Artificial Intelligence Provides Accurate Quantification of Thoracic Aortic Enlargement and Dissection in Chest CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition and Reconstruction
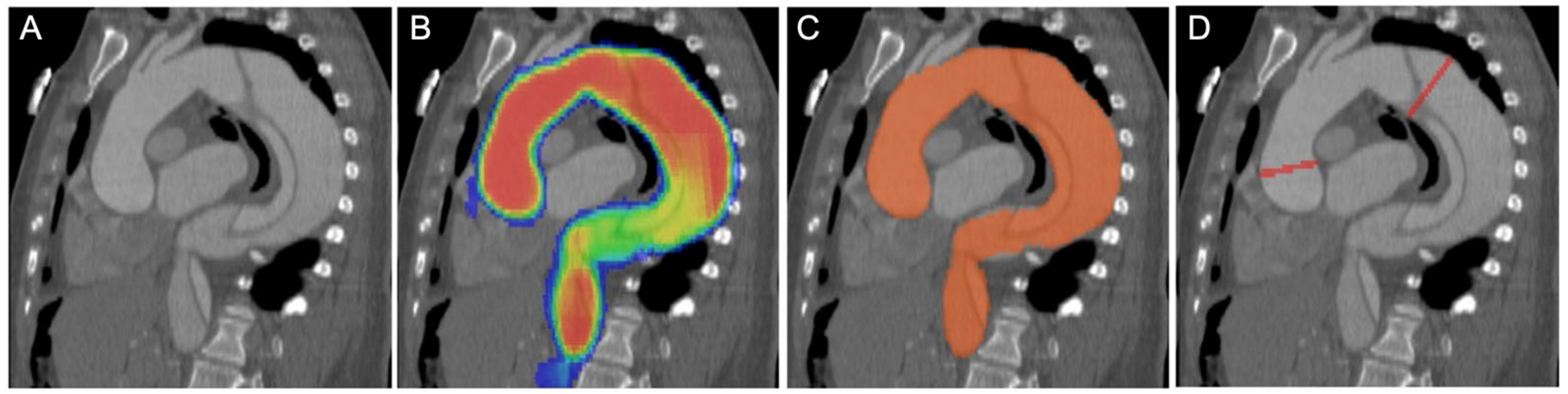
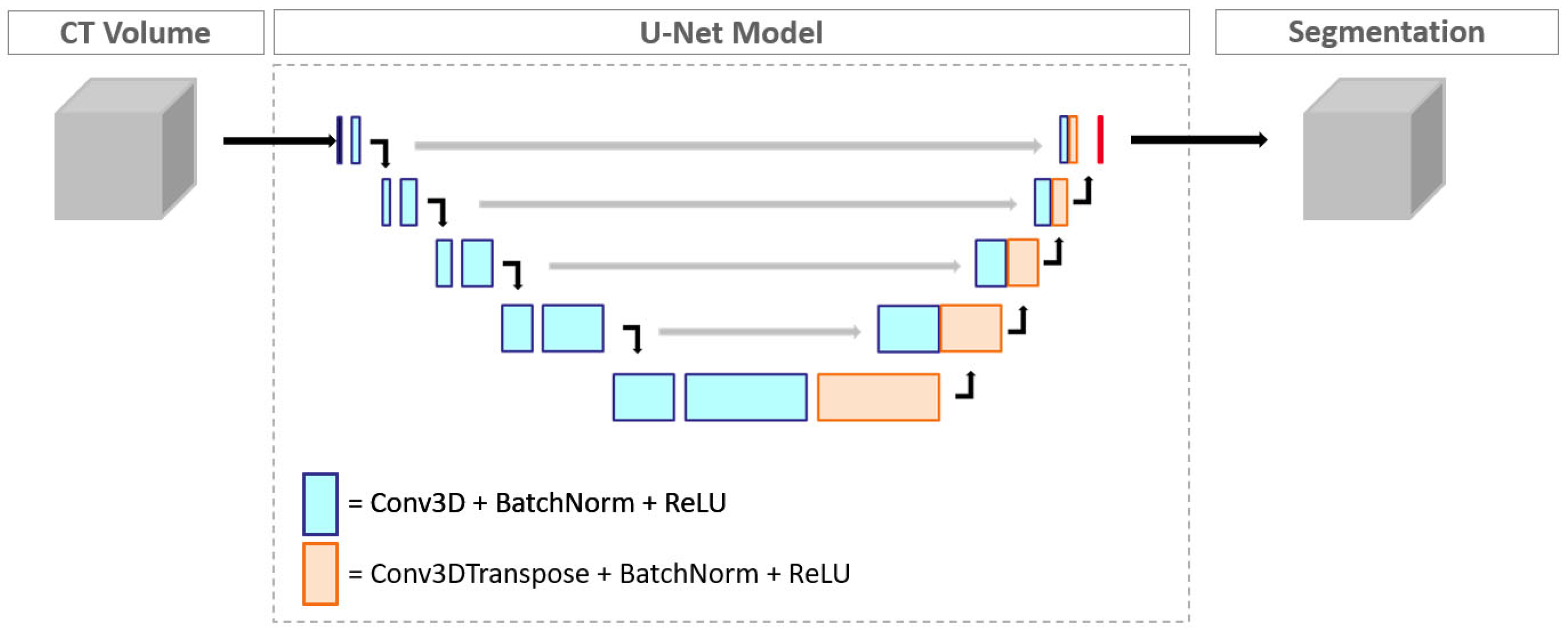
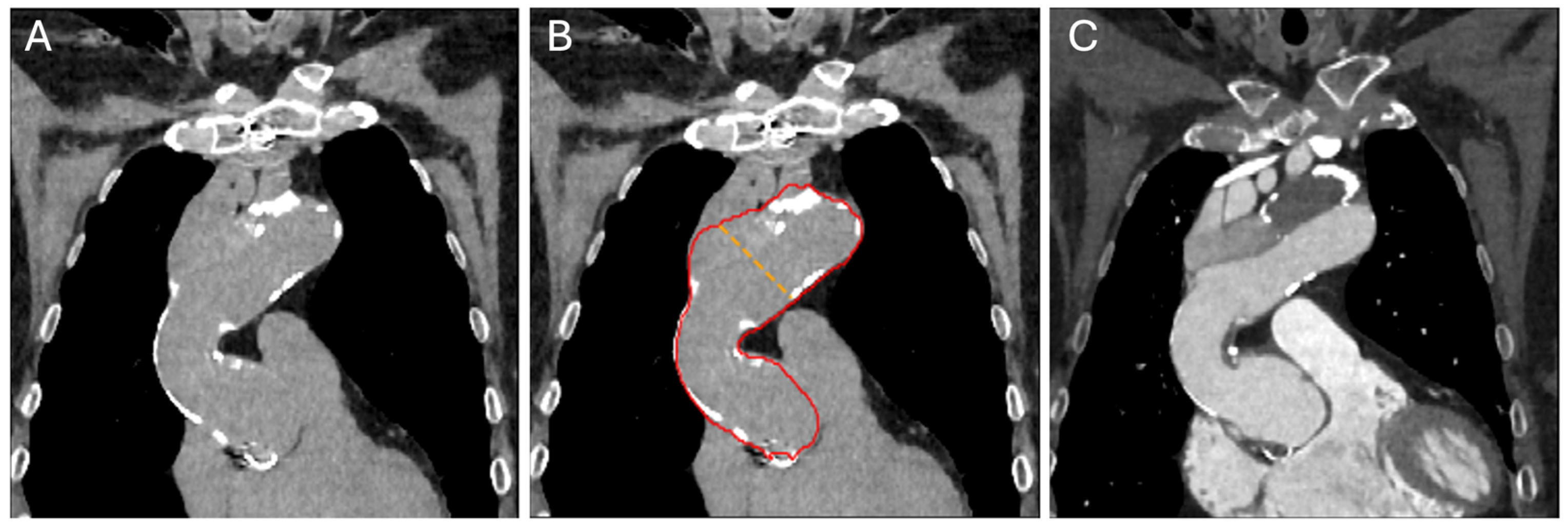
2.3. Convolutional Neural Network Model and Measurements
2.4. Image Reading
2.5. Statistical Analysis
3. Results
3.1. Patient Population
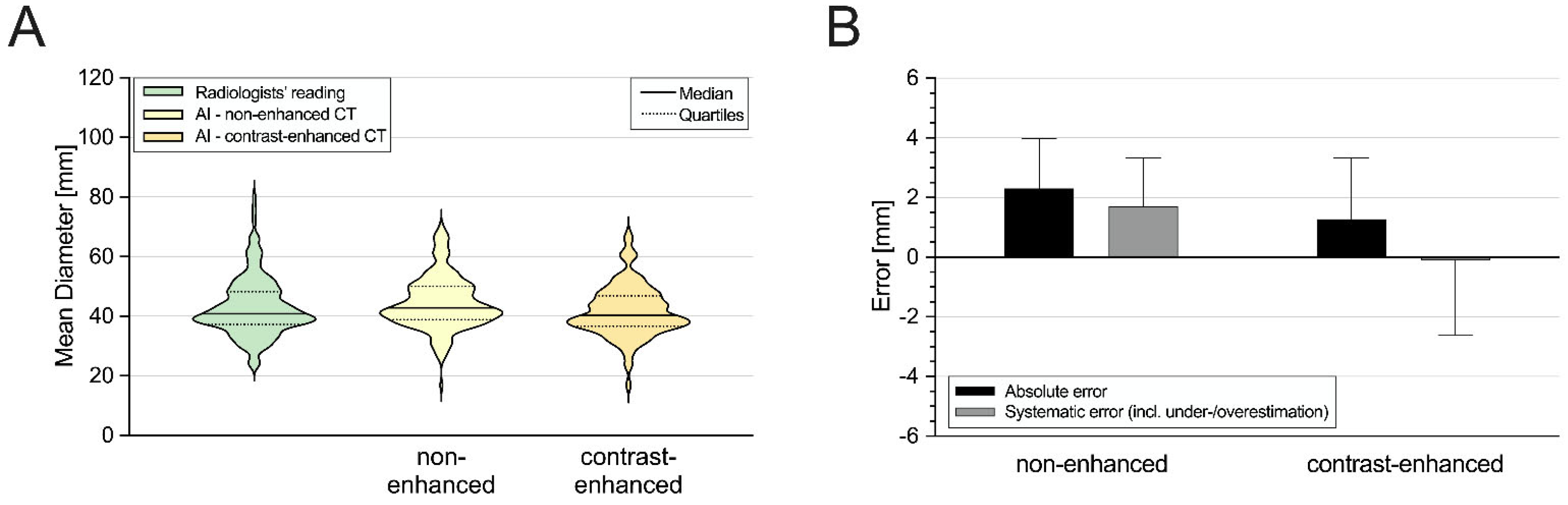
3.2. Aortic Diameter Quantification in the Entire Cohort
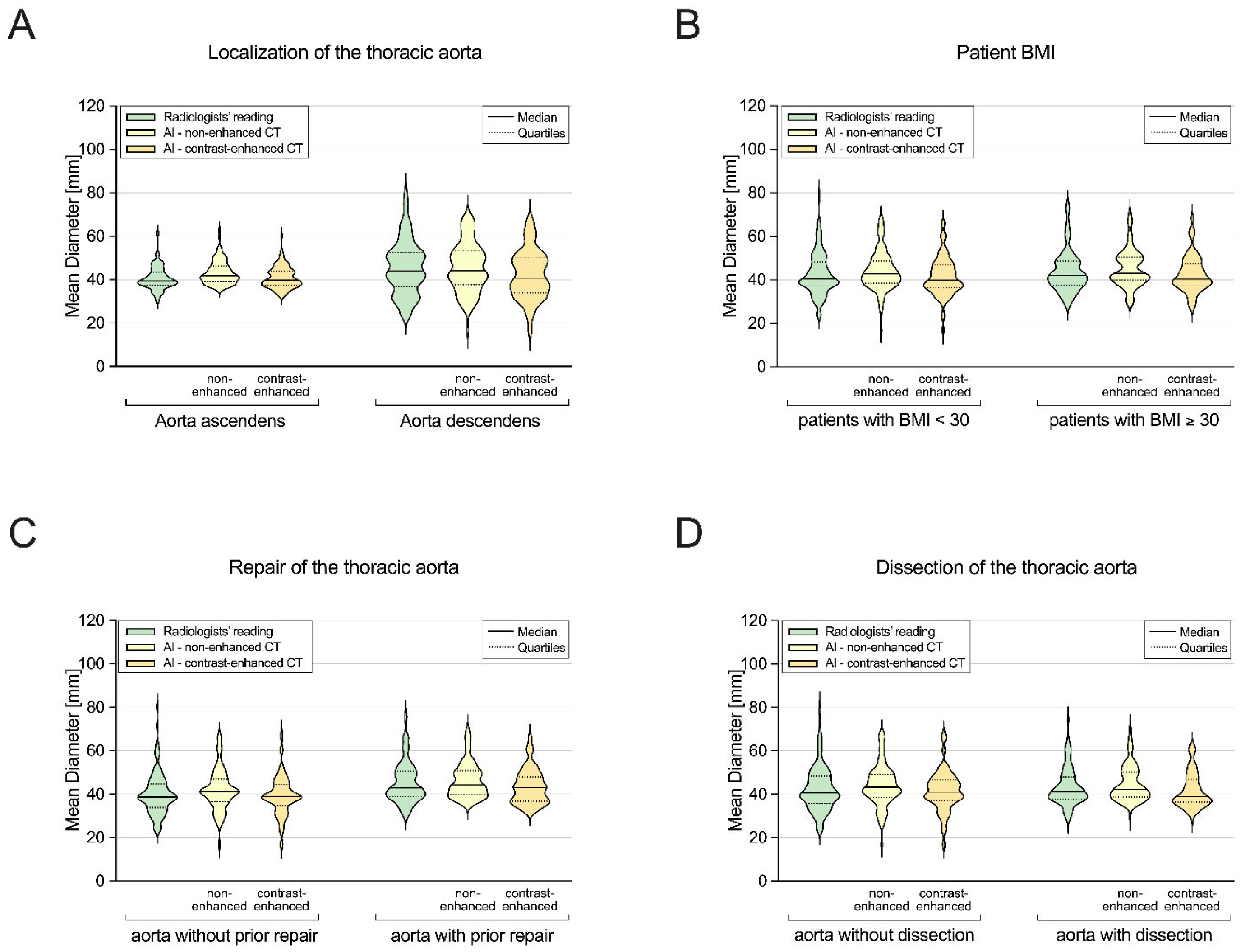
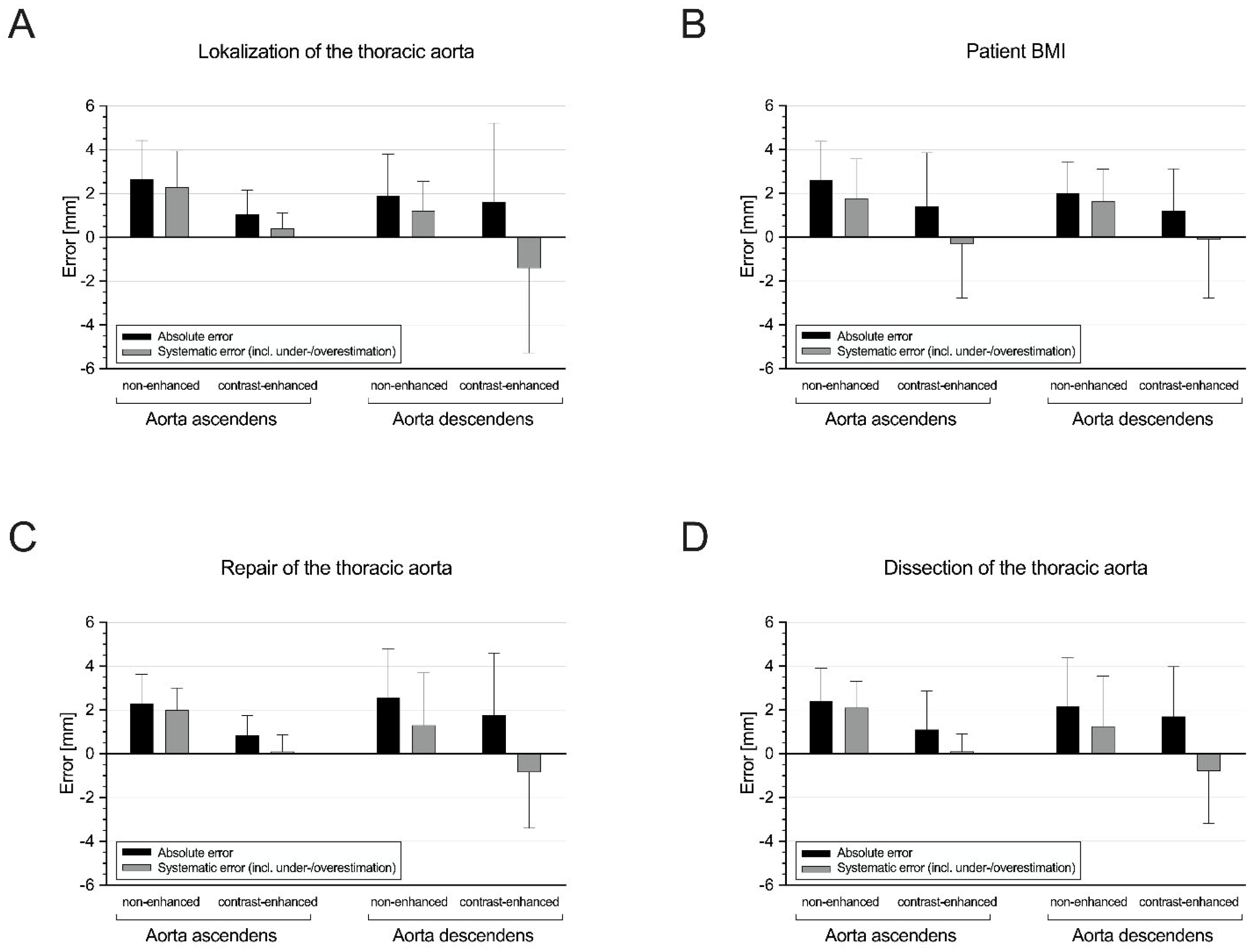
3.3. Subgroup I: Ascending Aorta vs. Descending Aorta
3.4. Subgroup II: Non-Obese vs. Obese Patients
3.5. Subgroup III: Patients without vs. with Prior Aortic Repair
3.6. Subgroup IV: Patients without vs. with Aortic Dissection
3.7. DNN-Based Detection of Aortic Dissection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuzmik, G.A.; Sang, A.X.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms. J. Vasc. Surg. 2012, 56, 565–571. [Google Scholar] [CrossRef]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. Circulation 2010, 121, e266–e369. [Google Scholar]
- Guo, M.H.; Appoo, J.J.; Saczkowski, R.; Smith, H.N.; Ouzounian, M.; Gregory, A.J.; Herget, E.J.; Boodhwani, M. Association of Mortality and Acute Aortic Events With Ascending Aortic Aneurysm: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e181281. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Di Eusanio, M.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Sverzellati, N.; Arcadi, T.; Salvolini, L.; Dore, R.; Zompatori, M.; Mereu, M.; Battista, G.; Martella, I.; Toni, F.; Cardinale, L.; et al. Under-reporting of cardiovascular findings on chest CT. Radiol. Med. 2016, 121, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Cayne, N.S.; Veith, F.J.; Lipsitz, E.C.; Ohki, T.; Mehta, M.; Gargiulo, N.; Suggs, W.D.; Rozenblit, A.; Ricci, Z.; Timaran, C.H. Variability of maximal aortic aneurysm diameter measurements on CT scan: Significance and methods to minimize. J. Vasc. Surg. 2004, 39, 811–815. [Google Scholar] [CrossRef]
- Rueckel, J.; Sperl, J.I.; Kaestle, S.; Hoppe, B.F.; Fink, N.; Rudolph, J.; Schwarze, V.; Geyer, T.; Strobl, F.F.; Ricke, J.; et al. Reduction of missed thoracic findings in emergency whole-body computed tomography using artificial intelligence assistance. Quant. Imaging Med. Surg. 2021, 11, 2486–2498. Available online: https://qims.amegroups.com/article/view/65941 (accessed on 1 January 2021). [CrossRef] [PubMed]
- Rueckel, J.; Reidler, P.; Fink, N.; Sperl, J.; Geyer, T.; Fabritius, M.; Ricke, J.; Ingrisch, M.; Sabel, B. Artificial intelligence assistance improves reporting efficiency of thoracic aortic aneurysm CT follow-up. Eur. J. Radiol. 2021, 134, 109424. [Google Scholar] [CrossRef]
- Sieren, M.M.; Widmann, C.; Weiss, N.; Moltz, J.H.; Link, F.; Wegner, F.; Stahlberg, E.; Horn, M.; Oecherting, T.H.; Goltz, J.P.; et al. Automated segmentation and quantification of the healthy and diseased aorta in CT angiographies using a dedicated deep learning approach. Eur. Radiol. 2022, 32, 690–701. [Google Scholar] [CrossRef]
- Ghesu, F.C.; Georgescu, B.; Zheng, Y.; Grbic, S.; Maier, A.; Hornegger, J.; Comaniciu, D. Multi-Scale Deep Reinforcement Learning for Real-Time 3D-Landmark Detection in CT Scans. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 41, 176–189. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Quint, L.E.; Liu, P.S.; Booher, A.M.; Watcharotone, K.; Myles, J.D. Proximal thoracic aortic diameter measurements at CT: Repeatability and reproducibility according to measurement method. Int. J. Cardiovasc. Imaging 2013, 29, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.C.; Rizzo, E.; Marques-Vidal, P.M.; von Segesser, L.K.; Dehmeshki, J.; Qanadli, S.D. Variability of ascending aorta diameter measurements as assessed with electrocardiography-gated multidetector computerized tomography and computer assisted diagnosis software. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 217–221. [Google Scholar] [CrossRef]
- Pradella, M.; Weikert, T.; Sperl, J.I.; Kärgel, R.; Cyriac, J.; Achermann, R.; Sauter, A.W.; Bremerich, J.; Stieltjes, B.; Brantner, P.; et al. Fully automated guideline-compliant diameter measurements of the thoracic aorta on ECG-gated CT angiography using deep learning. Quant. Imaging Med. Surg. 2021, 11, 4245–4257. Available online: https://qims.amegroups.com/article/view/73383 (accessed on 1 January 2021). [CrossRef]
- Monti, C.B.; van Assen, M.; Stillman, A.E.; Lee, S.J.; Hoelzer, P.; Fung, G.S.K.; Secchi, F.; Sardanelli, F.; De Cecco, C.N. Evaluating the Performance of a Convolutional Neural Network Algorithm for Measuring Thoracic Aortic Diameters in a Heterogeneous Population. Radiol. Artif. Intell. 2022, 4, e210196. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Boccalini, S.; Kitslaar, P.H.; Budde, R.P.J.; Tu, S.; Lelieveldt, B.P.F.; Dijkstra, J.; Reiber, J.H.C. A novel software tool for semi-automatic quantification of thoracic aorta dilatation on baseline and follow-up computed tomography angiography. Int. J. Cardiovasc. Imaging 2019, 35, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, C.; Tang, A.; Therasse, É.; Giroux, M.-F.; Elkouri, S.; Melanson, P.; Melanson, B.; Oliva, V.L.; Soulez, G. Measurements and detection of abdominal aortic aneurysm growth: Accuracy and reproducibility of a segmentation software. Eur. J. Radiol. 2012, 81, 1688–1694. [Google Scholar] [CrossRef]
- Biesdorf, A.; Rohr, K.; Feng, D.; von Tengg-Kobligk, H.; Rengier, F.; Böckler, D.; Kauczor, H.-U.; Wörz, S. Segmentation and quantification of the aortic arch using joint 3D model-based segmentation and elastic image registration. Med. Image Anal. 2012, 16, 1187–1201. [Google Scholar] [CrossRef]
- Bezerra de Carvalho Macruz, F.; Lu, C.; Strout, J.; Takigami, A.; Brooks, R.; Doyle, S.; Yun, M.; Buch, V.; Hedgire, S.; Ghoshhajra, B. Quantification of the Thoracic Aorta and Detection of Aneurysm at CT: Development and Validation of a Fully Automatic Methodology. Radiol. Artif. Intell. 2022, 4, e210076. [Google Scholar] [CrossRef]
- Sedghi Gamechi, Z.; Bons, L.R.; Giordano, M.; Bos, D.; Budde, R.P.J.; Kofoed, K.F.; Pedersen, J.H.; Roos-Hesselink, J.W.; de Bruijne, M. Automated 3D segmentation and diameter measurement of the thoracic aorta on non-contrast enhanced CT. Eur. Radiol. 2019, 29, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J.; Kim, S.; Lohr, J.; Towey, S.; Velichkovich, Z.; Kabachenko, T.; Driscoll, I.; Baker, B. Classification of Aortic Dissection and Rupture on Post-contrast CT Images Using a Convolutional Neural Network. J. Digit. Imaging 2019, 32, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-T.; Tsai, Y.-S.; Liou, C.-F.; Lee, T.-H.; Kuo, P.-T.P.; Huang, H.-S.; Wang, C.-K. Automated Stanford classification of aortic dissection using a 2-step hierarchical neural network at computed tomography angiography. Eur. Radiol. 2022, 32, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Afifi, R.O.; Casanegra, A.I.; Elefteriades, J.A.; Gleason, T.G.; Hanneman, K.; Roselli, E.E.; Willemink, M.J.; Fischbein, M.P.; on behalf of the American Heart Association Council on Cardiovascular Radiology; et al. Imaging and Surveillance of Chronic Aortic Dissection: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e000075. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Total (n = 100) | |
|---|---|---|
| Age (years) | 67.0 (55.3/73.0) | |
| Female | 40 (40.0) | |
| Body height (cm) | 173.0 ± 10.4 | |
| Body weight (kg) | 84.7 ± 23.1 | |
| BMI (kg/m2) | 27.9 ± 6.1 | |
| Aortic dilation | Predominantly ascending | 44 (44.0) |
| Predominantly descending | 56 (56.0) | |
| History of prior aortic repair | Overall | 56 (56.0) |
| Surgical | 29 (29.0) | |
| Endovascular | 17 (17.0) | |
| Both | 10 (10.0) | |
| Aortic dissection | 42 (42.0) | |
| Manual d (mm) | Automated d (mm) | Wilcoxon # | r # | ICC # | Bias # | LoA # | |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| 40.9 (37.3–48.2) | 42.9 (38.9–50.0) † | <0.05 | 0.86 | 0.93 | 1.5 | −7.8/10.8 | |
| 40.3 (36.6–46.9) ‡ | <0.05 | 0.89 | 0.94 | −1.3 | −9.8/7.2 | ||
| Subgroup I | |||||||
| Ascending aorta | 39.5 (37.3–43.5) | 39.8 (37.4–43.9) ‡ | 0.075 | 0.84 | 0.92 | 0.3 | −5.3/5.9 |
| Descending aorta | 44.0 (36.9–52.5) | 40.8 (34.1–50.0) ‡ | <0.05 | 0.94 | 0.94 | −3.0 | −12.7/6.7 |
| Subgroup II | |||||||
| BMI < 30 kg/m2 | 40.6 (37.3–48.3) | 39.8 (36.3–46.8) ‡ | <0.05 | 0.87 | 0.93 | −1.5 | −10.6/7.6 |
| BMI ≥ 30 kg/m2 | 42.1 (37.6–48.6) | 40.4 (37.1–47.5) ‡ | 0.113 | 0.93 | 0.95 | −1.2 | −8.3/5.9 |
| Subgroup III | |||||||
| Without aortic repair | 38.9 (33.9–44.8) | 39.1 (34.9–44.6) ‡ | 0.933 | 0.95 | 0.96 | −0.8 | −7.7/6.0 |
| With aortic repair | 43.0 (39.0–50.5) | 43.0 (36.8–48.2) ‡ | <0.05 | 0.84 | 0.91 | −1.7 | −11.2/7.8 |
| Subgroup IV | |||||||
| Without dissection | 40.9 (35.7–48.5) | 41.1 (37.1–46.8) ‡ | 0.352 | 0.94 | 0.94 | −1.1 | −10.1/7.9 |
| With dissection | 41.4 (37.7–48.2) | 39.0 (36.5–46.9) ‡ | <0.05 | 0.82 | 0.93 | −1.6 | −9.4/6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, N.; Yacoub, B.; Schoepf, U.J.; Zsarnoczay, E.; Pinos, D.; Vecsey-Nagy, M.; Rapaka, S.; Sharma, P.; O’Doherty, J.; Ricke, J.; et al. Artificial Intelligence Provides Accurate Quantification of Thoracic Aortic Enlargement and Dissection in Chest CT. Diagnostics 2024, 14, 866. https://doi.org/10.3390/diagnostics14090866
Fink N, Yacoub B, Schoepf UJ, Zsarnoczay E, Pinos D, Vecsey-Nagy M, Rapaka S, Sharma P, O’Doherty J, Ricke J, et al. Artificial Intelligence Provides Accurate Quantification of Thoracic Aortic Enlargement and Dissection in Chest CT. Diagnostics. 2024; 14(9):866. https://doi.org/10.3390/diagnostics14090866
Chicago/Turabian StyleFink, Nicola, Basel Yacoub, U. Joseph Schoepf, Emese Zsarnoczay, Daniel Pinos, Milan Vecsey-Nagy, Saikiran Rapaka, Puneet Sharma, Jim O’Doherty, Jens Ricke, and et al. 2024. "Artificial Intelligence Provides Accurate Quantification of Thoracic Aortic Enlargement and Dissection in Chest CT" Diagnostics 14, no. 9: 866. https://doi.org/10.3390/diagnostics14090866





