Optimizing Oxygen Delivery by Low-Flow Nasal Cannula to Small Infants: A Bench Study
Abstract
:1. Introduction
2. Materials and Methods
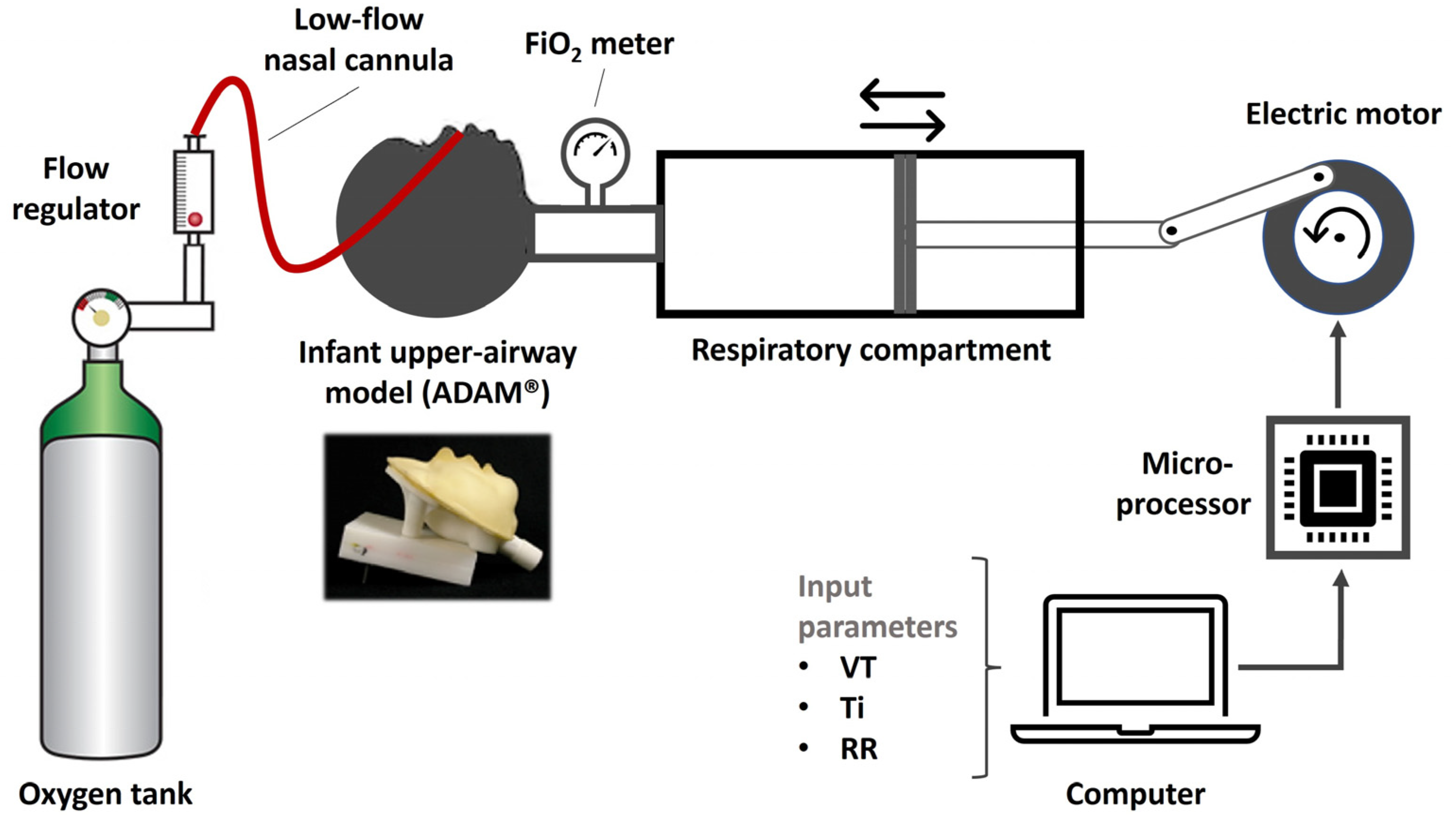
2.1. Experimental Setup
2.2. Simulations
2.3. Statistics and Charts
3. Results
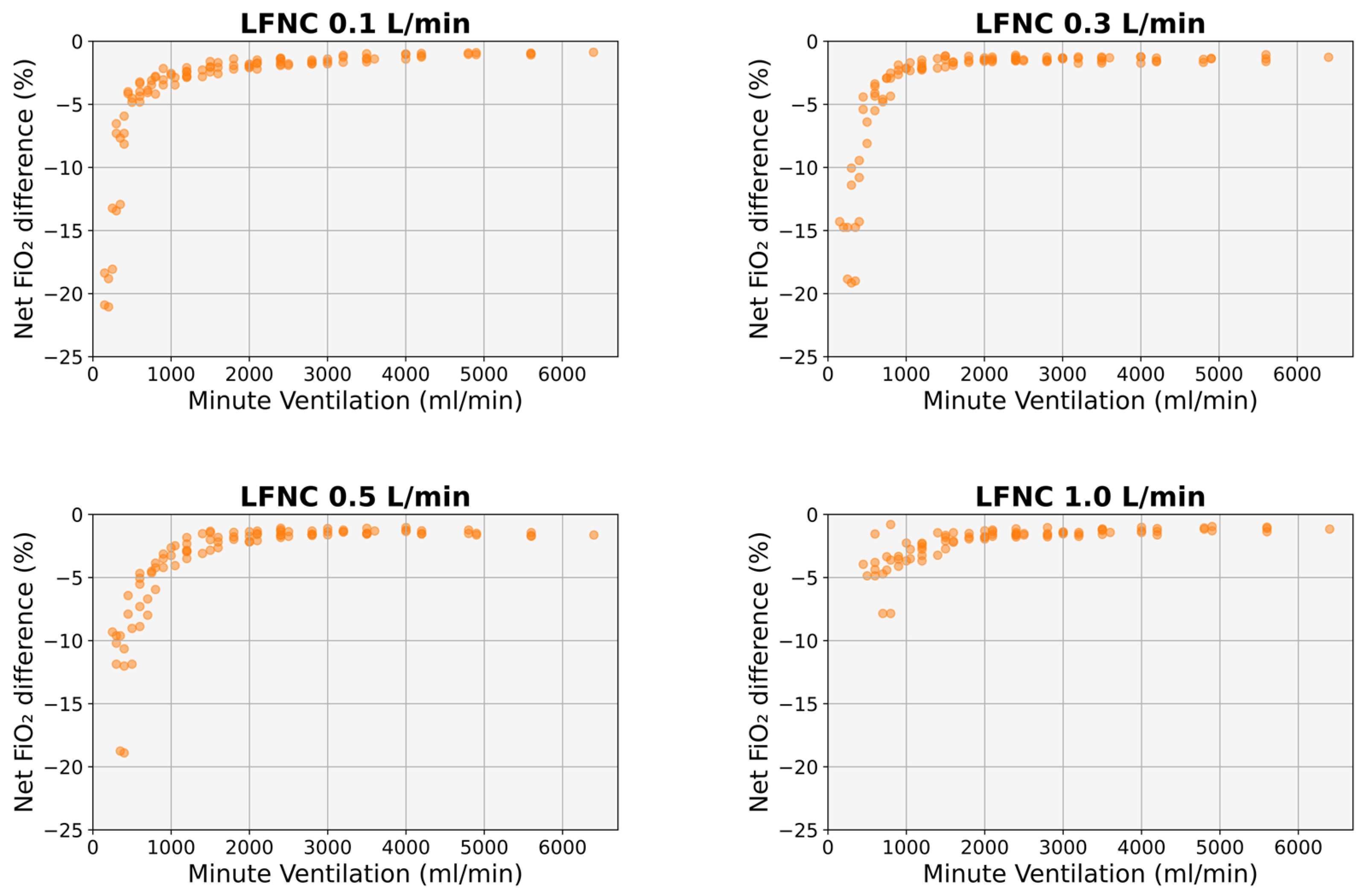
3.1. Determinants of Effective FiO2
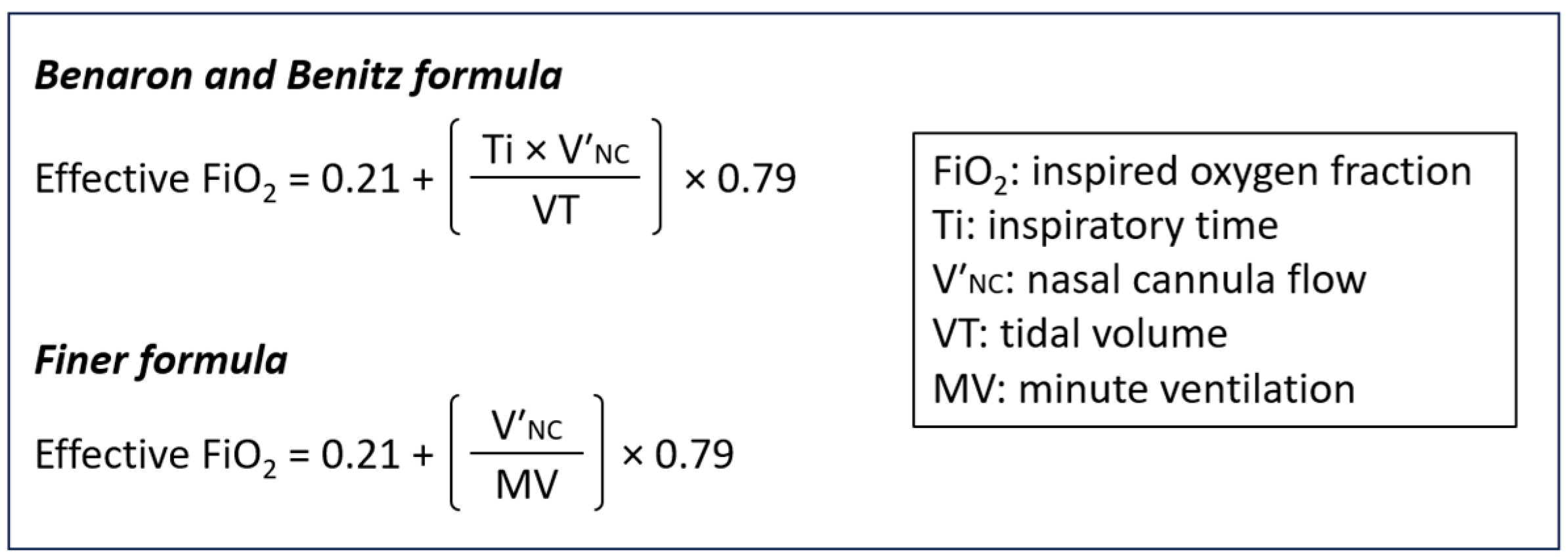
3.2. Comparison with Existing Mathematical Formulas
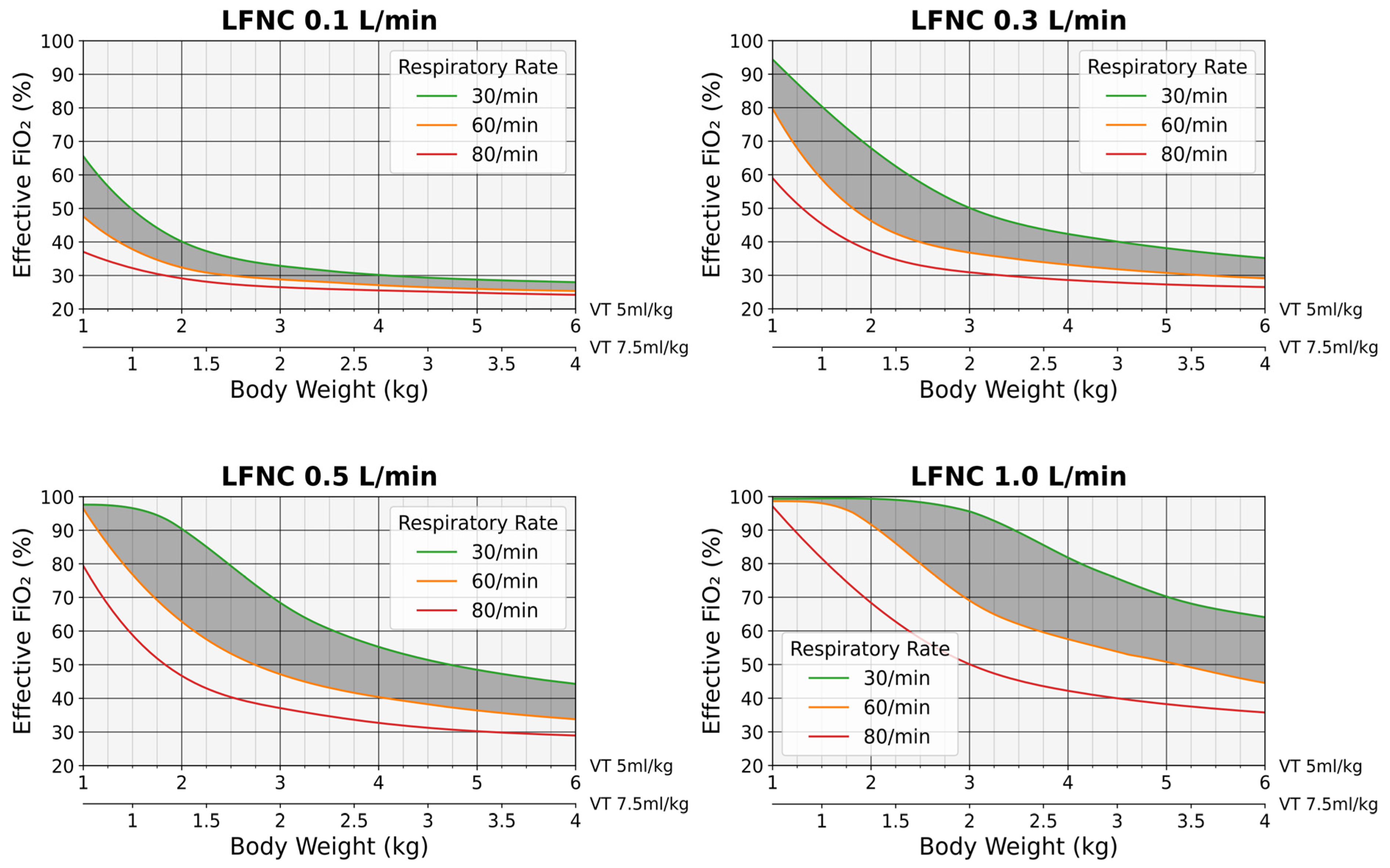
3.3. Predictive FiO2 Charts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, B.K.; Smallwood, C.D. Pediatric oxygen therapy: A review and update. Respir. Care 2017, 62, 645–661. [Google Scholar] [CrossRef]
- Wexler, H.R.; Levy, H.; Cooper, J.D.; Aberman, A. Mathematical model to predict inspired oxygen concentration: Description and validation. Can. Anaesth. Soc. J. 1975, 22, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Benaron, D.A.; Benitz, W.E. Maximizing the stability of oxygen delivered via nasal cannula. Arch. Pediatr. Adolesc. Med. 1994, 148, 294–300. [Google Scholar] [CrossRef]
- Finer, N.N.; Bates, R.; Tomat, P. Low flow oxygen delivery via nasal cannula to neonates. Pediatr. Pulmonol. 1996, 21, 48–51. [Google Scholar] [CrossRef]
- Vain, N.E.; Prudent, L.M.; Stevens, D.P.; Weeter, M.M.; Maisels, M.J. Regulation of oxygen concentration delivered to infants via nasal cannulas. Am. J. Dis. Child. 1989, 143, 1458–1460. [Google Scholar] [CrossRef]
- González, A.J.; Quinteros, A.; Luco, M.; Salinas, J.A.; Martínez, A.; Tapia, J.L. Hypopharyngeal oxygen concentration and pressures delivered by low flow nasal cannula in preterm infants: Relationship with flow, gas mixture, and infant’s weight. Pediatr. Pulmonol. 2019, 54, 1596–1601. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxidative stress in the newborn—A 30-year perspective. Neonatology 2005, 88, 228–236. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Schultz, M.J.; van der Voort, P.H.J.; de Jonge, E.; van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Engle, W.; Laptook, A.; Kazzi, S.N.J.; Buchter, S.; Rasmussen, M.; Yao, Q.; National Institute of Child Health and Human Development Neonatal Research Network. Oxygen delivery through nasal cannulae to preterm infants: Can practice be improved? Pediatrics 2005, 116, 857–861. [Google Scholar] [CrossRef]
- Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000, 105, 295–310. [CrossRef]
- Oden, N.; Phelps, D. STOP-ROP Effective FiO2 Conversion Tables for Infants on Nasal Cannula. 2000. Available online: https://api.semanticscholar.org/CorpusID:70975639 (accessed on 2 March 2024).
- Sabz, M.; Tavernini, S.; Pillay, K.; Christianson, C.; Noga, M.; Finlay, W.H.; Rouhani, H.; Martin, A.R. Variability in low-flow oxygen delivery by nasal cannula evaluated in neonatal and infant airway replicas. Respir. Res. 2022, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.W.; Suggett, J.A.; Coppolo, D.P.; Mitchell, J.P. Development and evaluation of a family of human face and upper airway models for the laboratory testing of orally inhaled products. AAPS PharmSciTech 2017, 18, 3182–3197. [Google Scholar] [CrossRef] [PubMed]
- Numa, A.H.; Newth, C.J. Anatomic dead space in infants and children. J. Appl. Physiol. 1985 1996, 80, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Arduino. Available online: https://www.arduino.cc/ (accessed on 2 March 2024).
- Stocks, J.; Dezateux, C.A.; Jackson, E.A.; Hoo, A.F.; Costeloe, K.L.; Wade, A.M. Analysis of tidal breathing parameters in infancy: How variable is TPTEF:TE? Am. J. Respir. Crit. Care Med. 1994, 150, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Ma, L.-Y.; Huang, N.-N. Measurement of tidal breathing pulmonary function in premature infants with different gestational ages. Chin. J. Contemp. Pediatr. 2015, 17, 449–452. (In Chinese) [Google Scholar]
- Paetow, U.; Windstetter, D.; Schmalisch, G. Variability of tidal breathing flow-volume loops in healthy and sick newborns. Am. J. Perinatol. 1999, 16, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Latzin, P.; Roth, S.; Thamrin, C.; Hutten, G.J.; Pramana, I.; Kuehni, C.E.; Casaulta, C.; Nelle, M.; Riedel, T.; Frey, U. Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS ONE 2009, 4, e4635. [Google Scholar] [CrossRef]
- Zhou, S.; Chatburn, R.L. Effect of the anatomic reservoir on low-flow oxygen delivery via nasal cannula: Constant flow versus pulse flow with portable oxygen concentrator. Respir. Care 2014, 59, 1199–1209. [Google Scholar] [CrossRef]
- Bates, J.H.; Schmalisch, G.; Filbrun, D.; Stocks, J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur. Respir. J. 2000, 16, 1180–1192. [Google Scholar] [CrossRef]
- Malmberg, L.P.; Seppä, V.-P.; Kotaniemi-Syrjänen, A.; Malmström, K.; Kajosaari, M.; Pelkonen, A.S.; Viik, J.; Mäkelä, M.J. Measurement of tidal breathing flows in infants using impedance pneumography. Eur. Respir. J. 2017, 49, 1600926. [Google Scholar] [CrossRef]
- Kuluz, J.W.; McLaughlin, G.E.; Gelman, B.; Cantwell, G.P.; Thomas, J.; Mahon, T.; Schleien, C.L. The fraction of inspired oxygen in infants receiving oxygen via nasal cannula often exceeds safe levels. Respir. Care 2001, 46, 897–901. [Google Scholar] [PubMed]
- Mantena, S.; Burke, T.F. Oxygen blending is urgently needed in resource-limited settings. J. Pediatr. 2021, 23, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Herrod, S.K.; Stevenson, A.; Vaucher, Y.E.; Lambert, S.R.; Isenberg, S.J.; Yap, V.L.; Ezeaka, V.C.; Carlo, W.A. Oxygen management among infants in neonatal units in sub-Saharan Africa: A cross-sectional survey. J. Perinatol. 2021, 41, 2631–2638. [Google Scholar] [CrossRef]



| Parameter | Simulated Range |
|---|---|
| Nasal cannula flow (L/min) | 0.1–1 |
| VT (mL) | 5–80 |
| RR (breaths/min) | 30–80 |
| Ti (ms) | 300–900 |
| Ti/Te ratio | 0.5–1.5 |
| MV (mL/min) | 150–6400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertzouanis, A.; Sinopidis, X.; Pelekouda, P.; Karatza, A.; Dimitriou, G.; Fouzas, S. Optimizing Oxygen Delivery by Low-Flow Nasal Cannula to Small Infants: A Bench Study. Diagnostics 2024, 14, 889. https://doi.org/10.3390/diagnostics14090889
Bertzouanis A, Sinopidis X, Pelekouda P, Karatza A, Dimitriou G, Fouzas S. Optimizing Oxygen Delivery by Low-Flow Nasal Cannula to Small Infants: A Bench Study. Diagnostics. 2024; 14(9):889. https://doi.org/10.3390/diagnostics14090889
Chicago/Turabian StyleBertzouanis, Aris, Xenophon Sinopidis, Polyxeni Pelekouda, Ageliki Karatza, Gabriel Dimitriou, and Sotirios Fouzas. 2024. "Optimizing Oxygen Delivery by Low-Flow Nasal Cannula to Small Infants: A Bench Study" Diagnostics 14, no. 9: 889. https://doi.org/10.3390/diagnostics14090889






