Tetralogy of Fallot. Adv. Cardiovasc. Dis. 2018, 39, 316–319. [Google Scholar] [CrossRef]
- Vijiiac, A.; Onciul, S.; Guzu, C.; Scarlatescu, A.; Petre, I.; Zamfir, D.; Onut, R.; Deaconu, S.; Dorobantu, M. Forgotten No More-The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics 2021, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, C.; D’Ascenzi, F.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Carrucola, C.; Cameli, P.; Bigio, E.; Mondillo, S.; Cameli, M. Traditional and Novel Imaging of Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. Curr. Heart Fail. Rep. 2020, 17, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, T.; Haines, P.; Li, M.; Wu, W.; Liu, M.; Chen, Y.; Jin, Q.; Xie, Y.; Wang, J.; et al. Prognostic Value of Right Ventricular Two-Dimensional and Three-Dimensional Speckle-Tracking Strain in Pulmonary Arterial Hypertension: Superiority of Longitudinal Strain over Circumferential and Radial Strain. J. Am. Soc. Echocardiogr. 2020, 33, 985–994.e981. [Google Scholar] [CrossRef] [PubMed]
- Landzaat, J.W.D.; van Heerebeek, L.; Jonkman, N.H.; van der Bijl, E.M.; Riezebos, R.K. The quest for determination of standard reference values of right ventricular longitudinal systolic strain: A systematic review and meta-analysis. J. Echocardiogr. 2022, 21, 1–15. [Google Scholar] [CrossRef]
- Helbing, W.A.; de Roos, A. Clinical applications of cardiac magnetic resonance imaging after repair of tetralogy of Fallot. Pediatr. Cardiol. 2000, 21, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Meca Aguirrezabalaga, J.A.; Silva Guisasola, J.; Díaz Méndez, R.; Escalera Veizaga, A.E.; Hernández-Vaquero Panizo, D. Pulmonary regurgitation after repaired tetralogy of Fallot: Surgical versus percutaneous treatment. Ann. Transl. Med. 2020, 8, 967. [Google Scholar] [CrossRef] [PubMed]
- Tatewaki, H.; Shiose, A. Pulmonary valve replacement after repaired Tetralogy of Fallot. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 509–515. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Kilner, P.J.; Hornung, T.S.; Li, W.; Francis, J.M.; Moon, J.C.; Smith, G.C.; Tat, T.; Pennell, D.J.; Gatzoulis, M.A. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: Detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J. Am. Coll. Cardiol. 2002, 40, 2044–2052. [Google Scholar] [CrossRef]
- Moiduddin, N.; Texter, K.M.; Cheatham, J.P.; Chisolm, J.L.; Kovalchin, J.P.; Nicholson, L.; Belfrage, K.M.; Janevski, I.; Cua, C.L. Strain Echocardiographic Assessment of Ventricular Function after Percutaneous Pulmonary Valve Implantation. Congenit. Heart Dis. 2012, 7, 361–371. [Google Scholar] [CrossRef]
- Hasan, B.S.; Lunze, F.I.; Chen, M.H.; Brown, D.W.; Boudreau, M.J.; Rhodes, J.; McElhinney, D.B. Effects of Transcatheter Pulmonary Valve Replacement on the Hemodynamic and Ventricular Response to Exercise in Patients With Obstructed Right Ventricle-to-Pulmonary Artery Conduits. Jacc-Cardiovasc. Interv. 2014, 7, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Opdahl, A.; Helle-Valle, T.; Skulstad, H.; Smiseth, O.A. Strain, Strain Rate, Torsion, and Twist: Echocardiographic Evaluation. Curr. Cardiol. Rep. 2015, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Halvorsrød, M.I.; Kiss, G.; Dahlslett, T.; Støylen, A.; Grenne, B. Automated tissue Doppler imaging for identification of occluded coronary artery in patients with suspected non-ST-elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2023, 39, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, J.R.; Cao, Y.; Cen, Y.N. Research of Quantitative Tissue Doppler Imaging on Assessing Left Ventricular Function in Patients with Early Stage Type2 Diabetes. Chin. J. Ultrasound Med. 2018, 34, 150–152. [Google Scholar] [CrossRef]
- Shang, Z.J.; Cong, T.; Sun, Y.H.; Wang, K.; Zhang, S.L. Assessment of right ventricular systolic function in patients with mitral stenosis using tissue Doppler imaging and strain rate imaging. J. Dalian Med. Univ. 2014, 36, 57–61. [Google Scholar] [CrossRef]
- Lin, N. Advances in Research in Right Heart Diseases with Echocardiography. Adv. Cardiovasc. Dis. 2013, 34, 702–707. [Google Scholar] [CrossRef]
- Pokharel, P.; Fujikura, K.; Bella, J.N. Clinical applications and prognostic implications of strain and strain rate imaging. Expert Rev. Cardiovasc. Ther. 2015, 13, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, S.; Wu, G.; Ahmad, M. Echocardiographic assessment of the right ventricle in the current era: Application in clinical practice. Echocardiography 2017, 34, 1930–1947. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular–arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef]
- Van Daele, C.M.; Chirinos, J.A.; De Buyzere, M.L.; Gillebert, T.C.; Rietzschel, E.R. Feasibility and agreement of a novel combined echocardiographic method to measure global longitudinal strain and strain rate compared to speckle tracking and tissue Doppler imaging. Acta Cardiol. 2020, 75, 191–199. [Google Scholar] [CrossRef]
- Eroğlu, A.G.; Uluğ, N.; Karakaş, H.; Yüksel, E.K.; Akyel, N.G.; Çığ, G.; Adaletli, İ.; Özdemir, G.N.; Türkkan, E.; Celkan, T.T. Evaluation of left ventricular function and myocardial deformation in children with beta-thalassemia major by real-time three-dimensional (four-dimensional) and speckle tracking echocardiography. Echocardiography 2022, 39, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Moiduddin, N.; Asoh, K.; Slorach, C.; Benson, L.N.; Friedberg, M.K. Effect of Transcatheter Pulmonary Valve Implantation on Short-Term Right Ventricular Function as Determined by Two-Dimensional Speckle Tracking Strain and Strain Rate Imaging. Am. J. Cardiol. 2009, 104, 862–867. [Google Scholar] [CrossRef]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zacà, V.; Ballo, P.; D’Andrea, A.; Muraru, D.; Losi, M.; et al. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Sudhakar, S.; Nanda, N.C.; Buckberg, G.; Pradhan, M.; Roomi, A.U.; Gorissen, W.; Houle, H. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography 2013, 30, 88–105. [Google Scholar] [CrossRef]
- Wang, T.T. Methods of quantitatively analyzing myocardial strain and stain rate. Int. J. Med. Radiol. 2017, 40, 277–281. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Alsharari, R.; Oxborough, D.; Lip, G.Y.H.; Shantsila, A. Myocardial Strain Imaging in Resistant Hypertension. Curr. Hypertens. Rep. 2021, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Kempny, A.; Diller, G.P.; Kaleschke, G.; Orwat, S.; Funke, A.; Radke, R.; Schmidt, R.; Kerckhoff, G.; Ghezelbash, F.; Rukosujew, A.; et al. Longitudinal left ventricular 2D strain is superior to ejection fraction in predicting myocardial recovery and symptomatic improvement after aortic valve implantation. Int. J. Cardiol. 2013, 167, 2239–2243. [Google Scholar] [CrossRef]
- Berglund, F.; Piña, P.; Herrera, C.J. Right ventricle in heart failure with preserved ejection fraction. Heart 2020, 106, 1798–1804. [Google Scholar] [CrossRef]
- Hassanin, M.; Ong, G.; Connelly, K.A. Right Ventricle Longitudinal Strain: A New Tool in Functional Tricuspid Regurgitation Prognostication. Can. J. Cardiol. 2021, 37, 945–948. [Google Scholar] [CrossRef]
- Hoit, B.D. Right Ventricular Strain Comes of Age. Circ. Cardiovasc. Imaging 2018, 11, e008382. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Cui, H.; Chang, W.; Li, Y.; Cui, X.; Li, G. Real-time three-dimensional echocardiography and two-dimensional speckle tracking imaging in the evaluation of left atrial function in patients with triple-vessel coronary artery disease without myocardial infarction. J. Clin. Ultrasound 2022, 50, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.; King, G.; Murphy, R.T.; Walsh, D. Myocardial strain: A clinical review. Ir. J. Med. Sci. 2022, 192, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: Special focus on speckle-tracking longitudinal strain. J. Echocardiogr. 2021, 19, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Delgado, V.; Marsan, N.A.; Bax, J.J. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur. J. Heart Fail. 2017, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Senior, R.; Pennell, D.J. Assessing left ventricular systolic function: From ejection fraction to strain analysis. Eur. Heart J. 2021, 42, 789–797. [Google Scholar] [CrossRef]
- Tanaka, H. Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J. Echocardiogr. 2019, 17, 17–24. [Google Scholar] [CrossRef]
- Gorcsan, J., 3rd; Tanaka, H. Echocardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011, 58, 1401–1413. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef]
- Stanton, T.; Leano, R.; Marwick, T.H. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ. Cardiovasc. Imaging 2009, 2, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Su, Y.X. Application Progress of Speckle Tracking Echocardiography in Cardiac Diseases. Med. Recapitul. 2021, 27, 560–565+570. [Google Scholar] [CrossRef]
- Cameli, M.; Righini, F.M.; Lisi, M.; Mondillo, S. Right ventricular strain as a novel approach to analyze right ventricular performance in patients with heart failure. Heart Fail. Rev. 2013, 19, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ayach, B.; Fine, N.M.; Rudski, L.G. Right ventricular strain: Measurement and clinical application. Curr. Opin. Cardiol. 2018, 33, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Lundorff, I.J.; Sengelov, M.; Jorgensen, P.G.; Pedersen, S.; Modin, D.; Bruun, N.E.; Fritz-Hansen, T.; Jensen, J.S.; Biering-Sorensen, T. Echocardiographic Predictors of Mortality in Women With Heart Failure With Reduced Ejection Fraction. Circ. -Cardiovasc. Imaging 2018, 11, e008031. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Haugaa, K.; Donal, E.; Stankovic, I.; Voigt, J.U.; Petersen, S.E.; Popescu, B.A.; Marwick, T. Right ventricular longitudinal strain in the clinical routine: A state-of-the-art review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 898–912. [Google Scholar] [CrossRef]
- Feigenbaum, H.; Mastouri, R.; Sawada, S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circ. J. 2012, 76, 1550–1555. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Gheorghiade, M.; Butler, J. Echocardiographic evaluation of left ventricular structure and function: New modalities and potential applications in clinical trials. J. Card. Fail. 2012, 18, 159–172. [Google Scholar] [CrossRef]
- El Amrousy, D.; Elgendy, E.; Awad, M.E.; El Razaky, O. Three-dimensional speckle tracking echocardiography for early detection of left ventricular dysfunction in children with non-alcoholic fatty liver diseases. Cardiol. Young 2021, 31, 562–567. [Google Scholar] [CrossRef]
- Wang, Y.B.; Huang, H.; Lin, S.; Hao, M.J.; He, L.J.; Liu, K.; Bi, X.J. Evaluation of Left Ventricular Function by Three-Dimensional Speckle-Tracking Echocardiography in Patients with Chronic Kidney Failure. Curr. Med. Sci. 2022, 42, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Tian, R.; Feng, J.; Zhai, Z.; Wang, Z.; Huang, L.; Lu, G.; Dong, S. Three-dimensional speckle tracking echocardiography to evaluate left ventricular function in patients with acute ST-segment elevation myocardial infarction after percutaneous coronary intervention following Tongxinluo treatment. J. Clin. Ultrasound 2022, 50, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Vachalcova, M.; Valočik, G.; Kurečko, M.; Grapsa, J.; Taha, V.A.; Michalek, P.; Jankajová, M.; Sabol, F.; Kubikova, L.; Orban, M.; et al. The three-dimensional speckle tracking echocardiography in distinguishing between ischaemic and non-ischaemic aetiology of heart failure. ESC Heart Fail. 2020, 7, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A. Three-dimensional speckle-tracking echocardiography offers complete volumetric and functional assessment of the left atrium. Int. J. Cardiovasc. Imaging 2021, 37, 2235. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, X.; Feng, H.J. Application of new echocardiography techniques in the evaluation of right ventricular function. J. Shenyang Med. Coll. 2020, 22, 587–590. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.F. The role of 3D wall motion tracking in heart failure. Nat. Rev. Cardiol. 2012, 9, 644–657. [Google Scholar] [CrossRef]
- Atsumi, A.; Seo, Y.; Ishizu, T.; Nakamura, A.; Enomoto, Y.; Harimura, Y.; Okazaki, T.; Abe, Y.; Aonuma, K. Right Ventricular Deformation Analyses Using a Three-Dimensional Speckle-Tracking Echocardiographic System Specialized for the Right Ventricle. J. Am. Soc. Echocardiogr. 2016, 29, 402–411.e402. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Atsumi, A.; Kawamura, R.; Aonuma, K. Three-Dimensional Speckle Tracking Echocardiography: A Promising Tool for Cardiac Functional Analysis. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 1290–1301. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Gao, Y.; Wan, X.; Xiao, Q.; Zhang, Y.; Sun, W.; Xie, Y.; Zeng, Q.; Chen, Y.; et al. Comprehensive Assessment of Right Ventricular Function by Three-Dimensional Speckle-Tracking Echocardiography: Comparisons with Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2021, 34, 472–482. [Google Scholar] [CrossRef]
- Li, Y.; Wan, X.; Xiao, Q.; Zhang, Y.; Sun, W.; Xie, Y.; Zeng, Q.; Sun, Z.; Yang, Y.; Wang, J.; et al. Value of 3D versus 2D Speckle-Tracking Echocardiography for RV Strain Measurement: Validation With Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 2056–2058. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wu, W.; He, L.; Gao, L.; Zhang, Y.; Lin, Y.; Qian, M.; Wang, J.; Zhang, L.; Xie, M.; et al. Right Ventricular Longitudinal Strain in Patients with Heart Failure. Diagnostics 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Hijazi, Z.M.; Fahey, J.T.; Rhodes, J.F.; Kar, S.; Makkar, R.; Mullen, M.; Cao, Q.-L.; Shirali, G.S. Speckle-Tracking Echocardiographic Measures of Right Ventricular Function Correlate With Improvement in Exercise Function After Percutaneous Pulmonary Valve Implantation. J. Am. Soc. Echocardiogr. 2015, 28, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Knirsch, W.; Dodge-Khatami, A.; Kadner, A.; Kretschmar, O.; Steiner, J.; Böttler, P.; Kececioglu, D.; Harpes, P.; Valsangiacomo Buechel, E.R. Assessment of myocardial function in pediatric patients with operated tetralogy of Fallot: Preliminary results with 2D strain echocardiography. Pediatr. Cardiol. 2008, 29, 718–725. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Hijazi, Z.M.; Rhodes, J.F.; Kar, S.; Makkar, R.; Mullen, M.; Cao, Q.L.; Mandinov, L.; Buckley, J.; Pietris, N.P.; et al. Changes in Speckle Tracking Echocardiography Measures of Ventricular Function after Percutaneous Implantation of the Edwards SAPIEN Transcatheter Heart Valve in the Pulmonary Position. Echocardiogr. J. Cardiovasc. Ultrasound Allied Tech. 2015, 32, 461–469. [Google Scholar] [CrossRef]
- Pascotto, M.; Caso, P.; Santoro, G.; Caso, I.; Cerrato, F.; Pisacane, C.; D’Andrea, A.; Severino, S.; Russo, M.G.; Calabrò, R. Analysis of right ventricular Doppler tissue imaging and load dependence in patients undergoing percutaneous closure of atrial septal defect. Am. J. Cardiol. 2004, 94, 1202–1205. [Google Scholar] [CrossRef]
- Firstenberg, M.S.; Greenberg, N.L.; Main, M.L.; Drinko, J.K.; Odabashian, J.A.; Thomas, J.D.; Garcia, M.J. Determinants of diastolic myocardial tissue Doppler velocities: Influences of relaxation and preload. J. Appl. Physiol. 2001, 90, 299–307. [Google Scholar] [CrossRef]
- Dincer, I.; Kumbasar, D.; Nergisoglu, G.; Atmaca, Y.; Kutlay, S.; Akyurek, O.; Sayin, T.; Erol, C.; Oral, D. Assessment of left ventricular diastolic function with Doppler tissue imaging: Effects of preload and place of measurements. Int. J. Cardiovasc. Imaging 2002, 18, 155–160. [Google Scholar] [CrossRef]
- Jategaonkar, S.R.; Scholtz, W.; Butz, T.; Bogunovic, N.; Faber, L.; Horstkotte, D. Two-dimensional strain and strain rate imaging of the right ventricle in adult patients before and after percutaneous closure of atrial septal defects. Eur. J. Echocardiogr. 2009, 10, 499–502. [Google Scholar] [CrossRef]
- La Gerche, A.; Jurcut, R.; Voigt, J.U. Right ventricular function by strain echocardiography. Curr. Opin. Cardiol. 2010, 25, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Solarz, D.E.; Witt, S.A.; Glascock, B.J.; Jones, F.D.; Khoury, P.R.; Kimball, T.R. Right ventricular strain rate and strain analysis in patients with repaired tetralogy of Fallot: Possible interventricular septal compensation. J. Am. Soc. Echocardiogr. 2004, 17, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Savu, O.; Jurcuţ, R.; Giuşcă, S.; van Mieghem, T.; Gussi, I.; Popescu, B.A.; Ginghină, C.; Rademakers, F.; Deprest, J.; Voigt, J.U. Morphological and functional adaptation of the maternal heart during pregnancy. Circ. Cardiovasc. Imaging 2012, 5, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, B.H.; Cikes, M.; Claus, P.; Sutherland, G.R. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur. J. Echocardiogr. 2009, 10, 216–226. [Google Scholar] [CrossRef]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’Hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef]


| Two-Dimensional Echocardiographic Parameters | Effects of Long-Term and Severe PRs | TDI Parameters | Effects of Long-Term and Severe PRs |
|---|---|---|---|
| RV Parameters | |||
| RVFAC | ↓ | TDI S′ | ↓ |
| TAPSE | ↓ | TDI RV strain | ↓ |
| RVEDD | ↑ | TDI RV strain rate | ↓ |
| LV Parameters | |||
| LVEF | ↓ | TDI S′ | ↓ |
| LVEDD | ↑ | TDI LV strain | ↓ |
| TDI LV strain rate | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.; Zhang, L.; Gao, L.; Lin, Y.; He, Q.; Xie, M.; Li, Y. Application of Speckle Tracking Echocardiography for Evaluating Ventricular Function after Transcatheter Pulmonary Valve Replacement. Diagnostics 2024, 14, 88. https://doi.org/10.3390/diagnostics14010088
Ji M, Zhang L, Gao L, Lin Y, He Q, Xie M, Li Y. Application of Speckle Tracking Echocardiography for Evaluating Ventricular Function after Transcatheter Pulmonary Valve Replacement. Diagnostics. 2024; 14(1):88. https://doi.org/10.3390/diagnostics14010088
Chicago/Turabian StyleJi, Mengmeng, Li Zhang, Lang Gao, Yixia Lin, Qing He, Mingxing Xie, and Yuman Li. 2024. "Application of Speckle Tracking Echocardiography for Evaluating Ventricular Function after Transcatheter Pulmonary Valve Replacement" Diagnostics 14, no. 1: 88. https://doi.org/10.3390/diagnostics14010088
APA StyleJi, M., Zhang, L., Gao, L., Lin, Y., He, Q., Xie, M., & Li, Y. (2024). Application of Speckle Tracking Echocardiography for Evaluating Ventricular Function after Transcatheter Pulmonary Valve Replacement. Diagnostics, 14(1), 88. https://doi.org/10.3390/diagnostics14010088









